Abstract
Acerentomon italicum is the most abundant species of Protura in Italy. In this paper, A. italicum is redescribed according to the most recent revision of diagnostic characters (morphometry, porotaxy), examining the type material and 59 specimens from 11 different localities in our collection. For some characters, scanning electron microscope (SEM) pictures are presented. An updated detailed catalogue extracted from the authors’ database is provided: A. italicum is reported from 91 Italian localities (mainly from Northern Italian regions) in a total of 520 ♂♂, 669 ♀♀, 28 pre-imagines, 69 maturi junior, 25 larvae II, four larvae I and eight undetermined. Few specimens were collected in Switzerland (two, about 5 km away from the Italian border), Austria (three), Slovenia (one) and Corsica (eight). The entire data set is analysed for information on phenology (juveniles detectable every month in Liguria and Tuscany, but only during spring–summer in the remaining regions of Northern Italy) and sex ratio (M:F = 0.78) of this species. A. italicum was collected in localities from 0 to 2000 m above sea level in the soil and litter of pure and mixed forest. The geological substratum, when recorded, was limestone, quartz/mica schists and conglomerates. The DNA barcode is newly provided for 21 representatives from three Italian populations of A. italicum.
Introduction
Acerentomon italicum Nosek, Citation1969 belongs to the doderoi group sensu Tuxen (Citation1964), characterised by the chaetotaxy formula 4/2 on sternite VIII and by the presence of seta x on tergite VII. In his cladistic analysis, François (Citation2006) placed this species in what he likewise called doderoi group (characterised by a single apomorphy: the presence of seta x on tergite VII), which, however, is more comprehensive than the homonymous group of Tuxen, and more precisely in his baldense subgroup (whose apomorphy is a very long clypeo-labrum – labrum ratio (LR) = 2.7–4.0).
A. italicum seems to be the most abundant Protura species in Italy: Galli et al. (Citation2011), out of a national total of 3929 Protura identifiable to species level, quoted 1060 specimens as belonging to this species (433 ♂♂, 573 ♀♀, 18 pre-imagines, 16 maturi junior, 14 larvae II and six undetermined) derived from many localities of mainland Italy and Tuscany.
In this paper, A. italicum is redescribed according to the most recent diagnostic characters (morphometry, porotaxy). An updated detailed catalogue extracted from the authors’ database is provided (118 collections from 91 localities in Italy, one in Switzerland, one in Austria, one in Slovenia and two in Corsica), some information about the phenology, sex ratios and ecology of this species are outlined, and the DNA barcode is newly provided for 21 representatives of three Italian populations of A. italicum.
Material and methods
Specimen sampling and extraction
Before 1990, some specimens were extracted just by sifting soil and litter samples, and preserved in 70% ethanol. More recent ones were extracted into 70% ethanol using Berlese–Tullgren funnels (2.5 mm mesh size) for 7 days from soil samples specially collected. Protura were incubated at 40–50°C for 24 hours in lactic acid to clear them, then mounted on slides in Marc André medium. Specimens were observed and identified to species and life-stage levels (larva I, larva II, maturus junior, preimago and imago) with the aid of an interference contrast microscope.
Specimens used for DNA analysis were extracted and preserved in absolute ethanol and sent to the laboratory for DNA extraction, before mounting them on slides according to the above procedure.
SEM analysis
Protura used for scanning electron microscope (SEM) analysis were taken from a 70% ethanol-preserved collection from Elba Island (Rio Marina, Capo Castello; see annex). Specimens were dehydrated in a graded ethanol series and then were transferred onto stubs, sputter coated with gold and observed under an SEM (Vega3_TESCAN Microscope type LMU).
DNA barcoding analysis
DNA barcodes were obtained for 21 representatives of Acerentomon italicum as part of a wider study on DNA barcoding of Protura. A non-destructive extraction method (Böhm et al. Citation2011) was applied, using the Blood & Tissue Kit (Qiagen), to extract whole genomic DNA while leaving the entire cuticular skeleton for determination. After DNA extraction the cuticle was transferred to 100% ethanol, before being used to create whole mounts. The thermocyler profile included an initial denaturation of 30 sec, 30 cycles of 1 min for 94°C, 1 min at 46°C and 1–1.5 min for 68°C, and a final extension step for 5 min at 68°C.
Each Polymerase Chain Reaction (PCR) consisted of 2 µL DNA, 5 µL PCR buffer (5× containing 18 mM Magnesium Chloride (MgCl2); BioLabs OneTaq), 0.7 µL dNTP (10 mM each; BioLabs Desoxynucleotide Solution Mix), 0.7 µL each primer (10 µM, VBC Biotech), 0.1 µL Polymerase (BioLabs OneTaq), 10.8 µL double distilled water (ddH2O). To increase the yield, the PCR step was repeated, applying the same conditions. PCR products were purified using GeneJET PCR Purification Kit (Thermo Scientific) and eluted in 20 µL with double distilled water (ddH2O). Sequencing was performed at LGC Genomics.
Primer pairs used to amplify the subunit I of the Cytochrome Oxidase complex (COI) are given in .
Table I. List of primer pairs used in the present study.
DNA samples will be deposited at the National History Museum Vienna (NHM), and vouchers at the Department of Earth, Environment and Live Sciences (DISTAV) of Genoa University.
Statistical analysis
PAST Software (Paleontological Statistics version 2.16; Hammer et al. Citation2001) was used to perform:
Principal component analysis on the full morphometric data set, and one-way analysis of variance (ANOVA) on body length and tarsus length to evidence significant difference between males and females.
Chi-square test to assess the statistical significance of the differences from the expected value (1) of the species sex ratio.
COI were checked for stop codons and aligned using default settings of the program MUSCLE implemented in MEGA version 6 (Tamura et al. Citation2013). MEGA version 6 was also used to construct a neighbour-joining (NJ) tree based on Kimura 2 parameter (K2P) distances, where missing data were completely excluded. Support values were calculated, performing 1000 bootstrap replicates.
To compare intraspecific and interspecific distances, we analysed our data with SpeciesIdentifier 1.7.8 (Meier et al. Citation2006).
Redescription
Material examined
The following abbreviations are used: LI = larva I, LII = larva II, MJ = maturus junior, PI = pre-imago, MHNG = Muséum d’Histoire naturelle de Genève.
