Abstract
Consecutive underwater observations carried out in shallow waters of Pontetto (Ligurian Sea) from June to the end of November 2013 revealed the presence of purple areas on the white surface of the asconoid calcareous sponge Clathina coriacea. Histological and ultrastructural investigations performed on the purple areas of the sponge showed the occurrence of a network of hyphae of an indeterminate fungus permeating the sponge tissues and presumably responsible for the unusual colour of the sponge. The hyphae, varying in size and morphology according to their location in the sponge body, are visible on the outermost sponge surface and, after crossing the mesohyl, penetrate inwards into the choanodermal layer, being mainly located along the apical border of the choanocytes. The occurrence of undamaged flagella and microvillar fringes, which are the organelles characterising the choanocyte cells, is consistent with a normal functionality of the sponge tissues, notwithstanding the presence of the fungal hyphae. The fungus develops only in summer because it disappears concomitantly with the approach of autumn. This feature is coherent with the consideration that the sponge acts as a suitable substrate for the developing fungus, which, in turn, does not interfere with the sponge filter-feeding activity.
Introduction
Porifera, one of the oldest metazoan groups, have colonised a wide range of freshwater and marine habitats, becoming fundamental components of the benthic communities. In different organisational models of sponges, the mesohyl has acquired a growing relevance for the increasing number of the encompassed cell types and for the differentiation of a variety of skeletal elements. Additionally, the mesohyl matrix has allowed the establishment of symbiotic associations with microorganisms, which in some species can constitute up to 40% of the cellular component (Vacelet Citation1975; Hentschel et al. Citation2006).
In fact, one of the most fascinating aspects of sponge ecology is the transphyletic interactions that these animals are able to establish. A remarkable number of papers have been devoted to documenting the relationships between sponges and symbionts, namely photosynthetic and heterotrophic bacteria (Taylor et al. Citation2007). In addition, due to the body organisation in which they are permeated by a complex canal system where a continuous water flow is induced by their filter-feeding activity, sponges are temporary or permanent substrata also for many metazoan species (Magnino & Gaino Citation1998; Puce et al. Citation2005; Calcinai et al. Citation2006a).
Symbiosis involving sponges shows a trend from relationships in which the two partners are independents, and live together to obtain a better chance for refuge or food supply, to relationships in which the two partners are deeply interconnected to form a unique symbiotic super-organism (Calcinai et al. Citation2006b).
The association with marine microorganisms represents the most outstanding feature because it is commonly known that their concentration within the sponge tissue exceeds by up to 2–4 orders of magnitude that of the microbes living in seawater (Hentschel et al. Citation2006). Sponge-associated algae have also been widely explored: although symbiotic zooxanthellae are mainly diffused in the species of the family Clionaidae (Granados et al. Citation2008), these organisms have been also sporadically recorded in other families (Scalera-Liaci et al. Citation1999).
The conveyance of unicellular autotrophic symbionts from generation to generation, by the intermediate of germinal cells, has been documented in Chondrilla australiensis Carter, 1873 (Usher et al. Citation2005), whereas in Tethya orphei Sarà, 1990, filamentous cyanobacteria were transferred from the mother tissue to the new individuals during the differentiation of asexually produced buds (Gaino et al. Citation2006).
Fungi associated with marine sponges have been frequently observed from temperate, tropical and polar waters (Gao et al. Citation2008; Wang et al. Citation2008; Baker et al. Citation2009; Lie & Wang Citation2009; Liu et al. Citation2010; Menezes et al. Citation2010; Paz et al. Citation2010; Ding et al. Citation2011; Wiese et al. Citation2011; Zhou et al. Citation2011; Suryanarayanan Citation2012; Thirunavukkarasu et al. Citation2012; Henriquez et al. Citation2013). Their diversity was mainly explored in order to assess the potential for the production of novel active secondary metabolites with biological activity (Höller et al. Citation2000; Henriquez et al. Citation2013) and, consequently, the real biological significance of the relationships remains unclear. In fact, from the isolation of a fungus from a marine sponge, it is not possible to assume if it is occasional or actually associated: it may grow there because sponges filter from the surrounding environment, which contains spores of terrestrial fungi that are facultative marine organisms (Höller et al. Citation2000). To date, only marine ascomycetes of the genus Koralionastes have been reported to have a unique physical association with encrusting sponges, developing their ascomata on or within these hosts (Kohlmeyer & Volkmann-Kohlmeyer Citation1990).
