Abstract
The Italian roe deer is classified as “vulnerable” in the International Union for Conservation of Nature Red List of Threatened Species, as the few specimens of this endemism may have a high risk of extinction. Conservation efforts for the Italian roe deer cannot prescind from the study of the feeding habits of the taxon. Therefore, in the present study, the spring diet composition of the Italian roe deer from two protected areas was estimated by using the micro-histological technique of faecal analysis. Univariate measures of alpha and beta diversity were computed to assess spatial differences in diet composition between the sites. A total of 79 different species of plants were identified, with few species (mainly woody plants) comprising over a quarter of the diet. The most consumed species were Rubia peregrina, Quercus suber and Osyris alba in Site 1, and Q. cerris, Carpinus betulus and Crataegus monogyna in Site 2. Alpha diversity analysis showed that diet composition was quite rich and diverse in both sites, with nearly all the shared species eaten to an equal extent. Moreover, the values of alpha diversity indices were not significantly different between the sites. The degree of dietary overlap ranged from “low” to “high”, as most of the identified plants were unshared, whereas the consumption of some shared plants differed between the sites. In conclusion, our results showed that that this subspecies of Capreolus is capable of exhibiting both a generalist and an opportunistic behaviour in relation of food resource availability.
Introduction
The Italian roe deer, Capreolus capreolus italicus Festa, Citation1925, is a recognised subspecies of the common European roe deer C. capreolus (Lorenzini et al. Citation2002; Gentile et al. Citation2009). Few populations of this Italian endemism are known to exist, and are relegated to only a few Mediterranean habitats in the central and southern parts of Italy (Gentile et al. Citation2009; Battisti et al. Citation2015). Accordingly, the International Union for Conservation of Nature (IUCN)’s Red List of Threatened Species has classified the Italian roe deer as ‘vulnerable’ (Rondinini et al. Citation2013). Lorenzini et al. (Citation2002) suggested some measures to conserve residual populations of the Italian roe deer, such as conducting research to determine its genetic structure, facilitating the expansion of remaining populations by reducing poaching and eliminating feral dogs, and establishing a re-introduction plan for southern Italy. However, conservation efforts for the Italian roe deer cannot prescind the study of an important aspect of its biology, such as that of the feeding habits of the taxon.
Diet is a key factor in the conservation of a threatened species, and knowledge about it has several uses. For instance, it may allow us to understand its composition as well as the nutritional value and the relative importance of its components. This, in turn, may lead to defining some food items as key plant species for identifying the elective habitat of the taxon and, hence, planning an effective re-introduction initiative. Additionally, the plants composing the diet may act as early warning indicators of food resource limitation, especially in relation to diet overlap with other animals.
To the best of our knowledge, no research has been conducted so far to understand the feeding habits of the Italian roe deer. Available information on its diet has been borrowed from studies on the European roe deer from forest areas of northern Europe (e.g. Duncan et al. Citation1998; Latham et al. Citation1999; Barančeková et al. Citation2010; Krasnov et al. Citation2015), where populations are more widespread and common, and vegetation characteristics are quite different compared to those of southern Europe. Since the feeding habits of roe deer may reflect the availability of food resources in a certain habitat (Gębczynska Citation1980; Cornelis et al. Citation1999), information obtained from the above studies may prove poorly appropriate for establishing a re-introduction plan for the Italian roe deer. In addition, it has been reported that, although the Italian and European forms may display similar behaviour patterns (e.g. social aggregation and large familiar areas during winter; dispersal and small familiar areas in summer), the Italian roe deer may prefer areas characterised by higher quality food, such as woodlands and scrublands (Focardi et al. Citation2009). Therefore, the present research was undertaken to provide a preliminary description of the use of plant resources by the Italian roe deer inhabiting two protected areas in the centre and south of Italy. Specifically, our aim was to determine the composition of spring diet by identifying, to the lowest possible taxonomic level, the indigestible plant fragments in faecal pellets. We also evaluated possible differences in diet composition between the sites using some alpha and beta diversity indices.
Materials and methods
Study sites
This study was carried out in two protected areas characterised by the presence of the Italian roe deer: in particular, preliminary direct observations as well as the presence of animal trails and pellets were the main criteria for selecting the study sites within each protected area.
