?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In the common sandpiper both sexes do not participate equally in brood rearing. The attendance of females progressively declines and by the third week after hatching, most of them leave their broods. To check if this unequal parental care results in different migration phenology of males and females, adult common sandpipers were caught at four ringing sites in Poland during their autumn migration and were sexed using a discriminant equation. The median date of female migration was 6 days earlier than that of males. In the first days of July, males comprised only 22% of common sandpipers caught, while later their percent share increased gradually and in August reached up to 80% of captured birds. However, birds of both sexes were present among migrants during the whole migration period and migration period of both sexes overlapped to a great extent. Most probably birds migrating through the study area came from a vast breeding area, where the period of egg laying differs between its northernmost and southernmost parts by at least 1 month.
Keywords:
Introduction
Detailed studies on migration of birds are difficult to conduct because of their complexity and different processes which shape migration patterns (Cristol et al. Citation1999; Jenni & Schaub Citation2003). Within a species, individuals of a given sex or age and also birds from different populations may reveal different timing or migration strategies (Ydenberg et al. Citation2005; Meissner Citation2015; Ożarowska & Zaniewicz Citation2015; Pinchuk et al. Citation2016). Different migration timing of males and females may have influence on the onset of other parts of the annual cycle, such as primary moult (Gill et al. Citation1995; Barshep et al. Citation2011). Moreover, it may be related to the amount of accumulated energetic reserves, which influences the migration strategy (Pinchuk et al. Citation2016). Hence, there is growing evidence of sex-based differences in bird migration phenology, also in waders in which sexual dimorphism is usually weakly expressed (e.g. McCloskey & Thompson Citation2000; Bishop et al. Citation2004; Meissner Citation2005, Citation2015; Ydenberg et al. Citation2005; Remisiewicz & Wennerberg Citation2006; Pinchuk et al. Citation2016).
The common sandpiper Actitis hypoleucos (Linnaeus, 1758) is a wader species widely distributed in Europe and Asia (Cramp & Simmons Citation1983). Its autumn migration through Europe is well described according to the results of regular counts (e.g. Harengerd et al. Citation1973; Winkler & Herzig-Straschil Citation1981; Meissner Citation1996; Mitrus et al. Citation1998; Laber Citation2003; Iwajomo & Hedenström Citation2011) and analyses of ringing recoveries (Stiefel et al. Citation1985; Meissner Citation1997; Cepák et al. Citation2008; Fransson et al. Citation2008; Saurola et al. Citation2013). It is known that adult birds precede juveniles when moving towards the wintering grounds, but migration dates of both age classes overlap to a great extent (Meissner Citation1996; Balmori Citation2003). However, there are no studies that examined possible differences in migration timing of adult males and females, because the common sandpiper is a monomorphic species and it is impossible to recognise the sex of the birds by their plumage characteristics (Meissner et al. Citation2015).
Observations from the breeding grounds suggest that in the early brood-rearing period, both sexes participate equally, and the attendance of males remains around 90% throughout the chick-rearing period. However, the attendance of females declines and by the third week most of them leave their broods (Yalden & Holland Citation1992). Hence, it might be expected that females migrate towards the wintering grounds earlier than males, but it is unknown how long females stay in the breeding area before they start autumn migration. The only attempt to distinguish migration timing of adult males and females was made in south-eastern Germany, where birds were sexed according to a wing length of 108 mm, which was thought to be a boundary for the wing length distributions of both sexes (Teubert & Kneis Citation1984). According to the data on birds sexed molecularly, individuals with wing length shorter than 111 mm and longer than 117 mm may be sexed safely as males and females, respectively (Meissner & Krupa Citation2016). Hence, using only one value of the wing length as a criterion for sexing birds can produce a bias towards one of the sexes. Moreover, a recently developed discriminant function for sexing adult common sandpipers according to linear measurements enables us to recognise the sex of birds caught and measured in previous years (Meissner & Krupa Citation2016). Thus, this study is aimed to validate the hypothesis of earlier migration of females of the studied species.
