Abstract
The aim of this paper is to document plant diversity in the metropolitan area of Rome (Italy) by providing a comprehensive inventory of the present-day vascular flora and an overview of its composition and species diversity. We compiled the floristic catalogue by including all vascular plant entities that occur spontaneously within the administrative boundaries of the Rome municipality. The data, which were gathered from extensive field surveys, from a broad review of the literature and from herbaria records, were updated and integrated in a comprehensive account. The inventory of the flora of metropolitan Rome lists 1649 entities, from 139 families and 677 genera. The flora contains 228 taxa that are non-native to the Italian flora, 81 of which are established in the study area. The overview of flora reveals a remarkable species diversity and outlines the main characteristics of the flora of Rome, which may be summarized as (1) a large number of taxa of high conservation value which occur in remnants of near-natural vegetation, (2) the loss or decline of some species, particularly of native freshwater plants, (3) a remarkably high native species richness within the urban area, which includes many native woody species and (4) a rich ruderal flora, prevalently composed of native species that are well adapted to human disturbance, along with a variety of taxa of non-native or uncertain origin. The large set of data and the overview presented in this paper represent a fundamental framework for future research and for the conservation of plant diversity in metropolitan Rome.
Maintaining biodiversity and wildlife in urban areas, where over half of the world's human population lives, is a major conservation issue that will become increasingly important in the future (Dearborn & Kark Citation2009; Jarošík et al. Citation2011; Kowarik Citation2011). In order to maintain such diversity, it is essential to provide environmental managers with up-to-date scientific information on species threats and conservation priorities, to support planning decisions and to develop effective strategies (Palmer et al. Citation2008). Hence, a major contribution to the conservation of urban biodiversity is the establishment of a system of information on species, and the first fundamental step in this direction is the compilation of complete inventories of currently present taxa (Shaminée et al. Citation2011; Sharrock Citation2011).
Comprehensive floristic surveys are of great value for both biodiversity management and research (Cadotte et al. Citation2006; Hahs et al. Citation2009). Up-to-date catalogues of vascular plants provide data for further studies, such as analyses aimed at exploring general patterns in urban ecology (e.g. La Sorte et al. Citation2008; Ricotta et al. Citation2008a, Citation2008b). Moreover, since cities are major centres of the introduction and spread of non-native species (Pyšek Citation1998; Kowarik Citation2011), these data-sets serve as rich sources of knowledge for research on plant invasion and biotic homogenization (Kühn & Klotz Citation2006; McKinney Citation2006; Ricotta et al. Citation2009, Citation2012; Lososová et al. Citation2012a, Citation2012b).
Despite the long history of urban ecology in Europe (Sukopp Citation2002), the flora of Mediterranean and southern European cities remains, with the exception of a few works (e.g. Chronopoulos & Christodoulakis Citation1996, Citation2006; Dana Citation2002; Krigas & Kokkini Citation2004), largely unstudied, thereby determining geographical biases in the knowledge of species patterns in this region. In Italy, information on urban plants is supported by a long-standing tradition of botanical research, which has focused on particular habitats and vegetation types. A few comprehensive catalogues of vascular plants have been compiled for temperate northern Italian cities (e.g. Siniscalco & Montacchini Citation1994; Banfi & Galasso Citation1998; Martini Citation2006). However, despite the progress made in recent years, complete inventories of urban floras in Mediterranean Italy are still scarce (e.g. Celesti-Grapow Citation1995; De Natale & La Valva Citation2000) and further research needs to be undertaken to gain a better understanding of urban plant diversity in this region.