The first protected area is the Castelporziano natural state reserve (headquarters coordinates: 41°44ʹ37.83″N, 12°24ʹ2.20″E), which is located in the Lazio region, centre of Italy. This preserve covers an area of 61 km2 containing several land-cover types (e.g. broad-leaved mixed oak forest, pasture, maquis, pseudo steppe, cork-oak forest, etc.) representative for the Mediterranean area (Manes et al. Citation1997). The mean annual temperature is +15.4°C and the annual precipitation is 740 mm. The populations of the Italian roe deer inhabiting the preserve have not been genetically contaminated by restocking with animals introduced from other areas (Focardi et al. Citation2006). Aside from the Italian roe deer, other ungulate species found in the preserve include fallow deer (Dama dama), wild boar (Sus scrofa majori), and red deer (Cervus elaphus) (Focardi et al. Citation2015). A study site (Site 1) of 0.66 km2, lying within 80–100 m above sea level (asl), was chosen in the north of the preserve (). The Italian roe deer is the only cervid species living in this site.
Figure 1. Localisation of the study sites within the two protected areas (Site 1, Castelporziano natural state reserve; Site 2, Regional Park of Gallipoli Cognato Piccole Dolomiti Lucane).
The second protected area is the Regional Park of Gallipoli Cognato Piccole Dolomiti Lucane (headquarters coordinates: 40°30′49.65″N, 16°8′35.70″E), which is situated in the Basilicata region, South of Italy. This park safeguards a wide area (270.27 km2) featuring different geomorphological and micro-climatic conditions. The annual average temperature is +11°C, whereas the average annual rainfall is 910 mm. The presence of the Italian roe deer in the park is due to a reintroduction programme initiated in 2008 (Regione Basilicata Citation2006). Within the park, a site (Site 2), situated at an elevation between 640 and 860 m asl and measuring about 0.60 km2, was chosen for sampling (). This area is extensively grazed by different native breeds of cattle, sheep and goats.
Information on vegetation characteristics of the sites were obtained from different sources. In particular, according to Pignatti et al. (Citation2001), Viburno-Quercetum ilicis and the Pruno-Crataegetum were the main plant communities characterising Site 1. A mixed-oak forest, consisting essentially of Quercus cerris and Quercus frainetto referring to Centaureo–Quercetum pubescentis communities, dominated the tree layer of Site 2 (Freschi et al. Citation2014). Meadows interspersed with thickets of dwarf bushes (e.g. C. monogyna, Prunus spinosa and P. amygdaliformis) were also available in this site.
Collection and processing of faecal pellets
Sampling took place monthly from May 2014 to July 2014 along replicate and permanent transects (2 m × 200 m), which were separated from one other by ~100 m (Torres et al. Citation2011; Valente et al. Citation2014), and spatially distributed throughout each study site.
Processing of fresh faecal pellets followed the method described by Freschi et al. (Citation2014, Citation2015, Citation2016). Briefly, pellets were first ground in a mortar, then cleared in a 0.05 M solution of sodium hydroxide (NaOH) for 2 h. Thereafter, the samples were washed with distilled water over a 63-µm sieve, and the retained material was collected over filter paper, dried and mounted in glycerol gelatine on five microscope slides. The first 10 non-overlapping plant fragments were counted in systematic transects in alternate rows.
Diet composition analysis
Botanical composition of diet was determined using the micro-histological analysis of faecal pellets (Baumgartner & Martin Citation1939; Dusi Citation1949). This technique has been widely used to investigate the diet composition of different herbivores, although its limitations related to differential digestibility of plant material are well documented (Holechek et al. Citation1982). Reviews of studies of roe deer feeding habits (Tixier & Duncan Citation1996; Cornelis et al. Citation1999) reported that this technique is the second most common method used for diet composition analysis of roe deer. Moreover, this technique is particularly useful for endangered species, since it does not interfere with the behaviour of the animals and does not require handling/collecting/killing individuals. This makes such technique suitable for analysing diet composition of the “vulnerable” Italian roe deer.
Identification of plant species was carried out by comparing the different features and dimensions of the epidermal cells and other valuable taxonomical structures (e.g. trichomes and stomata form) of the recovered fragments with those of a plant reference material prepared by collecting monthly leaves, stems, flowers and fruits of the plants found in the study site. Images of identified fragments were also acquired with a Leica EC3 digital camera (Leica Microsystems, Bannockburn, IL, USA) linked to software for image analysis (Leica LASV4.1).
The taxonomic nomenclature of the identified taxa follows Conti et al. (Citation2005). The fragments that were not identified to the species level were classified as “unidentified”, and were not included in our data set. Species abundance data were also aggregated to plant family in order to highlight botanical differences between the sites.
Statistical analysis
Diet composition was expressed as relative (rf) of a taxon (or family), i.e. by dividing the total number of fragments attributed to a given taxon (or family) by the total number of identified fragments (Freschi et al. Citation2014, Citation2015, Citation2016).