Interesting results in understanding the ecological role of fungi associated with sponges were obtained by molecular analysis of the two Hawaiian demosponges Suberites aurantiacus (de Laubenfels, 1936) and Mycale armata Thiele, 1903, where 23 and one fungal species were respectively found (Gao et al. Citation2008). This phylogenetic analysis indicated sponge-derived sequences were clustered into “marine fungus clades”.
How marine sponges and filamentous fungi establish ecological relationships remains unknown (Lie & Wang Citation2009). In order to confirm the real association between fungi and marine sponges, and to determine the vital functions of the fungi inside the sponge tissues, transmission electron microscopy (TEM) and non-cultivation-dependant diagnosis are needed (Passarini et al. Citation2013).
The aim of this paper was the description, at an ultrastructural level (TEM and scanning electron microscopy, SEM), of the association observed in the shallow waters of the Ligurian Sea between the calcarea Clathrina coriacea (Montagu, 1914), characterised by a simple, asconoid structure, and an unidentified purple fungus.
Figure 1. The white marine sponge Clathrina coriacea (a–c) in its natural environment, and (d) histological section of a body region. (a) typical anastomosed tubes ending in sharp conules bearing apical oscules (arrows); (b) patched purple areas on the sponge surface; (c) purple areas covering almost the entire sponge surface; (d) tangential histological section of a purple area of the sponge showing the network of fungal hyphae (arrows).
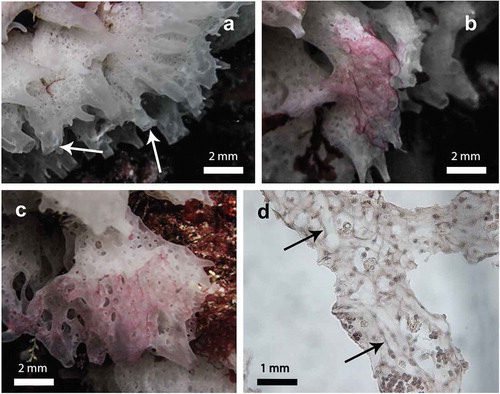
Figure 2. (a–c) histological sections, (d, f) scanning and (e, g) transmission electron microscopy of the purple areas of the sponge Clathrina coriacea. (a) cross section of a tube showing the position of the fungal hyphae (arrows) bordering the choanodermal layer (cl); m, mesohyl; pl, pinacodermal layer; (b) a fairly tangential section showing a hyphal filament in the sponge cavity (arrow); cl, choanodermal layer; (c) a hyphal filament (arrow) crossing the mesohyl (m), cl, choanodermal layer; (d) a hyphal filament on the choandodermal layer (cl); f, flagella of the choncocytes; (e) a hyphal filament showing its irregular pattern; (f) a fractured sponge tube showing a hyphal filament (hf) overgrowing a portion of the choanodermal layer (cl); (g) irregular shape of hyphal filaments (hf) interposed between two choanocytes (ch).
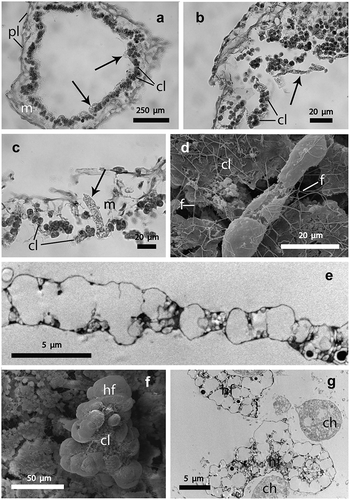
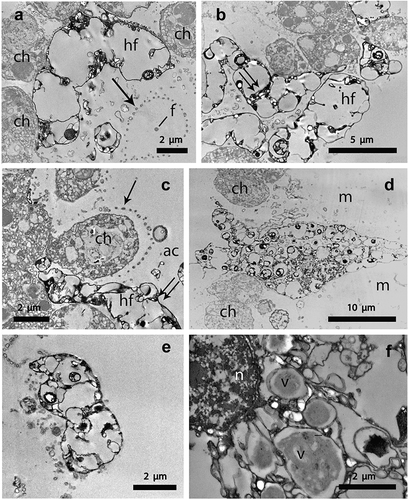
Figure 3. Transmission electron microscopy of the sponge/hyphal filament interaction in the purple areas of Clathrina coriacea. (a) region of a hyphal filament (hf) adhering to the choanocytes (ch). Note the presence of the choanocyte collar fringe (arrow) surrounding the central flagellum (f); (b) hyphal filaments (hf) showing their subtle elctron-dense border delimiting the outermost surface. Note the vacuolated central region crossed by thin septa (double arrow); (c) apical region of a hyphal filament (hf) penetrating into the choanodermal layer, whereas the remaining region (double arrow) protrudes towards the sponge atrial cavity (ac), ch, choanocyte; arrow points out the collar fringe; (d) region of a hyphal filament crossing the mesohyl (m) and penetrating into the choanodermal layer; ch, choanocyte; (e) region of a hyphal filament located on the outermost sponge surface; (f) a hyphal filament showing a portion of the nucleus (n) encircled by vacuoles (v) having an electron-dense core.