Rome, one of the largest and most populated southern European cities, located in the centre of the Mediterranean, is an ideal case study for urban flora because it has an exceptionally long history of human impact, a marked environmental heterogeneity (Blasi et al. Citation2005, Citation2008a) and a long tradition of botanical research. Botanists have, ever since ancient times, collected a large amount of data on the vascular flora of Rome; this information is, however, dispersed in local and grey literature and herbaria records, and deals almost exclusively with the urban area. The data available for the suburban area of Rome are instead distributed rather unevenly, with the few works that have been published being dedicated to the flora of individual sites, such as archaeological areas or nature reserves (e.g. Anzalone et al. Citation1990; Lattanzi & Tilia Citation2004a; Salerno et al. Citation2007). It is instead essential to document plant cover in the outskirts, where biodiversity is under increasing threat owing to the rapid sprawl of artificial surfaces (Frondoni et al. Citation2011). Indeed, it is the peripheral areas of the city that will expand most in the future and that will affect biodiversity by transforming critical sites of high-conservation value, and by fragmenting the remaining patches of natural habitats. A comprehensive knowledge of the flora of both the city and its environs has important implications for management, as effective conservation of natural resources requires an integrated approach that encompasses the metropolitan area as a whole (Blasi et al. Citation2008b, Citation2008c; Werner Citation2011).
Given the dual need to collect the botanical information available for the urban area in a comprehensive, up-to-date account and to extend the research to the suburban territory, a project was launched to collate a database of the present-day vascular flora of the metropolitan area of Rome, based on an extensive field survey and a broad review of the literature (Capotorti et al. Citation2013). The aims of this study, which was conducted within this project, are to present an inventory of the current flora found within the metropolitan area and to provide an overview of its composition and species diversity.
Study area
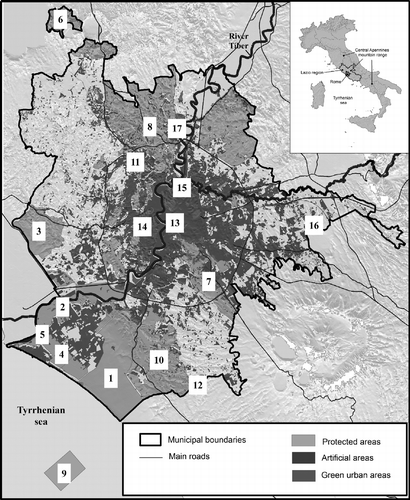
The study area (Figure ), which is located in the Lazio administrative region, corresponds to the municipality of Rome, extends over an area of 1287 km2 and has a population of 2,750,000 inhabitants (www.dati.istat.it). The area (hereafter referred to, indifferently, as either municipal or metropolitan Rome) has a remarkable ecosystem heterogeneity because it sits in the centre of the Mediterranean Basin, and has thus been moulded by both Western and Eastern biogeographic influences, it lies between the Tyrrhenian sea and the Apennine mountains and it has been subjected to a strong and long-lasting human impact (Blasi & Frondoni Citation2011; Frondoni et al. Citation2011; Capotorti et al. Citation2012).
The climate is broadly sub-Mediterranean, with a moderate summer drought, and includes five phytoclimatic types, varying from the Thermo-Mediterranean dry climate along the coast to the Meso-Mediterranean humid/subhumid inland climate (Blasi & Michetti Citation2001; Blasi et al. Citation2008a). The litho-morphology is determined by an extensive complex of volcanic hills and plateaux with lakes, secondary valleys and gorges, by the alluvial deposits of the river Tiber, and by a coastal strip that is approximately 20 km long and consists of sandy beaches and dunes, a drained clayey coastal plain and gravel terraces (Funiciello & Giordano Citation2008).
This varied biophysical setting determines the occurrence of several types of potential natural vegetation. The dominant types are sub-Mediterranean deciduous oak woods with Quercus cerris, Q. virgiliana, Q. frainetto,Q. robur, Q. dalechampii and Q. pubescens. The other types are potential evergreen oak woods with Quercus ilex, which are limited to the coastal areas, steep slopes and summits of the gorges, Q. suber woods in the inner dunes, psammophilous littoral vegetation types, and hygrophilous communities of the river network and subcoastal depressions (Acosta et al. Citation2003; Blasi & Capotorti Citation2005).
The current natural and semi-natural vegetation cover, the latter being induced by the anthropic use of land, includes evergreen and deciduous woods, Mediterranean maquis and garrigues, deciduous scrubs, riparian woods, reed beds and rush beds, hygrophilous vegetation of inland waters, vegetation of coastal dunes, halophilous vegetation of salt marshes, short grasslands of temporary ponds, pastures, therophytic grasslands, meadows, fallows, weed vegetation of arable land, herbaceous vegetation of walls, and ruderal and trampled habitats (Fanelli Citation2002; Blasi et al. Citation2005).