Holotype
♂, ITALY: Veneto, Colli Euganei near Padova (Marcuzzi) (MHNG).
Paratypes
One ♂, ITALY: Veneto, Colli Euganei near Padova (Marcuzzi) (MHNG); one ♂, three ♀♀ ITALY: Veneto, Cadore (Marcuzzi) (MHNG).
Other material
ITALY: one PI, two LII, Veneto, Belluno, Agordo, La Muda, 2 July 1985 (Gardini) (Genoa University – DISTAV); ITALY: six ♂♂, five ♀♀, one PI, two MJ, Veneto, Belluno Ponte delle Alpi, 29 July 1978 (Vit) (Genoa University – DISTAV); ITALY: one PI, Veneto, Treviso, Cison, S. Bolto Pass, 1 July 1985 (Paoletti) (Genoa University – DISTAV); ITALY: five ♀♀, Veneto, Treviso, Mansuè, Bosco di Basalghelle, 11 March 1982 (Paoletti) (Genoa University – DISTAV); ITALY: one ♀, Piedmont, Alessandria, Rocca Grimalda, S. Giacomo, 15 October 1979 (Torti) (Genoa University – DISTAV); ITALY: one ♀, Piedmont, Alessandria, Ovada, 18 October 1979 (Torti) (Genoa University – DISTAV); ITALY: one ♂, two ♀♀, Piedmont, Alessandria, Lerma, Cirimilla, 27 May 2009 (Torti) (Genoa University – DISTAV); ITALY: two ♂♂, two ♀♀, one MJ, one LII, Liguria, Savona, Bergeggi, 06 March 2007, (Capurro, Duradoni & Galli) (Genoa University – DISTAV); ITALY: one LI, Liguria, Savona, Bergeggi, 3 April 2007 (Capurro, Duradoni & Galli) (Genoa University – DISTAV); ITALY: two LII, Liguria, Savona, Bergeggi, 9 May 2007 (Capurro, Duradoni & Galli) (Genoa University – DISTAV); ITALY: one LI, Liguria, Savona, Bergeggi, 5 July 2007 (Capurro, Duradoni & Galli) (Genoa University – DISTAV); ITALY: two LI, one PI, Liguria, Genova, Cogoleto, Lerca, Rio Loaga, 3 December 2014 (Capurro & Ferretti) (Genoa University – DISTAV); ITALY: one PI, Liguria, Genova, Arenzano, 19 April 1981 (Gardini) (Genoa University – DISTAV); ITALY: three ♂♂, seven ♀♀, two MJ, Tuscany, Livorno, Elba Island, Rio Marina, Capo Castello, 3 November 2007 (Gardini) (Genoa University – DISTAV).
Diagnosis
Typical Acerentomon of the “doderoi” group (sensu François Citation2006) with a short and thin foretarsal sensillum b reaching the base of seta γ3. Sensillum a not reaching or barely reaching the base of d, c much longer than b, reaching or passing the base of e. Additional setae on the head (d6) absent. Absence of posterosubmedial (psm) pores and presence of single pores (asl = anterosublateral) near (anterior to or external) setae A3 on tergite VII. Female squama genitalis with long pointed acrostylus.
Imago
Principal component analysis on the full morphometric data set and one-way ANOVA on single characters (body length – F = 849,857, p > 0.05 – and tarsus length – F = 2075.31, p > 0.05) did not indicate any significant difference between males and females: mean and standard deviation values of the measurements and relative ratios have therefore been calculated on 39 specimens (25 females and 14 males). Body length 1675.3 ± 193.7 μm (range: 1130–1987).
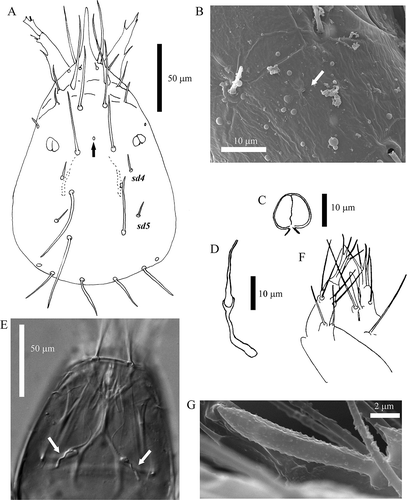
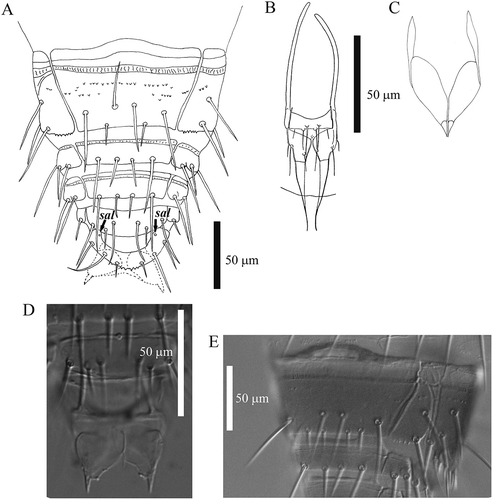
Head 164.6 ± 12.2 μm long in dorsal view (range: 136–187); setae sd4 and sd5 present, seta d6 (Rusek et al. Citation2012) absent (A). Frontal pore (fp) present (, ); clypeal pores (cp, between setae d1 and sd1) absent. Rostrum rather long: 45.4 ± 4.3 μm (range: 39–55). LR = 3.6 ± 0.3 (range: 2.9–4.2). Pseudoculus almost circular, diameter 10.4 ± 0.8 μm (range: 9–12), longitudinally divided (C); pseudoculus ratio (PR) = 15.8 ± 1.7 (range: 12.4–18.3). Canal of maxillary gland simple (D, E), the proximal part 18.7 ± 1.8 μm long (range: 15–25), CF (head length/hind part of maxillary gland length) = 9.0 ± 1.1 (range: 6.5–11.3). Maxillary palpus with seta-like sensilla (F). Labial palpus with apical tuft of setae and a “blunt saber”-shaped sensillum (F, G). Foremargin with 4–5 blunt teeth.