Materials and methods
Sponge collection
Specimens of the calcareous sponge Clathrina coriacea were collected by scuba diving in Pontetto (Eastern Ligurian Sea) (44°22’ 34.23 N; 9°04’ 24.10 E), at a depth varying from 1 to 5 m. Diving was performed from June to the end of November 2013. Once excided, sponge specimens were fixed underwater in 2.5% glutaraldehyde buffered with filtered artificial seawater (ASW) (pH adjusted to 7.5–7.8, with 0.1 N sodium hydroxide, NaOH) for 1 h, and transferred directly to sterilised locked bags containing ASW to prevent contact of sponge tissue with air. The samples were transported to the laboratory and immediately processed for histological and ultrastructural analyses. To obtain an estimation of the number of sponges hosting the purple symbiont, all the specimens of C. coriacea showing or not the purple colouration were counted during three sessions of 5’ of slow swimming (about 100 m long each).
Histological and ultrastructural analyses
For histological investigations, several fixed fragments of the purple areas (about 1 cm2 each) were repeatedly washed in ASW and processed according to the routinely used procedures; sections of 5–7 μm in thickness were then observed with a Leica microscope (Leica LMS Holdings GmH, Wetzlar, Germany).
For TEM, small fragments of the purple areas (about 2 mm2 each) were repeatedly washed in ASW and post-fixed in 1% osmium tetroxide, in ASW used as a buffer, for 1 h at 4°C; after repeated washes, the material was dehydrated in a series of ethanol dilutions and embedded in an Epon-Araldite mixture. Ultrathin sections were cut on a Leica EM UC6 ultracut (Leica Microsystem GmbH, Vienna, Austria), collected on formvar-coated copper grids, stained with uranyl acetate and lead citrate and examined with a Philips EM 208 (Philips).
Some fragments of the fixed material were processed for SEM. In this regard, they were repeatedly washed in ASW, dehydrated in a series of ethanol dilutions, critical point-dried, mounted on stubs with silver conducting paint, sputter-coated with gold-palladium in an Emitech K 550X (Emitech, Ashford, UK) sputter and observed with a Philips XL30 (Philips, Eindhoven, the Netherlands) microscope at an accelerating voltage of 18 kV.
Results
Clathrina coriacea is a white sponge living in the upper fringe of the rocky infra-littoral community. This sponge had a size ranging from 2 to 10 cm and was characterised by a net of contorted and anastomosed tubes of 0.5–1 mm in diameter, ending in sharp conules bearing apical oscules (). About 10% of the observed sponges showed patched purple areas, particularly outstanding on the white tissue of the sponge (). In some specimens, the purple areas widely invaded the sponge tissues occupying as much as 80% of the volume (–). In vivo, the sponge showed no sign of degeneration on its purple areas.
Histological tangential sections of the sponge tubes showed fungal hyphae permeating the mesohyl region (). In cross sections, the fungal hyphae were easily detectable as translucent elements overlapping the sponge choanocytes (). The shape of the hyphal filaments varied according to the location of these elements: from elongated in shape, when located inside the atrial cavity () or when crossing the thickness of the mesohyl (), to fairly roundish, when closer to the choanodermal layer ().
SEM images confirmed the occurrence of the hyphae along the choanodermal layer facing the atrial cavity. TEM analysis showed the irregular shape of the hyphae, the diameter of which varied along their length (). In some fractured sponge tubes, observed under SEM, it became evident that the hyphae were so large as to overgrow and partially envelop entire portions of the choanodermal layer (). This feature was also confirmed by TEM, which showed the hyphae interposed between adjacent choanocytes ().