The conservation status of the individual vegetation patches depends on the degree of anthropization of the surrounding landscape, which can be subdivided into three thematic areas: urban, agricultural and mixed agricultural-natural (Blasi et al. Citation2008a). The suburban areas make up the so-called Campagna Romana, which prevalently consists of arable land and pastures, locally interspersed with natural vegetation patches (Celesti-Grapow & Fanelli Citation1993). Some of these green areas penetrate the city and are, along with the coastal areas, protected by an extensive network of nature reserves and parks. The network consists of the nature reserves of Castelporziano and Litorale Romano (including the site of Community Importance Macchia Grande di Ponte Galeria, the Castel Fusano park and the archaeological site of Ostia Antica), the regional parks of the Bracciano-Martignano lakes, the Ancient Appian Way and Vejo, the nature marine protected area Secche di Tor Paterno and a system of nine regional nature reserves, including Decima Malafede and Insugherata (Figure ).
Methods
The inventory was compiled by including all vascular plant entities (i.e. sections, groups, species, hybrids, subspecies and varieties, hereafter referred to, indifferently, as either “taxa” or “species”) that occur spontaneously within the administrative boundaries of the Rome municipality. Data were gathered from the botanical literature and field surveys covering every type of habitat, i.e. both natural/semi-natural and human-made. In order to obtain an overall picture of the contemporary flora, we only took into account botanical records or publications from the last 30 years. This time span was chosen because the main source of information for this study was a comprehensive floristic database of the urban area of Rome resulting from an extensive field survey carried out between 1985 and 1994 and from floristic accounts published since 1984 (Celesti-Grapow Citation1995). For the purposes of this work, the species catalogue was extracted from this data-set, which was thoroughly updated in 2005 (Celesti-Grapow et al. Citation2006) and subsequently revised by including recent papers on new flora records (e.g. Iamonico & Lorenzetti Citation2008; Ceschin et al. Citation2010) and by adding new findings from our personal field research. The flora of the suburban area was recorded during an extensive survey campaign carried out within a project designed to produce the ecological maps of the New General Master Plan of the municipality (Blasi et al. Citation2004; Lattanzi et al. Citation2005) and through specific field surveys designed to cover the whole study area. Information was also obtained by reviewing all relevant recent floristic literature, including the flora of the Castelporziano reserve (Anzalone et al. Citation1990), as well as other recent botanical works (e.g. Bianco et al. Citation2002; Bartolucci et al. Citation2004; Salerno et al. Citation2007; Ceschin et al. Citation2012; Azzella et al. Citation2013; Troía & Azzella Citation2013) and single floristic records, which are listed in Appendix 1. For the determination of the species, we referred mainly to the Flora of Italy (Pignatti Citation1982) and to the Flora Europaea (Tutin et al. Citation1964–1980, Citation1993), as well as to more recent papers (e.g. Giuliani & Maleci Bini Citation2012; Scoppola & Lattanzi Citation2012; Zidorn Citation2012).
After the main data collation phase, the species list was further revised by checking information in the vast historical literature on the flora of Rome. Although our list does not include records of plants published prior to 1984, the long-term floristic research in the area provided a solid background for this phase of our study; earlier floristic works were used as a general source of information, especially those from the second half of the 20th century carried out by some of the leading Roman botanists in that period, first and foremost Anzalone (e.g. 1951, 1986). Crucial information was also gathered from the wealth of botanical records found in herbaria collections, particularly in the Herbarium of Rome (RO). Herbarium specimens and labels provide a large amount of information that is very useful for the compilation of floristic inventories (Fuentes et al. Citation2013), especially as a means of documenting floristic changes through time in response to urbanization (Dolan et al. Citation2011).
While compiling the list, we adopted a conservative approach and thus excluded any doubtful records from the inventory, such as entries for which no evidence exists because the respective herbaria specimen was not available, and species that were recorded in the past but are now considered to no longer be present. We excluded taxa that grow spontaneously exclusively as garden escapees in sites that are strongly influenced by human cultivation practices (e.g. watering and fertilizers), such as plant nurseries and flowerbeds, those that only occur close to the parental plant, and those that do not grow beyond the seedling stage.