Figure 1. Acerentomon italicum Nosek, Citation1969 Head. A, Dorsal view, setae sd4 and sd5 are labeled, arrow indicates pore; B, detail of frontal pore (scanning electron microscope, SEM); C, pseudoculus (from Nosek Citation1969); D, canal of maxillary gland (from Nosek Citation1969); E, maxillary glands (interference contrast microscope); F, maxillary and labial palpus (from Nosek Citation1969); G, labial palp sensillum (SEM).
Foretarsus length 113.6 ± 3.8 μm (range: 105–122), claw 42.2 ± 2.7 μm (range: 38–49), with a small inner tooth, tarsus ratio (TR) = 2.7 ± 0.2 (range: 2.3–3.0); empodium length 7.2 ± 1.3 μm (range: 5–11), empodium/unguis ratio (EU) = 0.17 ± 0.03 (range: 0.11–0.27); S-shaped seta longer than claw, 53.3 ± 3.1 μm (range: 44–57). Sensillum t1 claviform, BS (ratio between the distances of sensillum t1 from the base and the apex of tarsus, respectively) = 0.58 ± 0.05 (range: 0.47–0.70); t2 thin, t3 shaped like a willow leaf. Sensillum a (subequal to c in length) not reaching or barely reaching the base of d; c much longer than b, reaching or passing the base of e; b short and thin, reaching the base of seta γ3; tip of sensillum d reaching the base of g; e pretty long, surpassing the base of g, reaching the level of seta α7; base of sensillum f nearly at the same level of t3, close to g; apices of f and g passing base of claw; f longer than g. Bases of sensilla a’ and c’ situated at the same level of e and t3, respectively; a’ thin, reaching the level of S-shaped seta; c’ thin like a’, slightly shorter, passing base of claw (). Foretarsal pores present near sensillum c and between g and t3 (). Middle tarsus length 58.2 ± 4.0 μm (range: 52–66); claw length 29.1 ± 2.7 μm (range: 17–30). Hind tarsus length 66.3 ± 3.6 μm (range: 57–75); claw length 26.0 ± 2.6 μm (range: 20–30) ().
Figure 2. Acerentomon italicum Nosek, Citation1969 Foretarsus: main sensilla (a-g, a′-c′, t1, t3) and setae α7 and γ3 are labeled (see text). A, Exterior view, arrows indicate pores (from Nosek Citation1969); B, external side sensilla (interference contrast microscope); C, external side sensilla, arrow indicates pore near sensillum c (scanning electron microscope, SEM); D, detail of claw (SEM); E, pore near sensillum t3 (SEM); F, interior view.
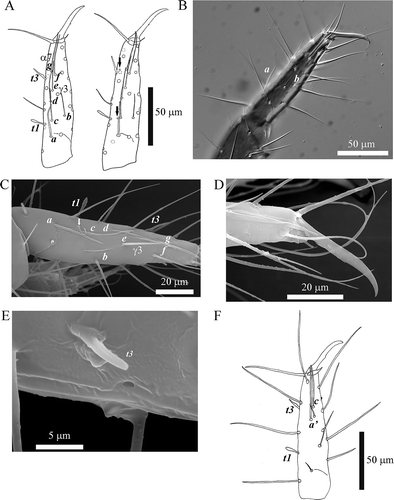
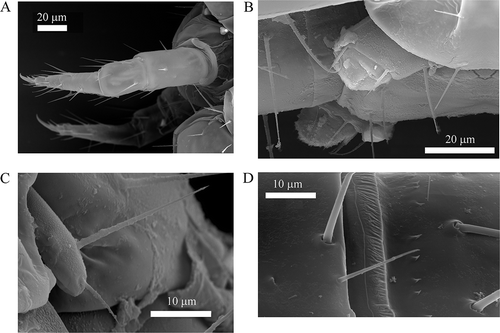
Figure 3. Acerentomon italicum Nosek, Citation1969. Scanning electron microscope (SEM) details of A, hind legs; B, abdominal appendage I; C, abdominal appendage III; D, striate band on abdominal segment VIII.
Chaetotaxy given in . Pronotum with two pairs of setae: seta 1 3 times longer than seta 2. Length ratio of setae P1:P1a:P2 on mesonotum as 1.05:1:1.38 (). Abdominal tergite I with three pairs of anterior setae (A1, A2, A3); length ratio of setae P1:P1a:P2 as 1.22:1:1.63; length of setae P2a, P3, P3a and P4 less than half that of P1a. Tergites II–VI each with five pairs of anterior setae (A1–A5), with seta A3 slightly posterior to the others; seta P3a absent, setae P1a and P2a nearer to P2 and P3 than to P1 and P2, respectively. Tergite VII () with five pairs of anterior setae (A1–A5) and seta x; P3a still absent. Tergites IX to XII with 14, 10, 6 and 9 setae, respectively (). Prosternum and mesosternum are shown in ; sternites VIII–XII in .
Table II. Chaetotaxy of Acerentomon italicum Nosek, Citation1969.
Figure 4. Acerentomon italicum Nosek, Citation1969. Tergites: pores (al = anterolateral, asl = anterosublateral, l = lateral, psl = posterosublateral, psm = posterosubmedial, sam = sternal anteromedial, spsl = sternal posterosublateral, spsm = sternal posterosubmedial), anterior (A) and posterior (P) setae are labeled. A, nota; B, chaetotaxy of larva II nota (interference contrast microscope); C, abdominal tergites V–VII; D, detail of pores on tergite VII (scanning electron microscope, SEM); E, porotaxy on abdominal segment VII (interference contrast microscope).
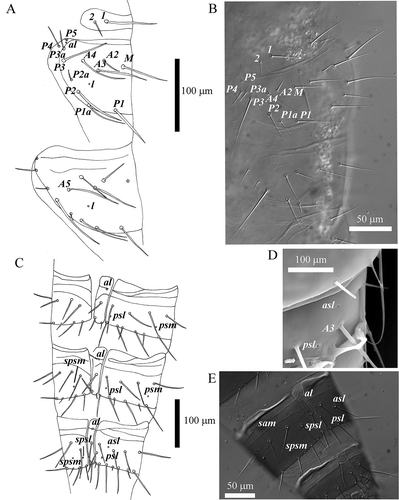
Figure 5. Acerentomon italicum Nosek, Citation1969. Last abdominal segments: pores are labeled (ac = anterocentral, psm = posterosubmedial). A, tergites VIII–XII; B, comb on tergite VIII (from Nosek Citation1969); C, hind border of pleurite VIII (from Nosek Citation1969); D, detail of pore psm on tergite VIII (scanning electron microscope, SEM); E, detail of hind border of pleurite VIII (SEM).