In addition, TEM investigations allowed the location of the fungal hyphae in the sponge tissue to be detailed. In the choanodermal region, the hyphae were almost adherent to the choanocytes that bordered the atrial cavity, where collar fringes encircling the central flagellum (), a characteristic of the choanocytes, were also evident. The hyphae presented a subtle electron-dense border delimiting a vacuolated central region irregularly crossed by thin septa (). The hyphae permeated the choanodermal layer in such a way that their apical region infiltrated among choanocytes and the rest of the filament protruded into the sponge atrial cavity (). Consequently, the continuity of the choanodermal layer was frequently interrupted by the presence of the hyphae reaching this tissue after having crossed the mesohyl matrix (). Some images showed fragments of the hyphae adherent to the outermost sponge surface (). On occasion, the nucleus of the hyphae was evident, encircled by vacuoles with a central electron-dense core delimited by an electron-translucent border ().
Discussion
Although demosponges are involved in a wide range of symbiotic relationships (Wulff Citation2006; Calcinai et al. Citation2013), calcareous sponges offer less opportunity for housing other organisms. This is probably due to their simple, asconoid structure and to the thinness of the mesohyl. Nevertheless, specimens of Clathrina luteoculcitella Wörheide & Hooper, 1999 from South China showed a great culturable fungal diversity represented by 12 genera among which the species Nigrospora oryzae (Berk & Br.) strain PF18, having a strong and broad spectrum of antimicrobial activities, could have potential for antimicrobial compound production (Ding et al. Citation2011).
At present, among the species of the genus Clathrina living the Liguria Sea, namely C. cerebrum (Haeckel, 1872), C. clathrus (Schmidt, 1864), C. contorta (Bowerbank, 1866) and C. rubra Sarà, 1958, C. coriacea represents the most diffused sponge (Longo & Pronzato Citation2011). In fact, C. clathrus, C. contorta and C. rubra are becoming rare representatives of the sponge community, and C. cerebrum, which in the past survived the summer heat crisis in minute forms (Gaino et al. Citation1996), has recently been strongly reduced (unpublished data).
In C. cerebrum, elongated bacteria, tentatively attributed to the genus Cytophaga, were recorded adherent to the choanodermal region and, more rarely, penetrating into the mesohyl (Burlando et al. Citation1988). These authors suggested this association was probably based on some species-specific mechanisms because such a relationship was lacking in other congeneric species.
In Sycon sp., characterised by a syconoid structure, two species of fungi (Emericellopsis minima Stolk and Scopulariopsis candida Vuill.) were isolated (Höller et al. Citation2000), but no information about the real nature of the association between the fungal strains and the sponge was provided.
This paper gives the first ultrastructural evidence of the relationship between a fungus and a marine sponge, allowing us to demonstrate the location of the fungal hyphae within both the mesohyl matrix and the choanodermal layer.
The occurrence of the fungal infection does not apparently affect the sponge organisation, as evidenced at the ultrastructural level by the integrity of both flagella and the collar fringes of the choanocytes. This evidence strongly suggests that the choanocyte functionality also remains unchanged.
The presence of the fungus does not constitute a stable relationship with the sponge tissues because repeated underwater observations, carried out for 6 months between summer and autumn, allowed us to verify the disappearance of the pigmented colonies of C. coriacea after the middle of October. This feature proves that the fungus develops inside the sponge body only during the summer season. The thin and typically not pigmented sponge tubes facilitated a rapid detection of the presence/absence of the fungal hyphae, without the need to apply the complex techniques usually utilised for isolating the fungal communities (Höller et al. Citation2000; Gao et al. Citation2008; Henriquez et al. Citation2013; Passarini et al. Citation2013). Additional surveys, carried out in early and late winter, confirmed the presence of the population of C. coriacea characterized by the typical white colour. This feature proves that the sponge does not have a seasonality development, being present all year round in the sampling site.
The sporadic presence of the fungus did not allow its characterisation to be performed. As the sponge/fungus association does not interfere with the survival of the sponge population, we can speculate that the fungus exploited the sponge body as a suitable substrate for its further development, without affecting sponge functionality. Nevertheless, the total lack of records concerning the presence of the fungus on the surrounding substratum or associated with other benthic organisms induces us to suppose a certain degree of specificity in this association.