As for the introduced species, we used the system standardized on a nationwide scale by the working group on the non-native flora of Italy (Celesti-Grapow et al. Citation2009, Citation2010), which follows the international approach and terminology (Pyšek et al. Citation2004) used to develop the database of the non-native flora of Europe (Lambdon et al. Citation2008). Accordingly, each species on the list was designated as native or non-native to the Italian flora, with the latter being further distinguished, on the basis of their residence time, as either archaeophytes or neophytes (non-native species introduced to Italy, respectively, before and after 1492). Besides, those taxa whose status as native or non-native to the Italian flora is still unclear owing to the lack of palaeobotanical and historic evidence were classified as doubtful aliens (see Celesti-Grapow et al. Citation2009 for details on the method).
Non-native species were also classified on the basis of their establishment status within the study area (i.e. on a local scale), as either casual species (those that do not form self-replacing populations and rely on repeated introductions for their persistence) or established species (naturalized, i.e. those that have become established and thus sustain self-replacing populations, regardless of the contribution of new propagules). Although the approaches to the treatment of casual species by different authors differ markedly, it is widely agreed that they should, even if treated in less detail, not be omitted from floristic inventories since they provide valuable information for plant invasion studies, e.g. by shedding light on the process leading to naturalization (Richardson & Pyšek Citation2012). In this catalogue, we therefore normally included casuals, while adopting the afore-mentioned cautious approach to cultivation escapees.
We standardized the taxonomy and nomenclature of the taxa across all data sources according to the Flora of the Lazio Region (Anzalone et al. Citation2010). For species not listed in that paper, we followed specific papers (e.g. Iamonico Citation2009, Citation2012; Scoppola et al. Citation2011; Fuentes-Bazan et al. Citation2012; Selvi & Greuter Citation2012) for the native species, and the inventory of the non-native flora (Celesti Grapow et al. Citation2009) for the introduced species.
Species diversity and composition of the vascular flora of Rome
The present-day flora of metropolitan Rome is listed alphabetically in Appendix 2. In total, it contains 1649 entities from 139 families and 677 genera. The families represented by the highest number of species are Poaceae (182 entities), Asteraceae (175) and Fabaceae (169), followed by Brassicaceae (69), Caryophyllaceae (65), Lamiaceae (63), Apiaceae (58) and Rosaceae (50). The genera represented most are Trifolium (35 entities), Carex (25), Vicia (24), Silene (19), Ranunculus (18), Juncus (17) and Allium (15). The flora contains 228 species non-native to the Italian flora, 42 of which are considered archaeophytes and the remaining 186 neophytes. The flora also contains 25 doubtful non-native species. With regard to their current establishment status in the study area, the 228 non-native taxa were classified as 147 casual and 81 established species. These data integrate and complete the preliminary information presented at the species level by Capotorti et al. (Citation2013).
Dynamic features of the flora of Rome
The rapidly changing features of urban flora are such that these figures should be considered an approximate estimate, particularly in view of the dynamic nature of large, bustling cities such as Rome: new exotic plants are introduced every year, while other species disappear as a result of human activities, such as new buildings or changes in land use.
Indeed, several taxa listed in previous accounts were not included in this inventory. Some species were no longer present, such as the native Euphorbia paralias in the Castelporziano reserve, Malcomia maritima on the Colosseum, Matthiola fruticulosa on the walls of the Palatine archaeological area and Trifolium hirtum in the Castel Fusano park. Not surprisingly, besides native species, taxa not recorded recently also include many non-native casual plants. While this may reflect the relatively conservative approach we adopted in the treatment of casual species when compiling this list, it is ascribable above all to the fact that casual species are, by definition, ephemeral and typically reflect transitory human preferences in ornamental or horticultural contexts (Kowarik Citation2005; Dehnen-Schmutz et al. Citation2007; Kowarik et al. Citation2013).