Three indices of alpha diversity were computed to compare the diets at species level: species richness (D) (Margalef Citation1958), diversity (H) (Shannon & Weaver Citation1949) and evenness (E) (Buzas & Gibson Citation1969). In the D index, the higher the value, the greater the richness. The value of H usually ranges between 1.5 and 3.5 and often does not exceed 4 (Margalef Citation1972). E value ranges between 0 and 1, where 1 indicates that all of the food items are used to an equal extent. Differences in diet richness, diversity and evenness between the sites were analysed by a Student’s t-test.
The qualitative Sørensen similarity index (CS) (Sørensen Citation1948) and the quantitative Morisita–Horn index (CMH) (Morisita Citation1959) were used to compare the dietary similarity or overlap between the diets. The values of both indices vary between 0 (no overlap) and 1 (complete overlap), and were classified according to the following scale: 0 < CS/CMH ≤ 0.29, small overlap; 0.30 ≤ CS/CMH ≤ 0.59, medium overlap; CS/CMH ≥ 0.60, high overlap.
Results
Diet composition
A total of 79 plant taxa belonging to 32 families were identified in the faecal pellets of the Italian roe deer (). The number of identified plants was higher in Site 2 than in Site 1 (60 vs. 57, respectively), whereas the overall ingestion rate (rf) ranged from 0.23 to 9.78% in Site 1, and from 0.04 to 10.47% in Site 2. Few taxa constituted over half of the diet: in particular, nine taxa accounted for 53.42% of the diet in Site 1, whereas 10 taxa accounted for 51.67% of the diet in Site 2. In both sites, most of the identified taxa (Site 1: 32 of 57; Site 2: 32 of 60) had a relative frequency of less than 1%, and represented 17.89 and 15.18% of the diet in sites 1 and 2, respectively.
Table I. Spring diet composition of the Italian roe deer from the two study sites (Site 1, Castelporziano natural state reserve; Site 2, Regional Park of Gallipoli Cognato Piccole Dolomiti Lucane). Data are relative frequencies of plant taxa identified in faecal pellets.
Of all the plant species we identified, 19 were found only in Site 1: Foeniculum vulgare, Olea europaea, Cynodon dactylon, Brachypodium sylvaticum, Trifolium campestre, Ulmus minor, Pistacia lentiscus, Quercus ilex, Rosa sempervirens, Rhamnus alaternus, Cytisus scoparius, Clematis flammula, Prunus spinosa, Arbutus unedo, Phillyrea latifolia, Tamus communis, Osyris alba, Quercus suber and Rubia peregrina. Conversely, the following 22 species were found only in Site 2: Acer campestre, A. monspessulanum, Cachrys ferulacea, Asphodelus ramosus, Briza maxima, Bromus erectus, Carpinus betulus, Cytisus hirsutus, Euonymus latifolius, Lonicera etrusca, Plantago serraria, Prunus cocomilia, Pyrus amygdaliformis, P. communis, Quercus cerris, R. canina, Rumex sanguineus, Sorbus domestica, S. torminalis, Symphytum bulbosum, Verbascum thapsus and Vicia sativa.
The most consumed species in Site 1 were R. peregrina (9.78%), Q. suber (8.62%), O. alba (7.27%), T. communis (5.22%) and P. latifolia (4.98%). Plants such as Q. cerris (10.47%), Carpinus betulus (9.54%), Crataegus monogyna (4.55%), P. amygdaliformis (4.55%) were the most consumed in Site 2.
The aggregation of abundance data to plant family showed that five families (i.e. Rubiaceae, Fagaceae, Rosaceae, Santalaceae and Cyperaceae) accounted for 53.38% of the diet in Site 1. The remaining part of the diet (46.62%) was represented by plants belonging to 24 families. The first family for consumption was Rubiaceae, which included only three taxa (i.e. R. peregrina, Galium verum, G. miollugo) comprising 14.11% of the diet. Fagaceae (three taxa accounting for 13.93%) and Rosaceae (seven taxa accounting for 11.18%) were the second and the third family for consumption, respectively. In Site 2, over half (53.64%) of the diet was composed of plants belonging to only three families (i.e. Rosaceae, Fagaceae and Corylaceae). In particular, Rosaceae was the family with the greatest number of species (11 of 60) and the highest relative frequency (30.37%). Among the plants belonging to this family, C. monogyna, P. amygdaliformis and R. canina occurred at high relative frequencies. The second and the third families for consumption were Fagaceae (two taxa accounting for 12.65%) and Corylaceae (two taxa accounting for 10.63%), respectively.