Material and methods
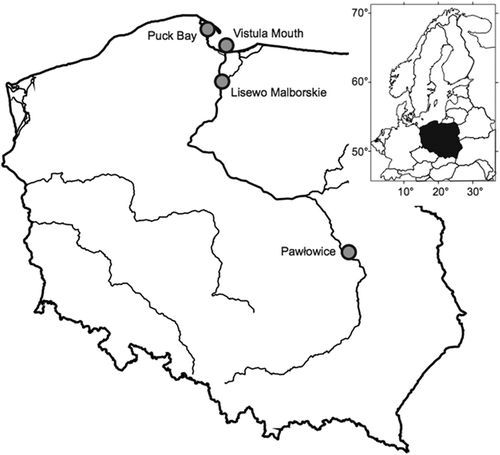
Birds were caught in walk-in traps (Busse & Meissner Citation2015) at four ringing sites in Poland () and aged according to plumage characteristics (Meissner et al. Citation2015). Only adult birds (older than 1 year) were analysed. Bird trapping started between the beginning of and mid-July and was finished in late September. This period (July–September) covers the majority of the migration of adult common sandpipers through Poland (Meissner Citation1996). The subsequent division of the study time into 5-day periods (pentades) followed Berthold (Citation1973), and the median test was used to assess differences between median migration dates of males and females.
Sexing was performed according to the equation given by Meissner and Krupa (Citation2016) based on the wing and tarsus plus toe lengths:
This equation allows correct sexing of 77.1% of birds (87.5% of males and 54.1% of females). Hence, it may produce sex bias in the analysed sample. To limit this error, the individuals with discriminant score D < −0.33 are identified as males and those with D > 1.26 as females, which allows us to reduce misclassification to 5% of males and 5% of females (Meissner & Krupa Citation2016).
In total, 1649 adult common sandpipers were caught and measured; however, only 926 of them were sexed using the equation given above with D value limits (). The percentage of unsexed birds varied between 41 and 50% in the following migration pentades, but these differences were statistically insignificant (G test, G = 4.52, df = 9, p = 0.87; the last four pentades were pooled due to a small sample size).
Table I. The total number of common sandpipers caught at different sites, and the number and percent share of individuals sexed by discriminant function. Study years at each site are given.
For assessing difference in median migration dates of males and females only data from Lisewo Malborskie and Pawłowice were taken into account, because the number of walk-in traps at these two ringing sites was stable; hence, it was assumed that the changes in the daily number of trapped birds roughly reflect species migration dynamics (Meissner Citation2008). Common sandpipers from these two sites comprised 60% of all caught birds and 62% with sex assigned. Hence, it was assumed that the data collected at these two sites reflect the overall migration pattern of the common sandpiper. All statistical procedures were performed using STATISTICA 12 software (StatSoft Inc.).
Results
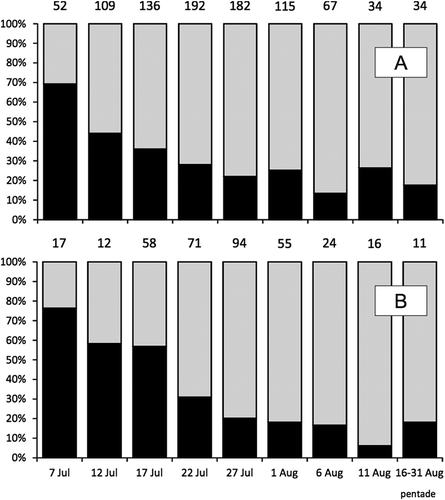
Migration periods of both sexes overlapped to a great extent, and males and females were present during the whole migration period. However, in the first days of July, males comprised only 23% of common sandpipers sexed molecularly and 31% among birds sexed by discriminant function. Later their percent share increased gradually, and in August between 74% and 94% of birds were males ().
Figure 2. The percent share of the common sandpiper females (black) and males (grey) caught in following pendates and in the second half of August. The middle day of each pentade was given. A: birds sexed according to discriminant function; B: bird sexed molecularly. Numbers above the bars indicate the sample size. Data from all ringing sites were pooled.
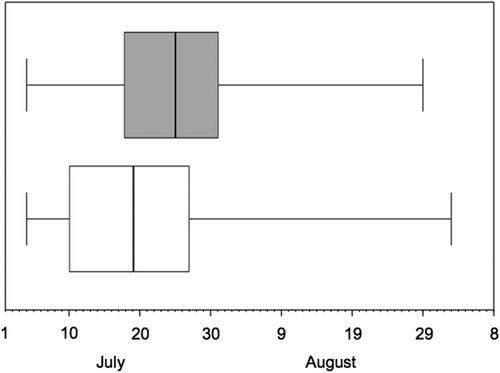
The median capture dates of females and males differed significantly (median-test, χ2 = 15.50, p = 0.001), being earlier in females, with a difference between medians of 6 days (19 July vs. 25 July) ().
Discussion
In the case of the common sandpiper, the possibility of sexing birds using linear measurements is limited due to a large overlap of linear measurement distributions of males and females (Meissner & Krupa Citation2016). Even though only small males and large females could be recognised, we assumed that obtained results showed actual differences in migration phenology of both sexes, especially as biometric variation among the common sandpiper populations originating from different parts of the breeding area has not been reported (Glutz Von Blotzheim et al. Citation1977; Cramp & Simmons Citation1983; del Hoyo et al. Citation1996) and the number of unsexed birds remained stable within the whole migration period.
Our study showed a temporal sex bias among adult common sandpipers captured when migrating through Poland towards wintering grounds. This supports predictions on earlier migration of females, which was described in some Arctic waders from the Scolopacidae family, in which one sex deserts the brood before fledging and migrates towards the wintering grounds earlier than the second sex (Butler & Kaiser Citation1995; Ydenberg et al. Citation2005; Barshep et al. Citation2012). However, migration terms of male and female waders breeding in the Arctic are well separated in time (e.g. Meissner Citation2005, Citation2015; Barshep et al. Citation2012), but the common sandpiper nests in the temperate zone, where the egg laying is less synchronised than in the Arctic (Wallander & Andersson Citation2003; McKinnon et al. Citation2012). Moreover, in the temperate zone there is enough time for repeated clutches after breeding failure, which occurs rarely in more northerly latitudes (Holland et al. Citation1982; Jamieson Citation2011). The common sandpiper migrating through Central Europe comes from a vast breeding area extending from the Eastern Baltic countries through Sweden and Finland to Northwestern Russia (Stiefel et al. Citation1985; Fransson et al. Citation2008; Saurola et al. Citation2013). The period of egg laying differs between the northernmost and southernmost parts of this area by at least 1 month (Glutz Von Blotzheim et al. Citation1977). That is why, although the brood attendance by females declines rapidly about 3 weeks before chicks fledge (Yalden & Holland Citation1992), migration periods of both sexes overlapped to a great extent. Nonetheless, the difference in median capture dates of males and females was 6 days, which is almost a quarter of the pre-fledging period.
In this paper we showed for the first time that in the common sandpiper, females precede males during migration towards their wintering grounds, which was expected due to progressive decline in females’ attendance during brood rearing. Our results show that the difference in migration time of both sexes in this species is less pronounced than that in Arctic breeding waders, and this may be due to the less synchronised period of egg laying in temperate zone. This may concern also other species with unequal parental care inhabiting vast non-Arctic breeding areas.
Acknowledgements
We would like to thank all ringers and volunteers working at WRG KULING ringing stations. Special thanks to Agnieszka Ożarowska for language correction and helpful comments, and to an anonymous reviewer for the suggestions that have greatly improved the first version of the manuscript. Publication of Waterbird Research Group KULING no. 160.
References
- Balmori A. 2003. Differential autumn migration of the Common Sandpiper (Actitis hypoleucos) in the Duero valley (north-west Spain). Ardeola 50:59–66.
- Barshep Y, Meissner W, Underhill LG. 2012. Timing of migration of the Curlew Sandpiper (Calidris ferruginea) through Poland in relation to Arctic breeding conditions. Ornis Fennica 89:120–129.
- Barshep Y, Minton C, Underhill LG, Remisiewicz M. 2011. The primary moult of Curlew Sandpipers Calidris ferruginea in North-western Australia shifts according to breeding success. Ardea 99:43–51. DOI:10.5253/078.099.0106.
- Berthold P. 1973. Proposals of standarization of the presentation of animal events, especially migratory data. Auspicium 5 Suppl:49–57.
- Bishop MA, Warnock N, Takekawa JY. 2004. Differential spring migration by male and female Western Sandpipers at interior and coastal stopover sites. Ardea 92:185–196.
- Busse P, Meissner W. 2015. Bird ringing station manual. Warsaw: De Gruyter Open Ltd.
- Butler RW, Kaiser GW. 1995. Migration chronology, sex ratio, and body mass of Least Sandpipers in British Columbia. Wilson Bulletin 107:413–422.
- Cepák J, Klvaňa P, Škopek J, Schröpfer L, Jelínek M, Hořák D, Formánek J, Zárybnický J eds 2008. Atlas migrace ptáků České republiky a Slovenska. Praha: Aventinum.
- Cramp S, Simmons KEL editors 1983. The birds of the Western Palearctic. Vol. 3. Oxford: Oxford University Press.
- Cristol DC, Baker MB, Carbone C. 1999. Differential migration revisited: Latitudinal segregation by age and sex class. Current Ornithology 15:33–87.
- del Hoyo J, Elliott A, Sargatal J eds. 1996. Handbook of the birds of the world. Vol. 3. hoatzin to auks. Barcelona: Lynx Edicions.
- Fransson T, Österblom H, Hall-Karlson S. 2008. Svensk ringmärkningsatlas. Vol. 2. Stockholm: Naturhistoriska riksmuseet. pp. 90–93.
- Gill JA, Clark J, Clark N, Sutherland WJ. 1995. Sex differences in the migration, moult and wintering areas of British-ringed Ruff. Ringing & Migration 16:159–167. DOI:10.1080/03078698.1995.9674107.
- Glutz Von Blotzheim UN, Bauer KM, Bezzel E. 1977. Handbuch der Vögel Mitteleuropas. Vol. 7. Wiesbaden: Aula-Verlag.
- Harengerd M, Prünte W, Speckmann M. 1973. Zugphänologie und Status der Limikolen in den RieseIfeldern der Stadt Münster. l. Teil: Haematopus bis Tringa. Vogelwelt 94:81–118.
- Holland PK, Robson JE, Yalden DW. 1982. The breeding biology of the Common Sandpiper Actitis hypoleucos in the Peak District. Bird Study 29:99–110. DOI:10.1080/00063658209476744.
- Iwajomo SB, Hedenström A. 2011. Migration patterns and morphometrics of Common Sandpipers Actitis hypoleucos at Ottenby, southeastern Sweden. Ringing & Migration 26:38–47. DOI:10.1080/03078698.2011.586509.
- Jamieson SE. 2011. Pacific Dunlin Calidris alpina pacifica show a high propensity for second clutch production. Journal of Ornithology 152:1013–1021. DOI:10.1007/s10336-011-0691-4.
- Jenni L, Schaub M. 2003. Behavioural and physiological reactions to environmental variation in bird migration: A review. In: Berthold P, Gwinner E, Sonnenschein E, editors. Avian migration. Berlin: Springer-Verlag. pp. 155–171.
- Laber J. 2003. Die Limikolen des österreichisch/ungarischen Seewinkels. Egretta 46:1–91.
- McCloskey JT, Thompson JE. 2000. Sex-related differences in migration chronology and winter habitat use of Common Snipe. The Wilson Bulletin 112:143–148. DOI:10.1676/0043-5643(2000)112[0143:SRDIMC]2.0.CO;2.
- McKinnon L, Picotin M, Bolduc E, Juillet C, Bêty J. 2012. Timing of breeding, peak food availability, and effects of mismatch on chick growth in birds nesting in the High Arctic. Canadian Journal of Zoology 90:961–971. DOI:10.1139/z2012-064.