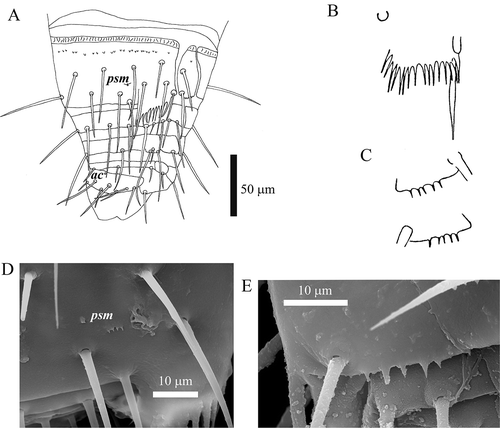
Figure 6. Acerentomon italicum Nosek, Citation1969. A, prosternum; B, mesosternum, sternal central (sc) pore is labeled; C, abdominal sternites V–VII, pores are labeled (sam = sternal anteromedial, sc = sternal central, spsl = sternal posterosublateral, spsm = sternal posterosubmedial); D, pleural pectines VI and VII (from Nosek Citation1969), anterolateral (al) pore is labeled; E, pleural pectines VI (interference contrast microscope).
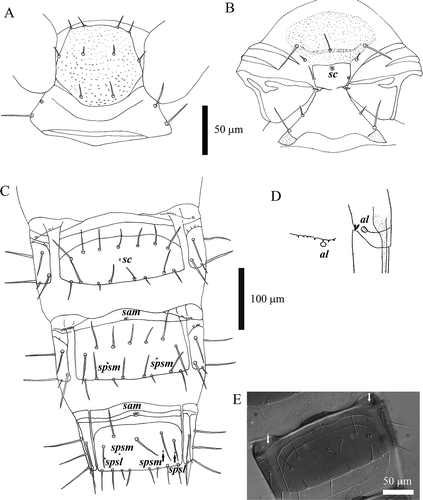
Figure 7. Acerentomon italicum Nosek, Citation1969. A, abdominal sternites VIII–XII, sternal anterolateral (sal) pores are labeled; B, male squama genitalis (from Nosek Citation1969); C, female squama genitalis; D, detail of female squama genitalis (interference contrast microscope); E, example of chaetotaxy anomaly of sternite VIII, with four posterior setae (interference contrast microscope).
Chaetotaxic variability (60 specimens examined):
Tergite III: asymmetrical absence of seta A5 (one pre-imago);
Tergite V: asymmetrical absence of seta P1a (one imago); asymmetrical absence of seta A1 (one larva II);
Tergite VII: asymmetrical absence of seta x (five imagines, one pre-imago);
Sternite II: presence of setae A1 (formula 6/5) (seven imagines); asymmetrical absence of seta A3 (one imago);
Sternite III: absence of seta Ac (formula 6/5) (33 imagines, including holotype); asymmetrical absence of seta A1 (one imago); presence of setae P1 (formula 6/6) (one imago);
Sternite VI: asymmetrical absence of seta A1 (one adult: the holotype); symmetrical absence of seta A1 (one pre-imago);
Sternite VII: asymmetrical absence of P1a seta (one imago); presence of seta Pc (formula 5/9) (five imagines);
Sternite VIII: asymmetrical absence of setae A2 and P1a (one imago); presence of a seta Ac (formula 5/2) (two imagines); symmetrical presence of four posterior setae (formula 4/4) (one imago) (); presence of a seta Ac and asymmetrical absence of seta P1a (one imago).
Porotaxy is given in . Pores designation follows Shrubovych (Citation2014). The most interesting and peculiar character is the absence of psm pores and the presence of single pores situated near (normally just a bit anterior and on the side) setae A3 on tergite VII (): we suggest to denominate these pores anterosublateral, asl. This pore is absent, while psm is present in specimens collected in Lombardy, Friuli-Venezia Giulia, Switzerland, Austria and Slovenia. Psm pores on tergite VIII are covered by a small notched lid ().
Table III. Porotaxy of Acerentomon italicum Nosek, Citation1969. Legend: ac = anterocentral, al = anterolateral, asl = anterosublateral, l = lateral, psl = posterosublateral, psm = posterosubmedial, sal = sternal anterolateral, sam = sternal anteromedial, sc = sternal central, spsl = sternal posterosublateral, spsm = sternal posterosubmedial.
Abdominal appendages with 4, 2, 2 setae (). Serration of pleural line VI () is quite variable within and between populations (sometimes markedly differing also on the two sides of the same specimen) from fine regular small teeth to conspicuous teeth like those described by Szeptycki (Citation1980) in A. fageticola; pleural line VII with a clumsy tooth followed by a group of smaller ones near the al pore (). Striate band on abd. VIII well developed, with a complete row of distinct teeth and, behind it, on the sternite, a second row of small teeth centrally interrupted (, , ); posterior margin of pleurite VIII with 5–6 regular pointed teeth (); comb with 14–17 long teeth (). Male squama genitalis with 6 + 6 setae (). Female squama genitalis with long, pointed acrostylus ().
Main measures and ratios of pre-imaginal stages are shown in ; chaetotaxy is given in (see also ).
Table IV. Main measures and ratios of pre-imaginal stages of Acerentomon italicum Nosek, Citation1969; measures are given in µm (mean values ± standard deviation, variation interval in parentheses). Legend: LR = labrum ratio, PR = pseudoculus ratio, CF = distal part of “filamento di sostegno” (= maxillary gland) ratio (see text), TR = tarsus ratio, BS = sensilla t1 position relative to the base of tarsus (see text).
Distribution
North Italy (Piedmont, Lombardy, Trentino-Alto Adige, Veneto, Friuli-Venezia Giulia, Liguria, Emilia-Romagna, Tuscany), Switzerland (about 5 km away from the Italian border), Austria, Slovenia and Corsica (See Annex I).
Remarks
Nosek (Citation1969, Citation1973) considered this species extremely close to A. gallicum, but this last species (after Szeptycki Citation1980) is known to be characterised by the presence of additional posterior setae on the head (d6, according Rusek et al. Citation2012). François (Citation2006), in his cladistic analysis of genus Acerentomon, put A. italicum in a clade including also A. noseki, A. skuhravyi, A. granulatum and A. brevisetosum. These species share a high LR ratio, the presence of 10–12 anterior setae on abdominal tergites II and III, the presence of seta x on abdominal tergite VII, the presence of six setae on abdominal tergite XI, the simple female acrostyle. Of all these characters, only the presence of seta x on tergite VII is considered to represent an apomorphy by François (Citation2006). A. noseki differs from A. italicum in the chaetotaxy of sternite VIII (4/0) and in comb VIII having many slender teeth. A. skuhravyi and A. granulatum differ in the length and shape of foretarsal sensillum b (thicker and longer, exceeding base of seta γ3) and in the presence of additional setae on the head (in the latter species, the chaetotaxy formula of sternite VIII is 6/0). A. brevisetosum lacks pleural pectines II–VII and has a long and spindle-shaped foretarsal sensillum a. In our opinion, the most similar species to Acerentomon italicum is A. fageticola Rusek, 1966 (see also its redescription in Szeptycki Citation1980); it differs from A. fageticola mainly in the shorter foretarsal sensillum a and in the presence of asl pores on tergite VII (character verified on A. fageticola specimens by J. Shrubovych). The variability of serration of pleural line VI observed in A. italicum invalidates it as a reliable diagnostic character to differentiate A. italicum from A. fageticola, contradicting the suggestion of Szeptycki (Citation1980).
Phenology and sex ratio
Monthly collecting 10 soil samples (about 1000 cc each) in a forest in northwest Italy, Galli et al. (Citation2012) recorded A. italicum juveniles (LII, MJ) from March to July and also in January. Extending the analyses to our whole collection, Mediterranean localities (Liguria and Tuscany) are characterised by the presence of juveniles (LI, LII, MJ) throughout the year (only in August they were missing; however, PI were collected then). In temperate sites (other regions), juveniles were collected only during spring–summer (March–August; PI until October). However, the monthly distribution of samplings is inhomogeneous.
Galli et al. (Citation2012) recorded a sex ratio unbalanced towards females (M:F = 0.74, χ2 = 5.7302, 1 d.f., p < 0.05). A similar value is found when analysing the whole database (M:F = 0.78, χ2 = 18.908, 1 d.f., p < 0.05).
Ecology
Through the analysis of data collected in the sampling localities (unfortunately in some cases not reported on the labels), it has been possible to record the presence of A. italicum from 0 to 2.000 m above sea level in the soil and/or litter of pure and mixed forests of the following plants: Quercus ilex, Quercus suber, deciduous Quercus spp., Robinia pseudoacacia, Corylus avellana, Fraxinus ornus, Fagus sylvatica, Castanea sativa and Pinus sp. In one case, specimens were collected in a reed grove near the seaside. The geological substratum has been recorded only in a few cases, and was limestone, quartz/mica schists and conglomerates.
DNA barcoding analysis
COI fragments from 21 representatives of Acerentomon italicum from Italy () were newly sequenced. The sequences are deposited at Barcode of Life Data Systems (BOLD) under project name ProtItal (). Analysing them together with the sequence from a population in Vienna (Resch et al. Citation2014) revealed big distances separating the Italian from the Austrian populations of Acerentomon italicum (maximum intraspecific pairwise distance: 31.98%).
Table V. List of representatives of Acerentomon italicum, used primer pair and Barcode of Life Data systems (BOLD) accession number analysed in the present study.
Figure 8. Acerentomon italicum Nosek, Citation1969. DNA Barcoding. Neighbour-joining (NJ) tree based on Kimura 2 Parameter (K2P) distances from 21 representatives of A. italicum from three collection sites in Liguria.
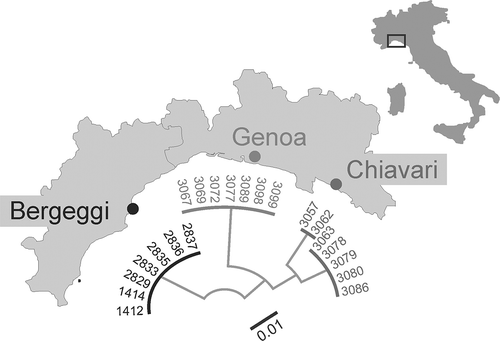
The big distance between the Austrian specimen and the three Italian populations at first sight indicates two clearly separate Molecular Operational Taxonomic Units (MOTUs). This seemingly correlates with differences in the porotaxy (asl missing and psm present in the specimen from Austria, but likewise in those from Lombardy, Friuli-Venezia Giulia, Switzerland and Slovenia). However, DNA barcodes from most of these populations are still missing. To decide at which point intraspecific variation is replaced by overlooked species diversity awaits a denser sampling of populations. Most importantly, it should be tested whether molecular distances correlate with geographic distances among populations of A. italicum. Until now, intraspecific distances have always been high, whenever more than a single population of a proturan species is covered by DNA barcoding. The high possibility of unrecognised diversity, however, points to the urgent need of searching for additional morphological characters, like the porotaxic characters presented in this contribution.
Acknowledgements
We express our sincere thanks to Dr. Peter Schwendinger (Geneva Natural History Museum) for providing the type material and many other specimens. We are grateful to Dr. Marco Bertolino for his kind help during the acquisition of SEM images.
References
- Böhm A, Bartel D, Szucsich NU, Pass G. 2011. Confocal imaging of the exo-and endoskeleton of Protura after non-destructive DNA extraction. Soil Organisms 83:335–345.
- Capurro M, Galli L, Torti C. 2009. Protura of Liguria (NW-Italy). Acta Zoologica Cracoviensia - Series B: Invertebrata 52:45–56. doi:10.3409/azc.52b_1-2.45-56.
- François J. 2006. Analyse cladistique du genre Acerentomon Silvestri, 1907 (Protura, Acerentomidae). Bulletin de la Société entomologique de France 111:5–10.
- Galli L, Capurro M, Torti C. 2011. Protura of Italy, with a key to species and their distribution. ZooKeys 146:19–67. doi:10.3897/zookeys.146.1885.