Spatial variation distribution between the diets
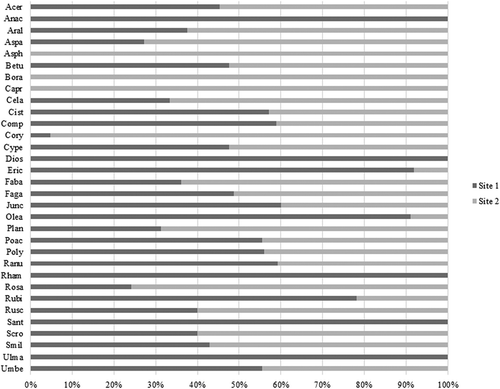
Eight of 32 families were not shared between the sites (). Among them, five families (i.e. Anacardiaceae, Dioscoreaceae, Rhamnaceae, Santalaceae and Ulmaceae) were part (15.09%) of the diet from Site 1, whereas the remaining three (i.e. Asphodelaceae, Boraginaceae and Caprifoliaceae) were part (6.48%) of the diet from Site 2. Families such as Ericaceae, Oleaceae and Rubiaceae were observed at high rates in Site 1. Conversely, higher frequencies of consumption of Corylaceae, Fabaceae and Rosaceae were observed in Site 2.
Figure 2. Relative frequencies of plant families identified in faecal pellets from both sites (Site 1, Castelporziano natural state reserve; Site 2, Regional Park of Gallipoli Cognato Piccole Dolomiti Lucane). 1. plant families: Acer = Aceraceae; Anac = Anacardiaceae; Aral = Araliaceae; Aspa = Asparagaceae; Asph = Asphodelaceae; Betu = Betulaceae; Bora = Boraginaceae; Capr = Caprifoliaceae; Cela = Celastraceae; Cist = Cistaceae; Comp = Compositae; Cory = Corylaceae; Cype = Cyperaceae; Dios = Dioscoreaceae; Eric = Ericaceae; Faba = Fabaceae; Faga = Fagaceae; Junc = Juncaceae; Olea = Oleaceae; Plan = Plantaginaceae; Poac = Poaceae; Poly = Polygonaceae; Ranu = Ranunculaceae; Rham = Rhamnaceae; Rosa = Rosaceae; Rubi = Rubiaceae; Rusc = Ruscaceae; Sant = Santalaceae; Scro = Scrophulariaceae; Smil = Smilacaceae; Ulma = Ulmaceae; Umbe = Umbelliferae.
Concerning alpha diversity analysis, although Margalef’s index showed higher values in Site 2 than in Site 1 (D = 5.27 in Site 1 and 5.02 in Site 2), the Student’s t-test revealed no significant difference (t = −1.24; df = 118; p = 0.218) in diet richness between the sites. Similarly, diet diversity was not significantly different (t = −1.81; df = 118; p = 0.073) between the sites as measured by the Shannon diversity index (H = 2.71 in Site 1, and 2.80). Diet evenness also did not vary significantly (t = 0.56; df = 118; p = 0.578) between the sites, as the E values were 0.84 and 0.83 for sites 1 and 2, respectively. The value of the Sørensen qualitative similarity index CS was 0.65, whereas the value of the Morisita–Horn quantitative index CMH was 0.20.
Discussion
The micro-histological analysis of faecal pellets allowed us to identify several plant taxa in both sites (i.e. 57 and 60 for sites 1 and 2, respectively). The number of identified taxa is comparable to that found in previous studies on the European roe deer (e.g. Tixier et al. Citation1997; Bartolomé et al. Citation2002; Krasnov et al. Citation2015). For instance, Bartolomé et al. (Citation2002), analysing the faecal pellets collected in a natural park located in Spain, found the diet of the European roe deer to be composed of 56 different plants. A similar result was reported by Tixier et al. (Citation1997), who found 58 plants eaten by roe deer in spring. Overall, these studies confirm that these cervids are generalist herbivores (Tixier & Duncan Citation1996; Barančeková et al. Citation2010), as their diet includes several plants.
On the other hand, it can be stated that they are also highly selective, as in both sites few species constituted over a quarter of the diet. Moreover, the most consumed plants were also not shared between the sites. The use of a small group of plants is in common with the European roe deer (Tixier et al. Citation1997; Latham et al. Citation1999; Bartolomé et al. Citation2002). Tixier et al. (Citation1997) reported that more than 20% of the diet of roe deer was composed of 1–3 preferred plant species. The authors concluded that these herbivores use a selective feeding strategy, which allows roe deer to first browse the most nutritious and palatable plants available (e.g. Illius et al. Citation2002; Ward et al. Citation2008), thus supporting the definition of “concentrated selector” (Hofmann Citation1989; Duncan et al. Citation1998). In the present study, the main components of the Italian roe deer’s diet in Site 1 were R. peregrina, Q. suber, O. alba, T. communis and P. latifolia. Plants such as Q. cerris, C. betulus, C. monogyna and P. amygdaliformis were the main components of the Italian roe deer’s diet in Site 2. The consumption of the above plants has been described in previous studies on the European roe deer (Gębczynska Citation1980; Jackson Citation1980; Tixier et al. Citation1997). For instance, Gębczynska (Citation1980) and Moser et al. (Citation2008) described C. betulus as one of the most important contributors to the diet of roe deer. The consumption of C. monogyna has been reported by Jackson (Citation1980) and Hearney and Jennings (Citation1983) by analysing rumen and pellets, respectively. The other plants identified in the present study have also been found to be components of the diet of the European roe deer. For example, the consumption of Hedera helix or various species of Rubus has been described in some previous studies (Jackson Citation1980; Tixier et al. Citation1997; Moser et al. Citation2008; Krasnov et al. Citation2015). These results seem to indicate that the diet of roe deer C. capreolus and that of its subspecies C. c. italicus are similar. The differences in diet composition are often related to the actual presence of a certain plant in a certain site, thus supporting the idea of Gębczynska (Citation1980) and Cornelis et al. (Citation1999) who suggested that the variation in the diet composition of roe deer is mainly due to the habitat in which the animals live. It has been reported that in dry Mediterranean habitats, the roe deer exhibited a polyphagic behaviour upon availability of resources (Duncan et al. Citation1998).
The plants identified in faeces from both sites belonged to 32 different families, most of which were shared between the diets (24 of 32, 75%). Families such as Rubiaceae, Fagaceae and Rosaceae occurred at high rates in faeces from Site 1 and accounted for 39.22% of the diet. In Site 2, the most frequent families were Rosaceae, Fagaceae and Corylaceae, which altogether constituted over half (53.65%) of the diet. These results seem to corroborate the findings of Focardi et al. (Citation2009), who suggested that the availability of high-quality food resources makes woods and scrubland the habitats most preferred by the Italian roe deer. However, open or agricultural fields may also become alternative habitats upon food availability, as shown by some studies on the European roe deer (e.g. Lamberti et al. Citation2006; Abbas et al. Citation2013; Sangiuliano et al. Citation2016). Overall, our results are consistent with previous studies on the diet composition of the European roe deer, showing that these cervids prominently consume woody plants (e.g. Gębczynska Citation1980; Cornelis et al. Citation1999; Bartolomé et al. Citation2002; Baranceková et al. Citation2004). For instance, Cornelis et al. (Citation1999) reported that half-woody plants, deciduous browse, and dwarf shrubs were among the most important food items for roe deer. Similarly, Baranceková et al. (Citation2004) found woody plants to be the main component of roe deer diet. The diet of the Italian roe deer also included a great proportion of forbs, whereas grasses were poorly represented. This result is in line with previous studies on roe deer (e.g. Gębczynska Citation1980; Latham et al. Citation1999; Baranceková et al. Citation2004), and it has been attributed to their different digestibility, with grasses being more hard to digest (Blair et al. Citation1977; Hofmann Citation1989) and less nutritious (Short & Epps Citation1976) than forbs.
The identification of fruits (Rosaceae) in faeces further confirms that these cervids seek out more palatable and high-quality food sources. Previous studies (Tixier et al. Citation1997; Wallach et al. Citation2010) have shown that fruits are the most preferred food items in some seasons. In particular, Wallach et al. (Citation2010) found that fruits were the dominant portion of roe deer diet in summer and early autumn. According to these authors, this feeding strategy demonstrates that roe deer can also exhibit opportunistic behaviour in some circumstances.
When comparing the diets by applying univariate measures of alpha diversity, no significant spatial differences in diet richness, diversity or evenness were found. The observed high average values of the indices indicated that, the composition of the diets was quite rich and diverse in spring; besides, nearly all the species composing the diets were eaten to an equal extent in both sites. These results were comparable with those of a previous study (Heroldová Citation1996), in which the diet of the European roe deer was compared with that of other ungulate species (i.e. Capra aegagrus and Ovis musimon) by analysing their dietary similarity and overlap. In particular, the author reported that the values of diet diversity and evenness indices of roe deer were 3.08 and 0.867, respectively, and concluded that these animals exhibited a higher dietary diversity and selectivity compared to other ungulates. Therefore, our results of alpha diversity analysis, along with those obtained by interpreting the relative frequencies of taxa composing the diet, further confirm that the Italian roe deer exhibited a selective behaviour. Beta diversity analysis showed that, the degree of dietary overlap was “high” and “low” as measured by the qualitative Sørensen similarity and the quantitative Morisita–Horn indices, respectively. These results were due to the conspicuous number of unshared plants between the diets (41 of 79, 51.90%), as well as the different consumption of some shared plants (e.g. C. monogyna, Q. virgiliana) between the sites.