Other species have not been found recently, though current knowledge is insufficient to exclude their presence. One such case is Cephalanthera rubra, an orchid that has “probably disappeared from its only coastal site in the Lazio region” in the Castel Fusano park, according to Bianco et al. (Citation2002). By contrast, some species that were regarded as extinct on the local, regional or even national scale have recently been rediscovered within the study area. The most noteworthy example is Trifolium latinum, which was considered extinct in Italy for a century (Conti et al. Citation1997) but was recently rediscovered in the Decima Malafede reserve by Fanelli et al. (Citation2012). Some species had not been found for decades because they were sheltering in sites that were inaccessible for a long time. For example, Asphodelus fistulosus, which had not been recorded in the city since the surveys on the wall flora conducted in the 1940s by Anzalone (Citation1951), was rediscovered when the scaffolding erected for the restoration of the Colosseum allowed botanists to access residual populations on top of the monument (Celesti-Grapow et al. Citation2001a).
It is precisely because of the dynamic character of the flora of Rome, as well as of the long history of botanical research in the area, that a series of studies have been conducted to explore changes over time in specific urban sites. For instance, Cornelini and Petrella (Citation1994) documented the turnover of species in Ostiense railway station by comparing early investigations by Cacciato (Citation1952) with the results of their more recent surveys. Other comparisons have spanned longer periods of time: for instance, the flora of the river Tiber, which was documented by Beguinot (Citation1899) and later by Anzalone (Citation1978, Citation1986), was again studied more recently by Ceschin et al. (Citation2010). However, the site that has been studied most by botanists over time is the Colosseum: Caneva et al. (Citation2003) analysed changes in the flora on this monument by comparing various inventories compiled over the past four centuries, ever since the first list published in the 1643 by the physician and botanist Panaroli (Citation1643).
Although it is beyond the scope of this paper to discuss all these changes in detail, evidence from our field survey and vast review of the literature indicates that the loss of species has not been uniform across taxa, with native freshwater plants being the group that has declined most in the last 30 years. One noteworthy example is Oenanthe lachenalii, which has disappeared since the temporary ponds in which it grew along the Ancient Appian Way dried up. Another remarkable case is Hydrocharis morsus-ranae, an aquatic plant that was reported to “grow along the banks of the river Tiber” by Anzalone (Citation1986) but was not found in the recent survey conducted by Ceschin et al. (Citation2010). Its reappearance cannot, however, be excluded in the future because information in the literature on the location in which it had been detected was too generic to be verified by field surveys throughout the municipality. The decline in the aquatic and bankside species of the flora of Rome, which had already been observed by Anzalone (Citation1978), is not an unexpected finding since a general decline in wetland and freshwater plants is a widely acknowledged trend in cities across the world (Preston et al. Citation2003; Kowarik Citation2011) and since aquatic and riparian habitats are among the most altered by human impact in Italy (Poldini et al. Citation2011; Croce et al. Citation2012).
Not all the exclusions of species from the catalogue are, however, due to verified or presumed local extinctions; some changes are the result of increasing knowledge, which has led, among other things, to variations in the systematics and/or taxonomy of some species and critical taxa, as in the case of the genera Rosa (Lattanzi & Tilia Citation2002, Citation2004b; Lattanzi et al. Citation2003), Rubus (Abbate et al. Citation2001, Citation2002) and Amaranthus (Iamonico Citation2008).
Corrections of misidentified species have also led to the exclusion of native species, such as Bromus inermis,Equisetum hyemale and Polygonum bellardii, as well as of non-native taxa, some of which were amended during the compilation of the inventory of the alien flora of Italy (Celesti-Grapow et al. Citation2009).
Flora of natural and semi-natural vegetation in the suburban area
Despite the loss of some taxa, the catalogue reveals a remarkable plant species diversity. High species richness is a common feature of settlement areas and is primarily due to the marked heterogeneity of their landscape (Sukopp Citation2004; Wania et al. Citation2006; Kowarik Citation2011). Many cities in the world are located in regions with a highly diverse pristine natural landscape (Kühn et al. Citation2004); besides, the wide variety of habitats found in the urban sector further enhances the range of different conditions available to plants (Sukopp Citation2004; Kowarik Citation2011).