References
- Baker PW, Kennedy J, Dobson ADW, Marchesi JR. 2009. Phylogenetic diversity and antimicrobial activities of fungi associated with Haliclona simulans isolated from Irish coastal waters. Marine Biotechnology 11:540–547. doi:10.1007/s10126-008-9169-7.
- Burlando B, Sabatini MA, Gaino E. 1988. Association between calcareous Clathrina cerebrum (Haeckel) and bacteria: Electron microscope study. Journal of Experimental Marine Biology and Ecology 116:35–42. doi:10.1016/0022-0981(88)90244-4.
- Calcinai B, Bavestrello G, Bertolino M, Pica D, Wagner D, Cerrano C. 2013. Sponges associated with octocorals in the Indo-Pacific, with the description of four new species. Zootaxa 3617:1–61. doi:10.11646/zootaxa.3617.1.1.
- Calcinai B, Cerrano C, Bavestrello G. 2006a. Sponges as hotspot of biodiversity. Biologia Marina Mediterranea 12:63–68.
- Calcinai B, Cerrano C, Totti C, Romagnoli T, Bavestrello G. 2006b. Symbiosis of Mycale (Mycale) vansoesti sp. nov. (Porifera, Demospongiae) with a coralline alga from North Sulawesi (Indonesia). Invertebrate Biology 125:195–204. doi:10.1111/j.1744-7410.2006.00052.x.
- Ding B, Yin Y, Zhang F, Li Z. 2011. Recovery and phylogenetic diversity of culturable fungi associated with marine sponges Clathrina luteoculcitella and Holoxea sp. in the South China Sea. Marine Biotechnology 13:713–721. doi:10.1007/s10126-010-9333-8.
- Gaino E, Bavestrello G, Cerrano C, Sarà M. 1996. Survival of the calcareous sponge Clathrina cerebrum (Haeckel, 1872) on a vertical cliff during the summer crisis. Italian Journal of Zoology 63:41–46. doi:10.1080/11250009609356105.
- Gaino E, Sciscioli M, Lepore E, Rebora M, Corriero G. 2006. Association of the sponge Tethya orphei (Porifera, Demospongiae) with filamentous cyanobacteria. Invertebrate Biology 125:281–287. doi:10.1111/j.1744-7410.2006.00061.x.
- Gao Z, Li B, Zheng C, Wang G. 2008. Molecular detection of fungal communities in the Hawaiian marine sponges Suberites zeteki and Mycale armata. Applied and Environmental Microbiology 74:6091–6101. doi:10.1128/AEM.01315-08.
- Granados C, Camargo C, Zea S, Sánchez JA. 2008. Phylogenetic relationships among zooxanthellae (Symbiodinium) associated to excavating sponges (Cliona spp.) reveal an unexpected lineage in the Caribbean. Molecular Phylogenetics and Evolution 49:554–560. doi:10.1016/j.ympev.2008.07.023.
- Henriquez M, Vergara K, Norambuena J, Beiza A, Maza F, Ubilla P, Araya I, Chàvez R, San-Martin A, Darias J, Darias MJ, Yaca I. 2013. Diversity of cultivable fungi associated with Antarctic marine sponges and screening for the antimicrobial, antitumoral and antioxidant potential. World Journal Microbiology Biotechnology doi:10.1007/s11274-013-1418–x.
- Hentschel U, Usher KM, Taylor MV. 2006. Marine sponges as microbial fermenters. FEMS Microbiology and Ecology 55:167–177. doi:10.1111/j.1574-6941.2005.00046.x.
- Höller U, Wright AD, Matthee GF, Konig GM, Draeger S, Aust H-J, Schulz B. 2000. Fungi from marine sponges: Diversity, biological activity and secondary metabolites. Mycological Research 104:1354–1365. doi:10.1017/S0953756200003117.
- Kohlmeyer J, Volkmann-Kohlmeyer B. 1990. New species of Koralionastes (Ascomycotina) from the Caribbean and Australia. Canadian Journal of Botany 68:1554–1559. doi:10.1139/b90-199.
- Lie Q, Wang G. 2009. Diversity of fungal isolates from three Hawaiian marine sponges. Microbiological Research 164:233–241. doi:10.1016/j.micres.2007.07.002.
- Liu WC, Li CQ, Zhu P, Yang JL, Cheng KD. 2010. Phylogenetic diversity of culturable fungi associated with two marine sponges: Haliclona simulans and Gelliodes carnosa, collected from the Hainan Island coastal waters of the South China Sea. Fungal Diversity 42:1–15. doi:10.1007/s13225-010-0022-8.
- Longo C, Pronzato R. 2011. Class Calcarea. In: Pansini M, Manconi R, Pronzato R, editors. Fauna d’Italia, Porifera I. Milano: Calderini. pp. 117–244.
- Magnino G, Gaino E. 1998. Haplosyllis spongicola Grube Polychaeta, Syllidae associated with two species of sponges from East Africa Tanzania, Indian Ocean. Marine Ecology 19:77–87. doi:10.1111/j.1439-0485.1998.tb00455.x.
- Menezes CBA, Bonugli-Santos RC, Miqueletto PB, Passarini MRZ, Silva CHD, Justo MR, Leal RR, Fantinatti-Garboggini F, Oliveira VM, Berlinck RGS, Sette LD. 2010. Microbial diversity associated with algae, ascidians and sponges from the north coast of São Paulo state, Brazil. Microbiological Research 165:466–482. doi:10.1016/j.micres.2009.09.005.
- Passarini MRZ, Santos C, Lima N, Berlinck RGS, Sette LD. 2013. Filamentous fungi from the Atlantic marine sponge Dragmacidon reticulatum. Archives of Microbiology 195:99–111. doi:10.1007/s00203-012-0854-6.
- Paz Z, Komon-Zelazowska M, Druzhinina IS, Aveskamp MM, Shnaiderman A, Aluma Y, Carmeli S, Ilan M, Yarden, O. 2010. Diversity and potential antifungal properties of fungi associated with a Mediterranean sponge. Fungal Diversity 42:17–26. doi:10.1007/s13225-010-0020-x.
- Puce S, Calcinai B, Bavestrello G, Cerrano C, Gravili C, Boero F. 2005. Hydrozoa (Cnidaria) symbiotic with Porifera: A review. Marine Ecology 26:73–81.
- Scalera-Liaci L, Sciscioli M, Lepore E, Gaino E. 1999. Symbiotic zooxanthellae in Cynachira tarentina a non-boring demosponge. Endocytobiosis & Cell Research 13:105–114.
- Suryanarayanan TS. 2012. The diversity and importance of fungi associated with marine sponges. Botanica Marina 55:553–564. doi:10.1515/bot-2011-0086.
- Taylor MW, Radax R, Steger D, Wagner M. 2007. Sponge-associated microorganisms: Evolution, ecology, and biotechnological potential. Microbiology and Molecular Biology Reviews 71:295–347. doi:10.1128/MMBR.00040-06.
- Thirunavukkarasu N, Suryanarayanan TS, Girivasan KP, Venkatachalam A, Geetha V, Ravishankar JP, Doble M. 2012. Fungal symbionts of marine sponges from Rameswaram, southern India: Species composition and bioactive metabolites. Fungal Diversity 55:37–46. doi:10.1007/s13225-011-0137-6.
- Usher KM, Sutton DC, Toze S, Kuo J, Fromont J. 2005. Inter-generational transmission of microbial symbionts in the marine sponge Chondrilla australiensis (Demospongiae). Marine and Freshwater Research 56:125–131. doi:10.1071/MF04304.
- Vacelet J. 1975. Étude en Microscopie Électronique de l’Association entre Bactéries et Spongiaires du Genre Verongia (Dictyoceratida). Journal de Microscopie et de Biologie Cellulaire 23:271–288.
- Wang G, Li Q, Zhu P. 2008. Phylogenetic diversity of culturable fungi associated with the Hawaiian sponges Suberites zeteki and Gelliodes fibrosa. Antonie van Leeuwenhoek 93:163–174. doi:10.1007/s10482-007-9190-2.
- Wiese J, Ohlendorf B, Blümel M, Schmaljohann R, Imhoff JF. 2011. Phylogenetic identification of fungi isolated from the marine sponge Tethya aurantium and identification of their secondary metabolites. Marine Drugs 9:561–585. doi:10.3390/md9040561.
- Wulff JL. 2006. Ecological interactions of marine sponges. Canadian Journal of Zoology 84:146–166. doi:10.1139/z06-019.
- Zhou K, Zhang X, Zhang F, Li Z. 2011. Phylogenetically diverse cultivable fungal community and polyketide synthase (PKS), non-ribosomal peptide synthase (NRPS) genes associated with the South China Sea sponges. Microbiology Ecology 62:644–654. doi:10.1007/s00248-011-9859-y.