In conclusion, the micro-histological analysis of faecal pellets allowed us to identify several plant taxa in both sites. The roe deer C. capreolus has been defined as a generalist highly selective feeder (Hofmann Citation1989; Duncan et al. Citation1998). This definition well applies to C. c. italicus, as the micro-histological analysis of the faecal pellets collected in two sites revealed that this subspecies is capable of exploiting several plant species, and of exhibiting opportunistic behaviour in relation to food resource availability. In particular, our results showed that, although these cervids heavily relied on woody plants and forbs, their diet was quite rich and diverse in both sites.
The results from the present study may be important to plan a successful re-introduction, since the taxa composing the diet may be used for identifying the elective habitat of the taxon. On the other hand, it must be acknowledged that our results do not allow us to draw an exhaustive description of the feeding habits of the Italian roe deer, since they are limited by the absence of information on the selection of the identified plants. Therefore, further studies are needed to investigate diet selection in relation to the amount and distribution of vegetation present at a site, as well as the seasonal variation in plant resources, in order to assess the relationship between preference and plant phenology.
Acknowledgements
We are grateful to the General Secretariat of Presidency of the Italian Republic and to the Direction of Castelporziano Presidential Estate (G. Calzolari and A. Tinelli) for the possibility to carry out the research. Thanks are also due to the Directive Staff (M. De Lorenzo and E. Mallia) of the Regional Park Gallipoli Cognato Piccole Dolomiti Lucane for their support.
References
- Abbas F, Picot D, Merlet J, Cargnelutti B, Lourtet B, Angibault J, Daufresne T, Aulagnier S, Verheyden H. 2013. A typical browser, the roe deer, may consume substantial quantities of grasses in open landscapes. European Journal of Wildlife Research 59: 69–75. doi:10.1007/s10344-012-0648-9
- Barančeková M. 2004. The roe deer diet: Is floodplain forest optimal habitat. Folia Zoologica 53: 285–292.
- Barančeková M, Krojerová-Prokešová J, Šustr P, Heurich M. 2010. Annual changes in roe deer (Capreolus capreolus L.) diet in the Bohemian Forest, Czech Republic/Germany. European Journal of Wildlife Research 56: 327–333. doi:10.1007/s10344-009-0321-0
- Bartolomé J, Rosell C, Bassols E. 2002. Diet composition of roe deer (Capreolus capreolus) in the natural park of the Garrotxa Volcanic Zone (Catalonia, Spain). Pirineos 157: 57–64. doi:10.3989/pirineos.2002.v157.61
- Battisti C, Di Gennaro A, Gippoliti S. 2015. Schematizing a historical demographic collapse on a large time span using local, secondary and grey data: The case of Italian roe deer Capreolus capreolus italicus in Central Italy. Journal for Nature Conservation 24: 63–67. doi:10.1016/j.jnc.2015.02.003
- Baumgartner LL, Martin AC. 1939. Plant histology as an aid in squirrel food-habit studies. The Journal of Wildlife Management 3: 266–268. doi:10.2307/3796113
- Blair RM, Short HL, Epps jr EA. 1977. Seasonal nutrient yield and digestibility of deer forage from a young pine plantation. The Journal of Wildlife Management 41: 667–676. doi:10.2307/3799988
- Buzas MA, Gibson TG. 1969. Species diversity: Benthonic foraminifera in western north Atlantic. Science 163: 72–75. doi:10.1126/science.163.3862.72
- Conti F, Abbate G, Alessandrini A, Blasi C. 2005. An annotated checklist of the Italian vascular flora. Rome: Palombi Editori.
- Cornelis J, Casaer J, Hermy M. 1999. Impact of season, habitat and research techniques on diet composition of roe deer (Capreolus capreolus): A review. Journal of Zoology (Lond.) 248: 195–207. doi:10.1111/j.1469-7998.1999.tb01196.x
- Duncan P, Tixier H, Hofmann RR, Lechner-Doll M. 1998. Feeding strategies and the physiology of digestion in roe deer. In: Andersen R, Duncan P, Linnell JDC, editors. The European roe deer: The biology of success. Oslo: Scandinavian University Press. pp. 91–116
- Dusi JL. 1949. Methods for the determination of food habits by plant microtechniques and histology and their application to cottontail rabbit food habits. The Journal of Wildlife Management 13: 295–298. doi:10.2307/3795871
- Festa E. 1925. Il Capriolo dell’Italia centrale. [The Roe deer of central Italy]. Bollettino del Museo zoologico e di Anatomia comparata dell’Università di Torino 40: 1–2.