- Galli L, Capurro M, Shrubovych J, Torti C. 2012. Phenology of Protura in a northwestern Italian forest soil (Hexapoda: Protura). Acta Zoologica Cracoviensia - Series B: Invertebrata 55:33–43. doi:10.3409/azc.55_1.33.
- Hammer Ø, Harper DAT, Ryan PD. 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4:1–9. Available: http://palaeo-electronica.org/2001_1/past/issue1_01.htm. Accessed Oct 2012 4.
- Meier R, Shiyang K, Vaidya G, Ng PKL. 2006. DNA barcoding and taxonomy in Diptera: A tale of high intraspecific variability and low identification success. Systematic Biology 55:715–728. doi:10.1080/10635150600969864.
- Nosek J. 1969. Three new species of Protura from Italy. Atti dell’Istituto Veneto di Scienze, Lettere ed Arti. Classe di Scienze matematiche e naturali 127:485–494.
- Nosek J. 1973. The European Protura, their taxonomy, ecology and distribution with keys for determination. Genève: Muséum d’Histoire Naturelle.
- Resch MC, Shrubovych J, Bartel D, Szucsich NU, Timelthaler G, Bu Y, Walzl M, Pass G. 2014. Where taxonomy based on subtle morphological differences is perfectly mirrored by huge genetic distances: DNA Barcoding in Protura (Hexapoda). PLoS ONE 9:e90653. doi:10.1371/journal.pone.0090653.
- Rusek J, Shrubovych J, Szeptycki A. 2012. Head porotaxy and chaetotaxy of order Acerentomata (Protura). Zootaxa 3262:54–61.
- Shrubovych J. 2014. Identification and character analysis of the Acerentomidae (Protura) of the northeastern Palearctic (Protura: Acerentomidae). Zootaxa 3755:136–164. doi:10.11646/zootaxa.3755.2.
- Szeptycki A. 1980. Polish Protura. I. Genus Acerentomon Silvestri, 1907. Bulletin entomologique de Pologne 50:311–392.
- Szeptycki A. 2007. Catalogue of the world Protura. Acta Zoologica Cracoviensia 50B:1–210.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30:2725–2729. doi:10.1093/molbev/mst197.
- Tuxen SL. 1964. The Protura. A revision of the species of the world. With keys for determination. Paris: Hermann.
Appendix I – Catalogue
In this catalogue, the following abbreviations are used: a.s.l. = above sea level, PI = pre-imago, MJ = maturus junior, LII = larva II, LI = larva I, undet. = undetermined, MHNG = Muséum d’Histoire Naturelle de Genève, MNHG = Museo Civico di Storia Naturale, Genova, Italy, MNV = Museo Civico di Storia Naturale, Verona, Italy. Unless otherwise stated, material is preserved in Genoa University – DISTAV.
ITALY
Piedmont – one ♀, Alessandria, Rocca Grimalda, S. Giacomo, locust-tree litter, 15 October 1979 (Torti); one ♀ Alessandria, surroundings of Ovada, chestnut forest, 18 October 1979 (Torti); one ♂, two ♀♀, Alessandria, Lerma, Cirimilla, 27 May 2009 (Torti); one ♀, Alessandria, Bosio, Capanne di Marcarolo, chestnut forest, 700 m a.s.l., 4 January 1981 (Torti).
Lombardy – one MJ, two LII, Bergamo, Fonteno, 7 May 1978 (Parodi & Zoia); one ♂, Brescia, Val Trompia, Bovegno, beech forest, 900 m a.s.l., 17 October 1982 (Zoia, Torti & Giusto); eight ♂♂, 14 ♀♀, one PI, one MJ, one LII, Brescia, Polaveno, 850 m a.s.l., 9 August 1979 (Zoia); two ♀♀, Brescia, Barghe, 19 April 1925 (Dodero); one ♂, Brescia, Gavardo, Casalicolo, 3 October 1981 (Zoia).
Trentino-Alto Adige – one ♂, Bolzano, Brunico, under ash strain, 19 August 1977 (Vit) (MHNG); two ♀♀, Trento, Levico Terme, wet slope, 24 July 1978 (Vit) (MHNG); one ♀, Trento, Fiera di Primiero, Passo Cereda, 1360 m a.s.l., 2 July 1985 (Gardini).
Veneto – 14 ♂♂, 15 ♀♀, one PI, four MJ, six LII, Belluno, Agordo, La Muda, 550 m a.s.l., 2 July 1985 (Gardini); 20 ♂♂, 19 ♀♀, one MJ, Belluno, Ponte delle Alpi, locust-tree litter, 24 July 1978 (Vit) (MHNG); 16 ♂♂, 16 ♀♀, one PI, two MJ, ibidem, 29 July 1978 (MHNG); seven ♂♂, 14 ♀♀, one PI, one undet., Belluno, Tambre, Pian del Cansiglio, beech forest, 1000 m a.s.l., 4 October 1985 (Zoia); one ♂, two ♀♀, one PI, Treviso, Cison, S. Bolto Pass, 760 m a.s.l., 1 July 1985 (Paoletti); one ♂, Treviso, Mansuè, Bosco di Basalghelle, 10 August 1980 (Paoletti); two ♂♂, ibidem, 2 December 1980; seven ♂♂, 11 ♀♀, two PI, one MJ, ibidem 11 March 1981.
Friuli-Venezia Giulia – five ♂♂, one ♀, Udine, Paluzza, hazelnut forest, 23 July 1978 (Vit) (MHNG); one ♂, Udine, surroundings of Tolmezzo, litter, 23 July 1978 (Vit) (MHNG); one ♂, two ♀♀, Pordenone, Tramonti di Sotto, Campone, 2 October 1985 (Zoia); one ♂, five ♀♀, Trieste, Aurisina, 29 May 1986 (Torti); one ♀, Trieste, Gabrovizza, 2 October 1983 (Zoia).