- Meissner W. 1996. Timing and phenology of autumn migration of Common Sandpiper (Actitis hypoleucos) at the Gulf of Gdansk. The Ring 18:59–72.
- Meissner W. 1997. Autumn migration and biometrics of the Common Sandpiper Actitis hypoleucos caught in the Gulf of Gdańsk. Ornis Fennica 74:131–139.
- Meissner W. 2005. Variation in timing of the Siberian Knot Calidris c. canutus autumn migration in the Puck Bay region (southern Baltic). Acta Ornithologica 40:95–101. DOI:10.3161/068.040.0205.
- Meissner W. 2008. To count or to catch: A comparison of two methods of determining wader migration phenology. Wader Study Group Bulletin 115:16–19.
- Meissner W. 2015. Immature dunlins Calidris alpina migrate towards wintering grounds later than adults in years of low breeding success. Journal of Ornithology 156:47–53. DOI:10.1007/s10336-014-1132-y.
- Meissner W, Hollland PK, Cofta T. 2015. Part 11: Ageing and sexing the Common Sandpiper Actitis hypoleucos. Wader Study 122:54–59. DOI:10.18194/ws.00009.
- Meissner W, Krupa R. 2016. Identifying the sex of the common sandpiper (Actitis hypoleucos) by linear measurements. Annales Zoologici Fennici 53:175–182. DOI:10.5735/086.053.0406.
- Mitrus C, Kuczborski R, Słupek J. 1998. Autumn passage of the Common Sandpiper Actitis hypoleucos in the Bug River valley – dynamics and biometry. Notatki Ornitologiczne 39:13–25. (in Polish with English summary).
- Ożarowska A, Zaniewicz G. 2015. Temporal trends in the timing of autumn migration of short- and long- distance migrating Blackcaps (Sylvia atricapilla). Ornis Fennica 92:144–152.
- Pinchuk P, Karlionova N, Meissner W. 2016. Biometry indicates sexual differences in spring migration strategy in Ringed Plovers Charadrius hiaticula tundrae captured in the southern Belarus. North-Western Journal of Zoology 12:319–324.
- Remisiewicz M, Wennerberg L. 2006. Differential migration strategies of the Wood Sandpiper (Tringa glareola) – genetic analyses reveal sex differences in morphology and spring migration phenology. Ornis Fennica 83:1–10.
- Saurola P, Valkama J, Velmala W. 2013. The Finnish bird ringing atlas. Vol. 1. Helsinki: Finnish Museum of Natural History and Ministry of Environment.
- Stiefel A, Priklonski SG, Postelnych AV. 1985. Common Sandpiper – Actitis hypoleucos (L.). In: Viksne JA, Michelson HA, editors. Migration of birds of Eastern Europe and Northern Asia. Moscow: Nauka. pp. 120–140. (In Russian).
- Teubert W, Kneis P. 1984. Geschlechtsspezifische Flügellängen adulter Fluβuferläufer, Actitis hypoleucos, nach Messungen aus dem Elbtal bei Riesa. Actitis 23:34–42.
- Wallander J, Andersson M. 2003. Reproductive tactics of the ringed plover Charadrius hiaticula. Journal of Avian Biology 34:259–266. DOI:10.1034/j.1600-048X.2003.03109.x.
- Winkler H, Herzig-Straschil B. 1981. Die Phänologie der Limikolen im Seewinkel (Burgenland) in den Jahren 1963 bis 1972. Egretta 24:47–69.
- Yalden DW, Holland PK. 1992. Relative contributions of Common Sandpiper Actitis hypoleucos parents to guarding their chicks. Ringing & Migration 13:95–97. DOI:10.1080/03078698.1992.9674025.
- Ydenberg RC, Niehaus AC, Lank DB. 2005. Interannual differences in the relative timing of southward migration of male and female western sandpipers (Calidris mauri). Naturwissenschaften 92:332–335. DOI:10.1007/s00114-005-0637-x.