- Focardi S, Aragno P, Montanaro P, Riga F. 2006. Inter-specific competition from fallow deer (Dama dama) reduces habitat quality for the Italian roe deer (Capreolus capreolus italicus). Ecography 29: 407–417. doi:10.1111/j.2006.0906-7590.04442.x
- Focardi S, Franzetti B, Ronchi F, Imperio S, Montanaro P, Aragno P, Toso S. 2015. Monitoring populations of a guild of ungulates: Implications for the conservation of a relict Mediterranean forest. Rendiconti Lincei 26: 535–544. doi:10.1007/s12210-015-0439-9
- Focardi S, Montanaro P, La Morgia V, Riga F. 2009. Piano d’azione nazionale per il Capriolo italico (Capreolus capreolus italicus). [National Action Plan of the Italian Roe deer (Capreolus capreolus italicus)]. Min. Ambiente — INFN, Quaderni di Conservazione della Natura 31:1–177.
- Freschi P, Fascetti S, Musto M, Cosentino C, Paolino R, Valentini V. 2016. Seasonal variation in food habits of the Italian hare in a south Apennine semi-natural landscape. Ethology Ecology Evolution 28: 148–162. doi:10.1080/03949370.2015.1022906
- Freschi P, Fascetti S, Musto M, Mallia E, Blasi AC, Cosentino C, Paolino R. 2014. Diet of the Apennine hare in a southern Italy Regional Park. European Journal of Wildlife Research 60: 423–430. doi:10.1007/s10344-014-0799-y
- Freschi P, Fascetti S, Musto M, Mallia E, Cosentino C, Paolino R. 2015. Diet of the Italian hare (Lepus corsicanus) in a semi-natural landscape of southern Italy. Mammalia 79: 51–59. doi:10.1515/mammalia-2013-0117
- Gębczynska Z. 1980. Food of the roe deer and red deer in the Białowieża Primeval Forest. Acta Theriologica 25: 487–500. doi:10.4098/AT.arch.80-44
- Gentile G, Vernesi C, Vicario S, Pecchioli E, Caccone A, Bertorelle G, Sbordoni V. 2009. Mitochondrial DNA variation in roe deer (Capreolus capreolus) from Italy: Evidence of admixture in one of the last C. C. italicus pure populations from central-southern Italy. Italian Journal of Zoology 76: 16–27. doi:10.1080/11250000802018725
- Hearney AW, Jennings TJ. 1983. Annual foods of the red deer (Cervus elaphus) and the roe deer (Capreolus capreolus) in the east of England. Journal of Zoology 201: 565–570. doi:10.1111/j.1469-7998.1983.tb05079.x
- Heroldová M. 1996. Dietary overlap of three ungulate species in the Palava Biosphere Reserve. Forest Ecology Management 88: 139–142. doi:10.1016/s0378-1127(96)03819-4
- Hofmann RR. 1989. Evolutionary steps of ecophysiological adaptation and diversification of ruminants: A comparative view of their digestive system. Oecologia 78: 443–457. doi:10.1007/BF00378733
- Holechek JL, Vavra M, Pieper RD. 1982. Botanical composition determination of range herbivore diets: A review. Journal of Rangeland Management 35: 309–315. doi:10.2307/3898308
- Illius AW, Duncan P, Richard C, Mesochina P. 2002. Mechanisms of functional response and resource exploitation in browsing roe deer. Journal of Animal Ecology 71: 723–734. doi:10.1046/j.1365-2656.2002.00643.x
- Jackson J. 1980. The annual diet of the roe deer (Capreolus capreolus) in the New Forest, Hampshire, as determined by rumen content analysis. Journal of Zoology 192: 71–83. doi:10.1111/j.1469-7998.1980.tb04220.x
- Krasnov V, Shelest Z, Boiko S, Gulik I, Sieniawski W. 2015. The diet of the roe deer (Capreolus capreolus) in the forest ecosystems of Zhytomirske Polesie of the Ukraine. Forest Research Papers 76: 184–190. doi:10.1515/frp-2015-0018
- Lamberti P, Mauri L, Merli E, Dusi S, Apollonio M. 2006. Use of space and habitat selection by roe deer Capreolus capreolus in a Mediterranean coastal area: How does woods landscape affect home range? Journal of Ethology 24: 181–188. doi:10.1007/s10164-005-0179-x
- Latham J, Staines BW, Gorman ML. 1999. Comparative feeding ecology of red (Cervus elaphus) and roe deer (Capreolus capreolus) in Scottish plantation forests. Journal of Zoology 247: 409–418. doi:10.1111/j.1469-7998.1999.tb01003.x
- Lorenzini R, Lovari S, Masseti M. 2002. The rediscovery of the Italian roe deer: Genetic differentiation and management implications. Italian Journal of Zoology 69: 367–379. doi:10.1080/11250000209356482
- Manes F, Grignetti A, Tinelli A, Lenz R, Ciccioli P. 1997. General features of the Castelporziano test site. Atmospheric Environment 31: 19–25. doi:10.1016/S1352-2310(97)00070-8
- Margalef R. 1958. Information theory in ecology. Journal of General Systems 3: 36–71.
- Margalef R. 1972. Interpretation not strictly statistical of representation of biological entities in multifactorial space. Investigación Pesquera 36: 183–190.
- Morisita M. 1959. Measuring of interspecific association and similarity between communities. Memoirs of the Faculty of science of Kyushu University. Serie E (Biology) 3: 65–80.
- Moser B, Schütz M, Hindenlang KE. 2008. Resource selection by roe deer: Are windthrow gaps attractive feeding places? Forest Ecology Management 255: 1179–1185. doi:10.1016/j.foreco.2007.10.023
- Pignatti S, Bianco PM, Tescarollo P, Scarascia Mugnozza GT. 2001. La vegetazione della Tenuta Presidenziale di Castelporziano. Il sistema ambientale della Tenuta Presidenziale di Castelporziano. Accademia Nazionale delle Scienze detta dei XL. Scritti E Documenti XXVI: 441–640.
- Regione Basilicata. 2006. Progetto esecutivo per la reintroduzione della specie Capreolus capreolus italicus nelle aree protette della regione Basilicata. [Executive project for the reintroduction of Capreolus capreolus italicus into protected areas of Basilicata Region]. DGR n. 728-15/05/2006. Available: http://www.regione.basilicata.it/giunta/files/docs/DOCUMENT_FILE_360990.pdf. Accessed Sep 2016 27.
- Rondinini C, Battistoni A, Peronace V, Teofili C. 2013. Lista Rossa IUCN dei Vertebrati Italiani [The IUCN Red List of Italian Vertebrates]. Rome: Comitato Italiano IUCN e Ministero dell’Ambiente e della Tutela del Territorio e del Mare.
- Sangiuliano A, Lovari S, Ferretti F. 2016. Dietary partitioning between European roe deer and European brown hare. European Journal of Wildlife Research 62: 527–535. doi:10.1007/s10344-016-1023-z
- Shannon CE, Weaver W. 1949. The mathematical theory of communication. [dissertation]. Urbana: University of Illinois Press.
- Short HL, Epps EA. 1976. Nutrient quality and digestibility of seeds and fruits from southern forests. The Journal of Wildlife Management 40: 283–289. doi:10.2307/3800427
- Sørensen TA. 1948. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content, and its application to analyses of the vegetation on Danish commons. Kongelige Danske Videnskabernes Selskabs Biologiske Skrifter 5: 1–34.
- Tixier H, Duncan P. 1996. Are European roe deer browsers? A review of variations in the composition of their diets. Revue d’Ecologie (La Terre et La Vie) 51: 3–17.
- Tixier H, Duncan P, Scehovic J, Yant A, Gleizes M, Lila M. 1997. Food selection by European roe deer (Capreolus capreolus): Effects of plant chemistry, and consequences for the nutritional value of their diets. Journal of Zoology 242: 229–245. doi:10.1111/j.1469-7998.1997.tb05799.x
- Torres RT, Carvalho JC, Panzacchi M, Linnell JDC, Fonseca C. 2011. Comparative use of forest habitats by roe deer and moose in a human-modified landscape in southeastern Norway during winter. Ecological Research 26: 781–789. doi:10.1007/s11284-011-0837-0
- Valente AM, Fonseca C, Marques TA, Santos JP, Rodrigues R, Torres RT. 2014. Living on the edge: Roe deer (Capreolus capreolus) density in the margins of its geographical range. Plos ONE 9: e88459. doi:10.1371/journal.pone.0088459
- Wallach AD, Shanas U, Inbar M. 2010. Feeding activity and dietary composition of roe deer at the southern edge of their range. European Journal of Wildlife Research 56: 1–9. doi:10.1007/s10344-009-0281-4
- Ward A, White PCL, Walker NJ, Critchley CH. 2008. Conifer leader browsing by roe deer in English upland forests: Effects of deer density and understorey vegetation. Orest Ecology and Management 256: 1333–1338. doi:10.1016/j.foreco.2008.06.034