Liguria – one ♂, Imperia, Baiardo, 800 m a.s.l., 17 June 1982 (Ansaldo, Torti & Zoia); seven ♂♂, three ♀♀, Imperia, Rezzo, Bosco di Rezzo, chestnut forest, 800 m a.s.l., 4 January 1998 (Poggi) (MNHG); one ♀, Savona, Bardineto, Roveirola, hornbeam forest, 750 m a.s.l., 29 March 1982 (Gardini & Rizzerio); four ♂♂, 11 ♀♀, one PI, one MJ, one LII, Savona, Bergeggi, cork oak forest on quartz/mica schists, 1 February 2007, (Capurro, Duradoni & Galli); 12 ♂♂, 37 ♀♀, one MJ, three LII, one undet., ibidem, 6 March 2007; eight ♂♂, nine ♀♀, six MJ, one LI, ibidem, 3 April 2007; 11 ♂♂, 14 ♀♀, four MJ, one LII, ibidem, 9 May 2007; 17 ♂♂, 23 ♀♀, two PI, four MJ, one LII, ibidem, 13 June 2007; 16 ♂♂, 15 ♀♀, 11 MJ, one LI, ibidem, 5 July 2007; two ♂♂, ibidem, 17 September 2007; 16 ♂♂, eight ♀♀, one PI, ibidem, 15 October 2007; four ♂♂, four ♀♀, one MJ, ibidem, 20 November 2007; four ♂♂, six ♀♀, one MJ, ibidem, 28 December 2007; 18 ♂♂, 27 ♀♀, one PI, three MJ, one LII, ibidem, 21 January 2008; three ♂♂ two ♀♀, one MJ, one LII, ibidem, 24 May 2012 (Galli & Zinni); four ♂♂, one ♀, Savona, Vado Ligure, Segno, Rocca dei Corvi, holm oak and hornbeam forest, 21 February 1985 (Torti & Zoia); one ♂, Savona, Varazze, holm oak forest, 19 February 1978 (Gardini); one ♂, Savona, Varazze, Deserto, holm oak forest, 22 August 1979 (Gardini); nine ♂♂, seven ♀♀, one PI, five MJ, one undet., Savona, Varazze, holm oak forest, 28 March 1978 (Gardini & Zoia); 10 ♂♂, 15 ♀♀, ibidem, 29 April 1981 (Gardini & Rizzerio) (MNHG); 21 ♂♂, 17 ♀♀, ibidem, 19 March 1981; nine ♂♂, 21 ♀♀, five PI, one MJ, ibidem, 29 May 1981 (MNHG); seven ♂♂, 10 ♀♀, one MJ, ibidem, 28 January 1982 (Rizzerio) (MNHG); two ♀♀, ibidem, 14 March 1982 (MNHG); one ♀, two MJ, Savona, Sassello, Foresta demaniale della Deiva, mixed forest dominated by locust-trees, 450 m a.s.l., 1 June 2005 (Biancardi & Galli); 25 ♂♂, 30 ♀♀, ibidem, 11 July 2007 (Poggi) (MNHG); one ♂, one ♀, Genova, Cogoleto, Lerca, Rio Loaga, holm oak forest, 15 November 1995 (Gardini); six ♂♂, 10 ♀♀, one PI, one MJ, two LI, two undet., ibidem, 3 December 2014 (Capurro & Ferretti); 12 ♂♂, 21 ♀♀, Genova, Arenzano, reed grove near the seaside, 28 March 1978 (Gardini & Zoia); 25 ♂♂, 19 ♀♀, three MJ, two LII, Genova, Arenzano, Villa Negrotto Cambiaso, 22 February 1980 (Gardini, Torti & Zoia); 23 ♂♂, 40 ♀♀, one PI, two MJ, Genova, Arenzano, 19 April 1981 (Gardini); one ♀, Genova, Masone, 4 October 1907 (Gestro) (MNHG); two ♂♂, four ♀♀, Genova, Voltri, Vesima, holm oak forest, 19 March 1981 (Gardini & Rizzerio); one MJ, ibidem, 16 February 1988; one ♀, ibidem, 14 March 1986 (Zoia); five ♀♀, Genova, Vesima, Rio Lupara, 24 December 1976 (Gardini & Peccenini); two ♂♂, five ♀♀, Genova, Isola del Cantone, Spinola, 5 November 1978 (Gardini); six ♂♂, six ♀♀, Genova, Campomorone, Isoverde, 2 November 1980 (Zoia & Antichi); one PI, Genova, Pegli, Torre Cambiaso, 17 September 1979 (Gardini); nine ♂♂, six ♀♀, one MJ, one LII, Genova, Sestri Ponente, Madonna del Gazzo, holm oak forest, 400 m a.s.l., 20 November 1981 (Gardini & Rizzerio); four ♂♂, three ♀♀, one PI, Genova, Borzoli, Cassinelle, 1 April 1908 (Gestro) (MNHG); one ♂, Genova, Bolzaneto, S. Lorenzo di Casanova, 21 September 1912 (Solari); one ♂, one ♀, Genova, Sant’Olcese, Busalletta, 6 November 1976 (Gardini); one ♂, Genova, Villetta Di Negro, 5 June 1907 (Dodero) (MNHG); two ♂♂, one ♀, ibidem, 13 August 1910; two ♂♂, ibidem, 12 February 1973 (Gardini); 13 ♂♂, 15 ♀♀, Genova [sic!] (Dodero); 11 ♂♂, four ♀♀, Genova, Nostra Signora del Monte, 26 November 1976 (Poggi); five ♂♂, six ♀♀, Genova, Montoggio, surroundings of Lago di Val Noci, oak forest, 14 December 1983; one ♂, one ♀, Genova, San Martino di Struppa, 550 m a.s.l., 16 May 1982 (Poggi) (MNHG); two ♀♀, Genova, Quezzi, oak and laurel forest, 10 April 1984 (Gardini) (MNHG); two ♂♂, 2 ♀♀, one MJ, two LII, Genova, St Martin Hospital, under holm oak, 1 April 2014 (Galli & Sarà); six ♂♂, two ♀♀, Genova, W slope of Mt. Fasce, mixed broadleaves forest dominated by chestnuts, 700 m a.s.l., 21 September 2006 (Galli); one ♂, one ♀, Genova, Quinto, E slope of Mt. Fasce, hornbeam forest, 400 m a.s.l., 18 May 2001 (Gardini); 12 ♂♂, 20 ♀♀, one MJ, Genova, Quinto, SW slope of Mt. Moro, mixed chestnut and oak forest, 150 m a.s.l., 20 February 2003 (Gardini) (MNHG); three ♂♂, 11 ♀♀, Genova, Quinto, Mt. Moro, oak forest, 150 m a.s.l., 30 March 2003 (Gardini); one ♀, Genova, Bogliasco, San Bernardo, mixed oak forest, 100 m a.s.l., 20 June 2007 (Costa); one ♀, Genova, Bargagli, Traso, 4 February 1973 (Poggi); two ♀♀, ibidem, 22 October 1978; three ♀♀, Genova, Propata, Caffarena, 22 January 1984 (Vit) (MHNG); one ♀, Genova, Lumarzo, Pannesi, Mt. Becco, 16 January 1983 (Zoia); one ♂, two ♀♀, Genova, Uscio, Colle Caprile, chestnut forest, 450 m a.s.l., 22 May 2006 (Poggi) (MNHG); one ♂, one ♀, Genova, Ognio, W slope Mt. Castello, chestnut forest, 500 m a.s.l., 17 March 1985 (Zoia); one ♀, Genova, Neirone, San Marco d’Urri, 30 December 1982 (Torti & Zoia); two ♂♂, one ♀, ibidem, chestnut forest, 2 November 1984; one ♀, Genova, Neirone, 17 March 1985, 400 m a.s.l. (Zoia); one ♀, one PI, Genova, Camogli, San Rocco, holm oak litter, 22 January 1984 (Vit) (MHNG); one ♂, ibidem, under a dead pine, (MHNG); one ♂, one ♀, Genova, Portofino, oak litter, 1 September 1979 (Vit), (MHNG); three ♂♂, six ♀♀, ibidem, hornbeam forest on conglomerate, 600 m a.s.l., 15 November 1981 (Gardini); two ♂♂, Genova, Rapallo, Montallegro, holm oak forest, 16 June 1907 (Dodero) (MNHG); one ♂, one undet., ibidem, 3 September 1973 (Poggi) (MNV); five ♂♂, four ♀♀, Genova, Fontanigorda, Mt. Collere, 1100 m a.s.l., 5 July 1982 (Torti & Zoia); one ♀, Genova San Colombano Certenoli, mixed forest, 18 September 2003 (Gardini); two ♂♂, three ♀♀, one MJ, ibidem, chestnut forest, 13 January 2004 (Gardini); three ♂♂, three ♀♀, two MJ, Genova, Chiavari, Madonna delle Grazie, holm oak forest, 17 May 2005 (Phytilis & Galli); two ♂♂, five ♀♀, ibidem, 1 April 2014 (Galli & Sarà); six ♂♂, six ♀♀, Genova, Chiavari, SW slope of Mt. Cucco, chestnut forest, 260 m a.s.l., 21 February 1994 (Zoia); one ♂, three ♀♀, ibidem, holm oak forest, 270 m a.s.l., 21 April 1994 (Zoia); two ♂♂, five ♀♀, Genova, Lavagna, 27 May 1979 (Zoia); two ♂♂, three ♀♀, one undet., La Spezia, Deiva Marina, cork oak litter, 21 November 1976 (Briganti, Gardini & Zoia); three ♂♂, six ♀♀, La Spezia, Framura, holm oak forest, 22 December 1984 (Zoia); three ♀♀, La Spezia, Levanto, holm oak forest, 7 September 1983 (Torti & Zoia); one ♂, one ♀, La Spezia, Portovenere, 8 February 1976 (Gardini); 13 ♂♂, 13 ♀♀, one PI, one undet., La Spezia, Portovenere, Tino Island, 4 May 2005 (Poggi) (MNHG); three ♂♂, two ♀♀, one MJ, La Spezia, La Spezia-Foce, holm oak forest, 2 October 1980 (Gardini, Rizzerio & Zoia); one PI, La Spezia, Lerici, S. Terenzo, 11 September 1985 (Torti); one ♂, six ♀♀, one PI, one LII, La Spezia, Ameglia, 3 March 1980 (Zoia).
Emilia-Romagna – three ♂♂, 11 ♀♀, one PI, one MJ, Parma, Bedonia, SW slope of Mt. Pelpi, 750 m a.s.l., 11 August 1982 (Ansaldo, Torti & Zoia); one ♀, Reggio Emilia, Carpineti, Valestra Mt., hornbeam forest, 900 m a.s.l., 3 July 1980 (Gardini, Giusto & Zoia).
Tuscany – three ♀♀, Massa Carrara, Pontremoli, N slope Mt. Orsaro, along the way to Passo del Cirone, beech forest, 1000 m a.s.l., 26 August 1983 (Torti & Zoia); two ♂♂, three ♀♀, ibidem, mixed hornbeam and chestnut forest, 26 October 1983; one ♀, Massa Carrara, Aulla, Masero, litter under Ostrya sp., 9 May 1984 (Torti & Zoia); four ♂♂, one ♀, one PI, Massa Carrara, surroundings of Campo Cecina, 10 August 1980 (Zoia); three ♂♂, seven ♀♀, two MJ, Livorno, Elba Island, Rio Marina, Capo Castello, holm oak forest, 3 September 2007 (Gardini); four ♂♂, Livorno, Elba Island, Capoliveri, Lacona, Colle delle Vacche, holm oak forest, 250 m a.s.l., 4 September 2007 (Gardini).
SWITZERLAND: one ♂, one PI, canton of Graubünden, Poschiavo, Albertuse, W slope Mt. Sessalbo, 2000 m, 16 August 1981 (Torti).
AUSTRIA: one ♂, one PI, one ♀, Vienna, Leopoldsberg (Institute of Systematics and Evolution of Animals, Polish Academy of Science).
SLOVENIA: one ♀, Oslu Cave (IZRK12332), 23 October 1982 (Novak).
FRANCE, Corsica: two ♂♂, three ♀♀, Bastia, Erbalunga, holm oak forest (on limestone), 22 May 1982, (Torchia & Zoia); two ♂♂, one ♀, Bastia, S. Maria di Lota, holm oak forest, 22 May 1982, (Torchia & Zoia).
Remarks
Some of the data from Liguria were already published in Capurro et al. (Citation2009). Records from Switzerland, Slovenia and Corsica (even if in the first case samples were collected pretty close – about 5 km – to the Italian border) might be considered new for these countries: until now this species was considered to be restricted to Italy and Austria (see Szeptycki Citation2007; Resch et al. Citation2014).