Also in metropolitan Rome, the rich floristic diversity may be attributed to the considerable heterogeneity (especially litho-morphological) of the physical environment. The geographical location of Rome in the Mediterranean Basin, a globally recognized biodiversity hotspot (Myers et al. Citation2000; Bacchetta et al. Citation2012), contributes to the enrichment of diversity, thanks to the large pool of native species (Anzalone et al. Citation2010). Indeed, cities located in biodiversity hotspots are acknowledged to play a prominent role in the conservation of these critically endangered ecosystems (Secretariat of the CBD Citation2012).
The catalogue in fact contains a high number of taxa of near-natural vegetation which have been recorded above all on the outskirts of the municipal area, where several near-natural environments retain significant components of native biodiversity, including biogeographically valuable, rare and threatened species. Many of these important taxa occur in a few sites located in the coastal and subcoastal sector; some key examples are listed in Table . The most important site by far is the Castelporziano reserve (Figure ), which preserves mesophilous woods, wetlands and temporary ponds that once covered extensive areas along the mid-Tyrrhenian coast, as well as remnants of dunal complexes, Mediterranean maquis and forests dominated by oaks (Quercus robur, Q. cerris, Q. frainetto, Q. virgiliana, Q. × pseudosuber, Q. suber and Q. ilex). Such areas have now become very fragmented owing to urban and agricultural pressure and land reclamations dating from the early 20th century. Other notable sites are the archaeological site of the ancient city of Ostia, the Site of Community Importance of Macchia Grande di Ponte Galeria and the marine protected area of Secche di Tor Paterno, where a seabed grassland of Posidonia oceanica, an endemic Mediterranean vascular plant whose presence along the coasts is declining throughout the Mediterranean, can still be found on top of a submerged rock formation (Ardizzone et al. Citation2006).
Table I Key examples of valuable plant species recorded in the coastal and subcoastal sector of the study area.
Peculiar environments within the inland sectors include the sulphur springs of Pomezia (in the Decima Malafede reserve), which contain the rare Agrostis monteluccii, and the volcanic gorges of the eastern sector close to the pre-Apennine mountains. Owing to the mesoclimatic conditions, these gorges foster extrazonal mesophilous communities characterized by typical beechwood flora and many species of ferns, such as the rare Pteris cretica, which is considered a tropical relict. Moreover, their geographical location determines the occurrence of woodlands and shrublands with a flora of high-biogeographic value that contains numerous Eastern elements, such as Styrax officinalis, an extremely rare shrub on the western edge of its distribution area.
On the shores of Lake Bracciano, Isoëtes sabatina, an endemic pteridophyte new to science, was recently found (Troía & Azzella Citation2013), and Eleocharis acicularis, which is common in the rice fields of northern Italy but is extremely rare in the Lazio region, was rediscovered after 50 years.
The outskirts also include several archaeological sites with a rich flora, such as the remains of the ancient city of Gabii (Salerno et al. Citation2007) and of the Villa di Livia close to river Tiber.
Further details on such species of high-conservation value and on their role in the plant diversity of metropolitan Rome are discussed by Capotorti et al. (Citation2013).
Flora of natural and semi-natural vegetation in the urban area
While the outskirts contribute substantially to the overall landscape diversity by retaining remnants of natural habitats, the stratification of different historical periods within the urban area further contributes to the variety of land cover, thereby enhancing the richness of the flora (Celesti-Grapow et al. Citation2006). Indeed, the history of a settlement is known to affect species richness and composition (McKinney Citation2001; Zerbe et al. Citation2003; Dehnen-Schmutz Citation2004). By offering a wide range of environmental conditions to plants in housing areas of different periods, from ancient remains to contemporary urban developments, long-standing settlement areas can harbour species with different habitat requirements and thus generally contain a higher degree of plant species richness than younger settlements (Secretariat of the CBD Citation2012).
The archaeological areas, historical parks, riverine habitats along the river Tiber and fragments of woodlands found within Rome's urban boundaries all provide suitable habitats for plants from natural and semi-natural vegetation. Although the residual woods of Rome are far from pristine, several studies have highlighted their important biodiversity value (Anzalone Citation1952, Citation1953; Montelucci Citation1953–1954; Blasi et al. Citation1995; