Abstract
The present paper describes the oligochaete assemblages of 12 Swiss Alpine lakes (1700–2500 m above sea level) in Canton Ticino. The lake catchment geology is dominated by gneiss. The scarcity of carbonate rocks leads to a low buffering capacity, increasing the lakes’ sensitivity to acidification. Because of their very low phosphorus concentrations, they are defined as ultraoligotrophic. Oligochaetes were identified from kick-samples taken from the outflow and littoral zone of the lakes one to three times per year in 1991–1994, 2003 and 2007, when the lake water chemistry was also characterized. Oligochaete assemblages consisted of 19 species in total, 18 of which were found in the littoral zone, and 10 in the outlets. Amongst them, Cernosvitoviella goodhui Healy, 1975 was recorded in Switzerland for the first time. Principal component analysis (PCA), performed excluding the highly alkaline Lake Bianco (pH = 7.8 and alkalinity = 566 µeq L−1) due to its atypical chemical composition, divided the remaining lakes into two groups with different sensitivity to acidity: the first group of four lakes was characterized by a higher average pH (6.4) and alkalinity (32 µeq L−1), whilst the second group of seven lakes was characterized by a lower average pH (5.7) and alkalinity (5 µeq L−1). Multivariate analysis performed on data collated from the littoral zone highlighted geo-lithology as a key driver in determining the species distribution among lakes. When applied to the lake outlet data, a similar distinction between acidic and calcareous waters was implied. Precipitation influenced the oligochaete assemblage in the littoral zone. During years with higher annual rainfall, the relative abundance of Enchytraeidae increased, probably because their semi-aquatic nature allows them to colonize the littoral zones that dry out periodically.
Introduction
Benthic fauna contribute to the biological diversity of surface waters and are considered an essential food resource for amphibians, fish and birds, as well as being crucial for the breakdown of organic matter. Oligochaetes have often been neglected in benthic macroinvertebrate studies in high-altitude freshwater environments, in spite of their importance and value as indicators of water quality, and their worldwide distribution. This is primarily due to the difficulty in identifying sexually immature organisms to the species level, which requires a high degree of taxonomic and ecological expertise. However, studies of the ecological response of aquatic oligochaetes to anthropogenic disturbances are numerous (Rodriguez & Reynoldson Citation2011). One of the most studied responses is that of trophic status (Rodriguez & Reynoldson Citation2011; Rashid & Pandit Citation2014), but many other factors, such as water chemistry (either as pollution or acidification), sediment particle size or water flow, and the invasion of alien species (e.g. Friday Citation1987; Rasmussen & Lindegaard Citation1988; Jeffries Citation1991; Stephenson et al. Citation1994; Brodersen et al. Citation1998; Abraham et al. Citation1999; Ilyashuk Citation1999; Heino Citation2000; Timm & Möls Citation2012; Boggero et al. Citation2014; Rota et al. Citation2014), can influence the biological diversity and the abundance of macroinvertebrates, including oligochaetes.
In European high-altitude lentic waters in different mountain ranges, oligochaetes and chironomids represent an important component of the benthic fauna (Laville Citation1971; Juget & Giani Citation1974; Stoichev Citation2000; Kownacki et al. Citation2006; Dumnicka & Boggero Citation2007). In lakes above the tree line, as well as in acidic mountain lakes, oligochaetes and chironomids are usually represented by the highest number of species (Kownacki et al. Citation2000) and reach the highest densities (Kownacki et al. Citation2006; Oertli et al. Citation2008) among taxonomic groups of benthic fauna. On the contrary, in high mountain streams, the relative abundance of oligochaetes is usually low, though they can make up 10–20% of the benthic community (Maiolini et al. Citation2006) at lake inlets and outlets. However, all over Europe, the composition of oligochaete fauna in alpine lakes differs from that found in the lowlands, and there are several species or genera that can be said to characterize such water bodies. Among them, Stylodrilus spp., Cernosvitoviella spp., Cognettia spp., Tubifex tubifex (Müller, 1774), Spirosperma ferox Eisen, 1879 and a few Nais species are the most common (Juget & Giani Citation1974; Uzunov & Varadinova Citation2000; Krno et al. Citation2006; Dumnicka & Boggero Citation2007; Dumnicka & Galas Citation2012). Several studies concerned with which abiotic factors, such as water acidification, affect the composition of oligochaete assemblages in alpine ponds and lakes were recently attempted (Kownacki et al. Citation2006; Dumnicka & Galas Citation2012). However, only a few recent studies have focused on oligochaetes living in Swiss high-altitude alpine ponds, lakes (Oertli et al. Citation2008) and rivers (Malard et al. Citation2001, Citation2003), so information on this taxonomic group in the Alps is scarce (Dumnicka Citation2004; Dumnicka & Boggero Citation2007; Dumnicka Citation2010).
Macroinvertebrates were sampled from 1991–1994 in nine high-altitude mountain lakes in southern Switzerland, with regular surveys carried out since 2000 centred on four of them. Due to oligochaete abundances in these environments and the general underestimation of their importance, the first data on their communities are presented in this paper. The aims of this study are (i) to extend the information available concerning the diversity of oligochaete fauna living in alpine lakes, and (ii) to understand their ecological requirements, for which information is severely lacking at high altitudes.
Materials and methods
Site description
Canton Ticino is one of the 26 cantons of the Swiss Confederation (). It lies on the southern side of the central Alps, and is mainly surrounded by Italy, except for the northern and northeastern borders that belong to the Swiss cantons of Grisons, Valais and Uri. 51% of the regional catchment area is forested and 14% is considered unproductive.
Figure 1. Switzerland and Canton Ticino with its main river basins. Lake names: (1) Bianco; (2) Matörgn; (3) Cristallina; (4) Piccolo Narèt; (5) Laghetto superiore; (6) Laghetto inferiore; (7) Laiòzz; (8) Tomè; (9) Zòta; (10) Muino superiore; (11) Muino inferiore; (12) Starlarèsc da Sgiof.
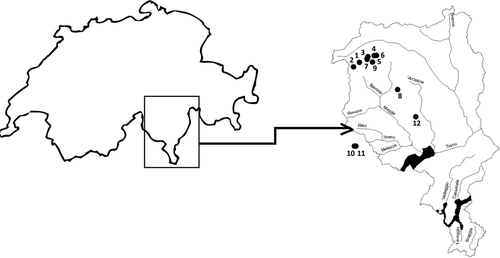
A total of 12 lakes were investigated. Nine were located in the Maggia River basin, and the remaining three were connected to the Melezza and Verzasca rivers. Three pairs of lakes represent cascading lakes, which means that the outlet of the upper lake constitutes the inlet of the lower lake: lakes Muino Superiore and Inferiore, lakes Cristallina and Piccolo Naret, and Laghetto Superiore and Inferiore. Lake Bianco is also a cascading lake, with a reservoir, Lake Cavagnöö, higher up, which was not considered in this work.
The freshwater drainage system of the lakes comprises a few small streams, sometimes just “flowing waters”, fed by the meltwaters of nearby snow, which covers the area for 9 months of the year. All of the sites chosen are permanent mountain lentic ecosystems of altitudes varying between 1700 and 2450 m above sea level (a.s.l.) ().
Table I. Studied lakes and their geographic position, morphometry, typology and macroinvertebrate sampling period.
The timberline is placed at about 2000 m a.s.l. The lakes’ watersheds consist of sparsely vegetated and unproductive grassland, landslides and rock. Three of the lakes (Bianco, Laiozza and Piccolo Naret) enclose a glacier within their basin. Geo-lithology is mainly characterized by acid metamorphic rocks, such as gneiss, but also amphibolite, while calcareous rocks are rare (Posch et al. Citation2007). Disturbance factors include recreational activities, cattle grazing and the presence of a low-traffic road near the Laghetto lakes.
Sampling design
The sampling procedures for aquatic macroinvertebrates followed the common protocol developed during previous projects (NIVA Citation1987; ICP waters Programme Centre Citation1996, Citation2010). Major near-shore habitats, identified by walking the entire perimeter of the lake and recognizing the dominant substrate types present, were inspected using a semi-quantitative kick-and-sweep technique with a 250-μm-mesh kick net (Frost et al. Citation1971). At the outflow the net was placed downstream, while the operator was kicking the substratum in an upstream position. Samples were then preserved with 80% alcohol in labelled bottles and sorted on return to the laboratory using a stereoscopic microscope. Samples were collected during the ice-free season alongside water samples for hydro-chemical analyses (). Samples taken between 1991 and 1994 in lakes Laiozza, Zota, Matorgn, Piccolo Naret, Cristallina and Muino Superiore were identified by Dr. Anna Maria Nocentini, with the majority of organisms identified to the family level, except for Stylodrilus heringianus Claparède, 1862 (Lumbriculidae) and Haplotaxis gordioides (Hartmann, 1821) (Haplotaxidae). Whenever possible, individuals were mounted on microscopic slides in Canada balsam for identification to the species level. Due to bad preservation or mechanical damage of small, newly hatched individuals of certain taxa, mainly from the genus Nais, precise determination was not always possible, and specimens were determined to the genus level or were treated as a species complex (Nais communis/N. variabilis). The following taxonomic guides were used: Timm (Citation2009), Schmelz and Collado (Citation2010).
For water chemistry, a standard procedure was followed by taking samples at the waterline level (0.1–0.5 m) at greater lake depths or at the lake mouth near the outlet (ICP waters Programme Centre Citation2010). One-litre polyethylene bottles were used and then stored at low temperatures for subsequent analysis. Sampling occurred mainly in autumn, and the following physico-chemical parameters were measured: water temperature, pH, alkalinity, conductivity, cations (calcium, magnesium, sodium, potassium, ammonium) and anions (sulphates, nitrates, chloride), and reactive silica (RSi). Variables were analyzed following the methodology in use (http://www.idrolab.ise.cnr.it, Steingruber & Colombo Citation2007–2013; Steingruber et al. Citation2011).
The annual precipitation taken from Robiei pluviometric station of MeteoSwiss was calculated considering the hydrological year to be from 1 October to 30 September, since the precipitation that falls in late autumn and winter in the form of snow affects the hydrology and chemistry of the next spring and summer (Rogora et al. Citation2013).
Statistical approaches
Principal component analysis (PCA – CANOCO 4.5 program; ter Braak & Šmilauer Citation2002) was performed in order to cluster lakes with a similar chemical composition, excluding the alkaline Lake Bianco, because of its atypical chemistry. Chemical parameters were centred and standardized and their scores divided by their standard deviation. Canonical correspondence analysis (CCA – CANOCO 4.5 program; ter Braak & Šmilauer Citation2002) was performed on the entire set of lakes to detect any relations amongst the sites based on chemical characteristics, yearly precipitation, altitude, season and the relative abundance of the oligochaete assemblage composition either in the lake littoral zone or lake outlet. The most suitable explanatory variables were selected by manual forward selection. Samples with only a few individuals (< 5 ind.) were excluded (from outlets: LS 8/91, LS 7/03, LI 7/03, TOM 9/03, TOM 6/07, STA 8/03, STA 9/07 [for lake codes see ]) as were not considered representative of the entire lake fauna. Rare species in samples, where other species were present, were down-weighted. All environmental variables, except pH, were log-converted, while species data were square root transformed.
Lake surface water samples were used to characterize both the littoral zone and the outlet zone. For both statistical analyses, the average autumn chemical values collected during the sampling periods were used, when possible (see ).
Table II. Average chemical water characteristics based on autumn samplings of Swiss Alpine lakes in Canton Ticino in different time periods. Years correspond to different sampling periods: ES “1993÷1995” means that the values shown are the average of all the occasional samples collected in autumn from 1993 to 1995, while “2003, 2007” means that the values are the average of all the periodic samples collected in autumn during 2003 and 2007.
In the CCA, Enchytraeidae were treated as a single taxon (i.e. family level), as individuals were identified to the family level in the 1990s, and to the species level thereafter. To evaluate the significance of the CCA analysis, a Monte Carlo permutation test with 999 permutations under a reduced model was performed on both the first axis and all the axes collectively.
Results
Lake water chemistry
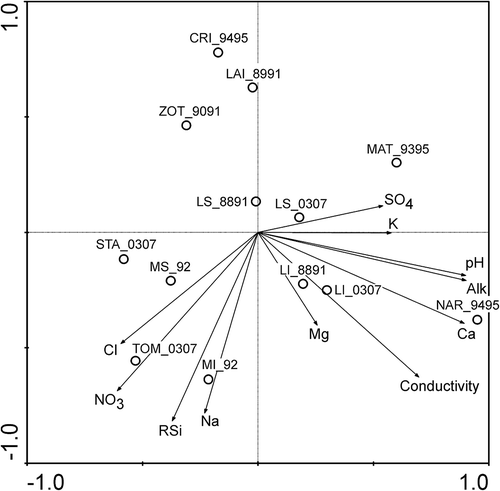
The Alpine Swiss lakes studied generally showed a low solute content () with conductivity values below 15 µS cm−1. Cations were mainly represented by calcium, with increasing importance when calcareous rocks were present in the watershed, while magnesium, sodium and potassium ions were present at low values. Ammonium ions were negligible in most lakes. The main acidic anions were sulphates and nitrates. The pH was lower than 6.0 in five of the lakes, while the pH ranged between 6.0 and 7.0 in six lakes. Lake Bianco had a pH of around 8 and an alkalinity > 300 µeq L−1 due to the higher fraction of calcareous rocks (15%) in its watershed.
The first axis of the PCA analysis () divided the lakes into two groups with a different sensitivity to acidity. The first group (LS, LI, NAR, MAT [see for lake codes]) was characterized by a higher average pH (6.4) and alkalinity (32 µeq L−1) values, while in the second group (CRI, ZOT, LAI, MS, MI, TOM, STA) the average values of pH (5.7) and alkalinity (5 µeq L−1) were lower. The second axis was determined by altitude-dependent chemical parameters, especially reactive silica, but also nitrate. In fact, the more acid-sensitive lakes were divided into two groups: lakes at lower altitudes with higher concentrations of RSi and nitrates (STA, MS, TOM, MI: 0.96 mg RSi L−1 and 0.43 mg N L−1) and lakes at higher altitudes with lower concentrations of RSi and nitrates (CRI, LAI, ZOT: 0.54 mg RSi L−1 and 0.19 mg N L−1).
Species distribution
Nineteen taxa were identified to the species level in the study sites. The lakes were mainly inhabited by Lumbriculidae and Enchytraeidae, and by the subfamilies Tubificinae and Naidinae (on average 37, 30, 19 and 15%, respectively), while in the outlets a combination of Naidinae, Enchytraeidae and Lumbriculidae (on average 49, 35 and 15%, respectively) were more common (). The higher population diversity in lake samples was also reflected in the number of taxa. In fact, a higher number of taxa was collected in the littoral zone (n = 18) than in the outlets (n = 10). Eleven taxa were restricted to the littoral zone and two to stream waters, and six were common between the two environments. Three out of the 19 taxa found were only observed in Lake Bianco.
Figure 3. Oligochaete assemblages in the studied lakes and their outlets (expressed as percentages).
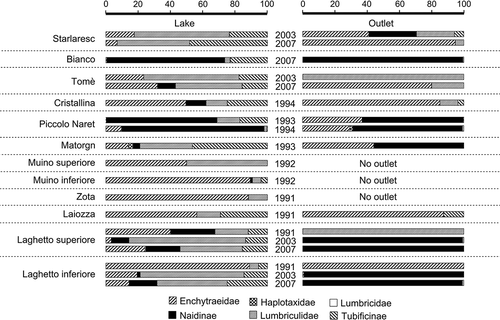
In all investigated lakes, Stylodrilus sp. (determined as S. heringianus Claparede, 1862 on the basis of mature specimens) was recorded (). In five lakes, Tubifex tubifex (Müller, 1774) was found, the only species from the subfamily Tubificinae, whereas juvenile specimens with hair setae that were probably from the same species were caught in 10 lakes ().
Table III. List of species found in the studied lakes, divided according to the observed relative abundances in the 1990s and in the 2000s. 0: not found, X: common, R: rare (< 5 individuals).
Nais communis Piguet, 1906 and N. variabilis Piguet, 1906 were the most common species, representing 57% of the subfamily Naidinae. Among the Enchytraeidae, the genera Cernosvitoviella and Cognettia were the most abundant and widespread, with the former represented by five species. Rare species were also found. Cernosvitoviella goodhui Healy, Citation1975 was found for the first time along the littoral of Lake Laghetto Inferiore, Cernosvitoviella microtheca Rota & Healy, Citation1999 in Lake Muino Inferiore, Haplotaxis gordioides (Hartman, 1821) in Lake Matorgn in 1993, and Cernosvitoviella immota (Knöllner, 1935) was collected in the outlet of Lake Starlaresc da Sgiof.
Key factors in taxon distribution
The influence of abiotic variables on the biological composition of the two habitat types, the lake littoral zones and stream outlets, was tested through a CCA ().
Figure 4. Biplots based on canonical correspondence analysis applied to environmental vs biological lake littoral assemblages. A, samples and environmental variables; B, species and environmental variables. SO4: sulphates; Ca: calcium. Lake codes are given in .
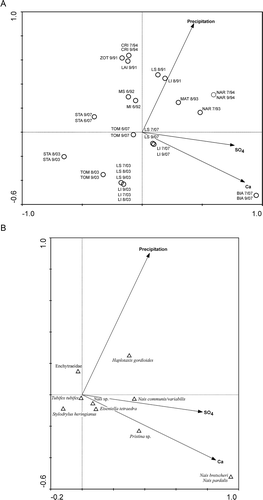
The first two axes of the CCA performed on the lake littoral and environmental variables explained 36.4% of the total variance (first axis: 25.4%, second axis: 11.0%). The relation between the canonical axes of oligochaete species composition and environmental parameters was significant (p = 0.001). The two canonical axes explained 61.1 and 26.5%, respectively, of the variance of the species–environment relation. The first axis represented an acid–base gradient with samples characterized by higher pH and calcium ion concentration on the right (BIA, NAR, MAT, LI, LS) and lower values on the left (BIA, NAR, MAT, LI, LS), while the second axis was related to annual precipitation with higher values during 1991, 1992 and 1994 and lower values during 2003 and 2007. Regarding the distribution of the oligochaete taxa, less acid-sensitive lakes seemed to be populated mainly by Naidinae, while in more acidic lakes Enchytraeidae, Lumbriculidae and Tubificinae were more common. In addition, in more acid-sensitive lakes Enchytraeidae seemed to be more abundant in sites with higher annual precipitation compared to lakes with lower annual precipitations, with Lumbriculidae and Lumbricidae as dominant families.
The same analysis carried out on data from the lake outlets showed again a split between acidic and more alkaline outlets on the first axis ().
Figure 5. Biplots based on canonical correspondence analysis applied to environmental vs biological lake outlet assemblages. A, samples and environmental variables; B, species and environmental variables. SO4: sulphates; Ca: calcium. Lake codes are given in .
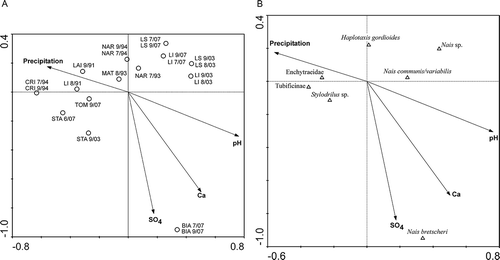
The first two axes of the CCA performed explained 57.7% of the total variance (first axis: 47.9 %, second axis: 9.3%). The relation between the canonical axes of oligochaete species composition and environmental parameters was significant (p = 0.001). The two canonical axes explained 67.4 and 13.1%, respectively, of the variance of the species–environment relation. Similarly to the situation observed for littoral samples, in outlet samples, Naidinae were also restricted to habitats with a higher pH (BIA, NAR, LI, LS, MAT), while in highly acidified habitats (TOM, STA) Enchytraeidae, Tubificinae and Lumbriculidae were more frequent.
Next to species composition, the number of taxa of oligochaetes was also correlated with the pH: in the lake littoral zones the correlation coefficient was 0.62 (p = 0.0002), and in the lake outlets it was 0.56 (p = 0.0017).
Discussion
Due to alkalinity values lower than 50 µeq L−1, most of the studied lakes should be considered at risk of acidification (Steingruber & Colombo Citation2010a). However, a significant chemical recovery due to decreasing sulphate concentrations and increasing pH and alkalinity levels has occurred in most of the lakes in the monitoring area during the last 30 years (Rogora et al. Citation2013). In addition to this, as total phosphorus values are below or at the detection limit (5 µg L−1), chlorophyll a values are < 2 µg L−1 (S. Steingruber pers. comm.), and there is complete water transparency, all lakes should be classified as ultra-oligotrophic (Vollenweider & Kerekes Citation1982).
The PCA analysis divided the lakes into two groups: lakes in carbonate-poor catchments with lower alkalinity and pH values, and lakes with higher pH and alkalinity in catchments with a higher percentage of carbonate. Adding the alkaline Lake Bianco to the PCA analysis would separate it from the other lakes, without obtaining a clear distinction between lakes that are more or less sensitive to acidity.
Lakes were also divided on the basis of their reactive silica concentrations and altitude. A decrease in the reactive silica and nitrate concentrations with altitude was previously observed by Marchetto et al. (Citation1995). The decrease in RSi was associated with the lower weathering rates at lower temperatures (Zobrist & Drever Citation1990), while the decrease in nitrate was explained by the lower atmospheric nitrogen deposition at higher altitudes (Steingruber & Colombo Citation2010b).
In these lakes, very common oligochaete species of high-altitude fresh waters were found, that were characteristic of aquatic as well as terrestrial and semi-aquatic environments. Despite the wide distribution of the main species present, the composition of the oligochaete fauna of the studied lakes was specific and different from that reported for Swiss Alpine ponds (Oertli et al. Citation2008), as well as from that of the Italian Alpine lakes (Dumnicka & Boggero Citation2007). Conversely, the number of taxa found in the studied lakes was similar to that observed in alpine lakes on the Italian Alps and Tatra Mountains in Poland (Dumnicka & Boggero Citation2007), and was noticeably greater than in the high-altitude ponds studied by Oertli et al. (Citation2008). Probably, a smaller number of species and a lack of Naidinae in the ponds of the Macun region may be caused by exceptionally harsh environmental conditions (Oertli et al. Citation2008).
Moreover, the higher number of oligochaete taxa in the littoral compared to those found in outlet sites is typical for Alpine water bodies (Dumnicka & Boggero Citation2007) because of the oligochaetes’ general preference for standing waters. Not surprisingly, Lumbriculidae and Tubificinae are known to prefer fine sediments (Dumnicka Citation1994; Verdonschot Citation2001) and were generally more abundant along the lake littorals where such substrates are more likely to occur, while Naidinae, which seemed to prefer coarse substrates (Learner et al. Citation1978), were more frequently found in the lake outlets. Otherwise, the distribution of Enchytraeidae seemed to be determined by parameters other than substrate type and current velocity, because they could be numerically important in both littoral and outlet samples. The high relative abundance of Enchytraeidae in the littoral zone of acid-sensitive lakes characterized by higher annual precipitations may be related to their semi-aquatic nature. In fact, due to a high amount of precipitation, lake water levels could have increased, or water fluctuation levels could have been more noticeable, submerging periodically dry littoral zones and thus favoring Enchytraeidae over strictly aquatic families (Haplotaxidae and Lumbriculidae) and subfamilies (Tubificinae, Naidinae), as was observed in other lakes with high water fluctuation levels (Dumnicka & Galas Citation2012).
Considering species distribution, the common coexistence of Nais communis and N. variabilis in Swiss Alpine lakes has been known for a long time, but N. variabilis was usually found in lakes situated at higher altitude (Piguet & Bretscher Citation1913). On the Italian Alps, N. communis was found alone, whereas only N. variabilis was present in Polish high mountain lakes (Dumnicka & Boggero Citation2007). Both species were found sharing the same habitat in River Roseg on Bernina Massif (Malard et al. Citation2001), confirming their co-occurrence in Swiss Alpine territories. Furthermore, the presence of Nais bretscheri Michaelsen, 1899 and N. pardalis Piguet, 1906 exclusively found in Lake Bianco, was probably related to the higher water conductivity and/or higher pH values in the lake.
Another difference between the oligochaete fauna of ponds in the Macun region was the presence of Lumbriculus variegatus (Müller, 1774), which was found often by Oertli et al. (Citation2008) but not confirmed in the present study. Among the Enchytraeidae, Cernosvitoviella goodhui (Healy Citation1975) was reported for the first time in Switzerland. Cernosvitoviella immota is generally found along European and North American marine coastal areas and is considered amphibious (Schmelz & Collado, Citation2010). The same morphological species had already been found in three ponds of the Macun region (Oertli et al. Citation2008) and in Polish mountain streams (Kasprzak & Szczęsny Citation1976). Cernosvitoviella microtheca was found only in Lake Muino Inferiore in 1992 in low numbers and was therefore considered rare. Notwithstanding the fact that in recent years Schmelz and Collado (Citation2010) synonymized Cernosvitoviella microtheca with C. atrata (Bretscher, 1903), thus creating confusion, the specimens belonging to these species can be easily distinguished due to differences in the structure of their genital organs, namely the shape of the spermatheca and sperm duct (Rota & Healy Citation1999).
CCA evidenced a clear separation between faunistic records in the littoral zone and outlets of calcareous and acidic lakes, as was stated for the first time by Boggero et al. (Citation1996), Boggero and Nobili (Citation1998), and more recently by Steingruber et al. (Citation2013). This was contrary to what was reported by Wiederholm and Eriksson (Citation1977), and by Wathne et al. (Citation1997). In general, Naidinae were more abundant in less sensitive lakes, while in more acidic waters, Enchytraeidae, Lumbriculidae and Tubificinae were mostly present in the littoral zone, and Enchytraeidae at the lake outlets. In addition, at higher pH, the number of oligochaete taxa also increased. The absence of Naidinae in acidic waters was already reported in Smith et al. (Citation1990) and the tolerant nature of Enchytraeidae and Lumbriculidae species in regards to pH level was discussed in Ilyashuk (Citation1999), while a decreasing number of oligochaete species and abundances were discussed in Ilyashuk (Citation1999), Stephenson et al. (Citation1994) and Keller et al. (Citation1990).
Acknowledgements
We are grateful to Dr. Diego Fontaneto (CNR-ISE, Italy) for a first critical reading of the paper and for the valuable comments received, to Chiara Pradella Caissutti for biological sampling. Special thanks are due to Drs. Chiara Napolitano (Scuola Universitaria Professionale della Svizzera Italiana [SUPSI], Switzerland) and Ruth Rawcliffe (University College London [UCL], United Kingdom) who reviewed the manuscript language. The present research was conducted within the International Cooperative Programme on Assessment and Monitoring Effects of Air Pollution on Rivers and Lakes, partly funded by the Swiss Federal Office for the Environment (FOEN).
References
- Abraham J, Allen P, Dunbar J, Dworkin S. 1999. Sediment type distribution in reservoirs: sediment source versus morphometry. Environmental Geology 38:101–110. doi:10.1007/s002540050406.
- Boggero A, Basset A, Austoni M, Barbone E, Bartolozzi L, Bertani I, Campanaro A, Cattaneo A, Cianferoni F, Corriero G, Martin Dörr A, Elia AC, Ficetola GF, Kamburska L, La Porta G, Lauceri S, Ludovisi A, Gaino E, Goretti E, Lorenzoni M, Manca M, Marchetto A, Morabito G, Nonnis Marzano F, Oggioni A, Pierri C, Riccardi N, Rossetti G, Ungaro N, Volta P, Zaupa S, Fontaneto D. 2014. Weak effects of habitat type on susceptibility to invasive freshwater species: an Italian case study. Aquatic Conservation: Marine and Freshwater Ecosystems. doi:10.1002/aqc.2450.
- Boggero A, Nobili M. 1998. Macrobenthic community and chemical characteristics of four Alpine lakes in Canton Ticino. Bollettino della Società Ticinese di Scienze Naturali 86:17–23.
- Boggero A, Nocentini AM, Nobili M, Gianatti M. 1996. Ricerche sulla fauna macrobentonica litorale in laghi d’alta quota nel bacino imbrifero del Lago Maggiore. Atti VII Congresso Nazionale S.It.E., 11–14 Settembre 1996, Napoli.
- Brodersen K, Dall P, Lindegaard C. 1998. The fauna in the upper stony littoral of Danish lakes: macroinvertebrates as trophic indicators. Freshwater Biology 39:577–592. doi:10.1046/j.1365-2427.1998.00298.x.
- Dumnicka E. 1994. Communities of oligochaetes in mountain streams of Poland. Hydrobiologia 278:107–110. doi:10.1007/BF00142317.
- Dumnicka E. 2004. A description of Cernosvitoviella tridentina, a new species of Enchytraeidae (Oligochaeta) from the Italian Alps. Annales de Limnologie - International Journal of Limnology 40:133–137. doi:10.1051/limn/2004011.
- Dumnicka E. 2010. Two new freshwater enchytraeid species (Oligochaeta) from the Italian Alps. Italian Journal of Zoology 77:38–43. doi:10.1080/11250000902855505.
- Dumnicka E, Boggero A. 2007. Freshwater Oligochaeta in two mountain ranges in Europe: the Tatra Mountains (Poland) and the Alps (Italy). Fundamental and Applied Limnology / Archiv für Hydrobiologie 168:231–242. doi:10.1127/1863-9135/2007/0168-0231.
- Dumnicka E, Galas J. 2012. Temporal changes in oligochaete fauna of three alpine ponds in the Tatra Mountains (Poland). Boreal Environment Research 17:252–262.
- Friday L. 1987. The diversity of macro invertebrate and macrophyte communities in ponds. Freshwater Biology 18:87–104. doi:10.1111/j.1365-2427.1987.tb01297.x.
- Frost S, Huni A, Kershaw WE. 1971. Evaluation of a kicking technique for sampling stream bottom fauna. Canadian Journal of Zoology 49:167–173. doi:10.1139/z71-026.
- Healy B. 1975. A description of five new species of Enchytraeidae (Oligochaeta) from Ireland. Zoological Journal of the Linnean Society 56:315–326. doi:10.1111/j.1096-3642.1975.tb00273.x.
- Heino J. 2000. Lentic macroinvertebrate assemblage structure along gradients in spatial heterogeneity, habitat size and water chemistry. Hydrobiologia 418:229–242. doi:10.1023/A:1003969217686.
- ICP Waters Programme Centre. 1996. Programme manual. NIVA report SNO 3547–96. 37 pp.
- ICP Waters Programme Centre. 2010. ICP Waters Programme Manual 2010. NIVA SNO 6074–2010.ICP Waters report 105/2010. 91 pp.
- Ilyashuk BP. 1999. Littoral oligochaete (Annelida: Oligochaeta) communities in neutral and acidic lakes in the Republic of Karelia, Russia. Boreal Environment Research 4:277–284.
- Jeffries M. 1991. The ecology and conservation value of forestry ponds in Scotland, United Kingdom. Biological Conservation 58:191–211. doi:10.1016/0006-3207(91)90119-T.
- Juget J, Giani N. 1974. Répartition des oligochètes lacustres du Massif de Néouvielle (Hautes-Pyrénées) avec la description de Peloscolex pyrenaicus n. sp. Annales de Limnologie 10:33–53. doi:10.1051/limn/1974006.
- Kasprzak K, Szczęsny B. 1976. Oligochaetes (Oligochaeta) of the River Raba. Acta Hydrobiologica 18:75–87.
- Keller W, Molot LA, Griffiths RW, Yan ND. 1990. Changes in the zoobenthos community of acidified bowland lake after whole-lake neutralization and lake trout (Salvelinus namaycush) reintroduction. Canadian Journal of Fisheries and Aquatic Sciences 47:440–445. doi:10.1139/f90-047.
- Kownacki A, Dumnicka E, Kwandrans J, Galas J, Ollik M. 2006. Benthic communities in relation to environmental factors in small high mountain ponds threatened by air pollutants. Boreal Environment Research 11:481–492.
- Kownacki A, Galas J, Dumnicka E, Mielewczyk S. 2000. Invertebrate communities in permanent and temporary high mountain lakes (Tatra Mts). Annales de Limnologie - International Journal of Limnology 36:181–188. doi:10.1051/limn/2000016.
- Krno I, Šporka F, Galas J, Hamerlík L, Zaťovičová Z, Bitušík P. 2006. Littoral benthic macroinvertebrates of mountain lakes in the Tatra Mountains (Slovakia, Poland). Biologia, Bratislava 61:S147–S166. doi:10.2478/s11756-006-0127-4.
- Laville H. 1971. Recherches sur les Chironomides [Diptera] lacustres du Massif de Néouvielle (Hautes-Pyrénées). Annales de Limnologie 7:335–414. doi:10.1051/limn/1971005.
- Learner MA, Lochhead G, Hughes BD. 1978. A review of the biology of British Naididae (Oligochaeta) with emphasis on the lotic environment. Freshwater Biology 8:357–375. doi:10.1111/j.1365-2427.1978.tb01457.x.
- Maiolini B, Lencioni V, Boggero A, Thaler B, Lotter AF, Rossaro B. 2006. Zoobenthic communities of inlets and outlets of high altitude Alpine lakes. Hydrobiologia 562:217–229. doi:10.1007/s10750-005-1812-y.
- Malard F, Galassi D, Lafont M, Doledec S, Ward JV. 2003. Longitudinal patterns of invertebrates in the hyporheic zone of a glacial river. Freshwater Biology 48:1709–1725. doi:10.1046/j.1365-2427.2003.01118.x.
- Malard F, Lafont M, Burgherr P, Ward JV. 2001. A comparison of longitudinal patterns in hyporheic and benthic oligochaete assemblages in a glacial river. Arctic, Antarctic, and Alpine Research 33:457–466. doi:10.2307/1552556.
- Marchetto A, Mosello R, Psenner R, Bendetta G, Boggero A, Tait D, Tartari GA. 1995. Factors affecting water chemistry of alpine lakes. Aquatic Sciences 57:81–89. doi:10.1007/BF00878028.
- NIVA. 1987. International cooperative programme for assessment and monitoring of acidification of rivers and lakes: Programme Manual. Programme Centre, NIVA. 23 pp.
- Oertli B, Indermuehle N, Angélibert S, Hinden H, Stoll A. 2008. Macroinvertebrate assemblages in 25 high alpine ponds of the Swiss National Park (Cirque of Macun) and relation to environmental variables. Hydrobiologia 597:29–41. doi:10.1007/s10750-007-9218-7.
- Piguet E, Bretscher K. 1913. Catalogue des Invertébrés de la Suisse. Oligochètes 7:1–215.
- Posch M, Eggenberger U, Kurz D, Rihm B. 2007. Critical Loads of Acidity for Alpine Lakes. A weathering rate calculation model and the generalized First-order Acidity Balance/FAB) model applied to Alpine lake catchments. Environmental studies no. 0709. Federal Office for the Environment, Berne. 69 pp.
- Rashid R, Pandit AK. 2014. Macroinvertebrates (oligochaetes) as indicators of pollution: A review. Journal of Ecology and the Natural Environment 6:140–144. doi:10.5897/JENE2014.0443.
- Rasmussen K, Lindegaard C. 1988. Effects of iron compounds on macroinvertebrate communities in a Danish lowland river system. Water Research 22:1101–1108. doi:10.1016/0043-1354(88)90004-8.
- Rodriguez P, Reynoldson TB. 2011. The pollution biology of aquatic oligochaetes. Netherlands: Springer. 265 pp.
- Rogora M, Colombo L, Lepori F, Marchetto A, Steingruber S, Tornimbeni O. 2013. Thirty years of chemical changes in alpine acid-sensitive lakes in the Alps. Water, Air, & Soil Pollution 224:1746–1766. doi:10.1007/s11270-013-1746-3.
- Rota E, Bartoli M, Laini A. 2014. First time in Italy. Is the elusive aquatic megadrile Sparganophilus Benham, 1892 (Annelida, Clitellata) accelerating its dispersal in Europe? Journal of Limnology. doi:10.4081/jlimnol.2014.939.
- Rota E, Healy B. 1999. A taxonomic study of some Swedish Enchytraeidae (Oligochaeta), with descriptions of four new species and notes on the genus Fridericia. Journal of Natural History 33:29–64. doi:10.1080/002229399300461.
- Schmelz RM, Collado R. 2010. A guide to European terrestrial and freshwater species of Enchytraeidae (Oligochaeta). Soil Organisms 82:176 pp.
- Smith ME, Wyskowski BJ, Brooks CM, Driscoll CT, Cosentini C. 1990. Relationships between acidity and benthic invertebrates of low-order woodland streams in the Adirondack Mountains, New York. Canadian Journal of Fisheries and Aquatic Sciences 47:1318–1329. doi:10.1139/f90-151.
- Steingruber SM, Boggero A, Pradella Caissutti C, Dumnicka E, Colombo L. 2013. Can we use macroinvertebrates as indicators of acidification of high-altitude Alpine lakes? Bollettino della Società Ticinese di Scienze Naturali 101:23–34.
- Steingruber SM, Colombo L. 2007–2013. Results from the participation of Switzerland to the International Cooperative Programme on Assessment and Monitoring effects of air pollution on rivers and lakes. Annual reports 2005–2012. Dipartimento del territorio del Canton Ticino.
- Steingruber SM, Colombo L. 2010a. Effect of acid deposition on chemistry and biology of high-altitude Alpine lakes. In: Bundi U, editor. Alpine Waters. Handbook of Environmental Chemistry 6:119–140.
- Steingruber SM, Colombo L. 2010b. Acidifying deposition in Southern Switzerland (Assessment of the trend 1988–2007). Environmental studies no. 1015. Bern: Federal Office for the Environment.
- Steingruber SM, Colombo L, Pradella C. 2011. Switzerland: Recovery of macroinvertebrates in acid sensitive freshwaters in southern Switzerland. In: Skjelkvåle BL, de Wit HA, editors. Trends in precipitation chemistry, surface water chemistry and aquatic biota in acidified areas in Europe and North America from 1990 to 2008. NIVA-Report SNO 6218–2011. ICP-Waters Report 106/2011:81–92.
- Stephenson M, Mierle G, Reid RA, Mackie GL. 1994. Effects of experimental and cultural lake acidification on littoral benthic macroinvertebrate assemblages. Canadian Journal of Fisheries and Aquatic Sciences 51:1147–1161. doi:10.1139/f94-114.
- Stoichev S. 2000. The zoobenthos from several glacial lakes in the Rila Mountains, Bulgaria. In: Golemansky V, Naidenow W, editors. Biodiversity and evolution of glacial water ecosystems in the Rila Mountains. Sofia: Institute of Zoology. pp. 155–162.
- ter Braak CJF, Šmilauer P. 2002. CANOCO reference manual and CanoDraw for Windows. User’s guide (version 4.5). Biometrics, Wageningen and České Budějovice. 499 pp.
- Timm T. 2009. A guide to the freshwater Oligochaeta and Polychaeta of Northern and Central Europe. Lauterbornia 66:235 pp.
- Timm H, Möls T. 2012. Littoral macroinvertebrates in Estonian lowland lakes: the effects of habitat, season, eutrophication and land use on some metrics of biological quality. Fundamental and Applied Limnology / Archiv für Hydrobiologie 180:145–156. doi:10.1127/1863-9135/2012/0203.
- Uzunov Y, Varadinova E. 2000. Oligochaeta limicola from glacial lakes of the Rila Mountains National Park (Bulgaria). In: Golemansky V, Naidenow W, editors. Biodiversity and evolution of glacial water ecosystems in the Rila Mountains. Sofia: Institute of Zoology. pp. 45–48.
- Verdonschot PFM. 2001. Hydrology and substrates: determinants of oligochaete distribution in lowland streams (The Netherlands). Hydrobiologia 463:249–262. doi:10.1023/A:1013132514610.
- Vollenweider RA, Kerekes J. 1982. Eutrophication of waters, monitoring, assessment and control. Paris: OECD. 154 pp.
- Wathne BM, Patrick S, Cameron N. 1997. AL:PE Acidification of mountain lakes: Palaeolimnology and Ecology. Part 2. Remote mountain lakes as indicators of air pollution and climate change. EU environment programme. Report 3638–97. Oslo, Norwegian Institute for Water Research.
- Wiederholm MT, Eriksson L. 1977. Benthos of an acid lake. Oikos 29:261–267. doi:10.2307/3543612.
- Zobrist J, Drever JJ. 1990. Weathering processes in Alpine watersheds sensitive to acidification. Proceedings of the EEC Workshop Acidification processes in remote mountain lakes, 20–22 June 1989, Pallanza. Air Pollution Research Report 20:179–161.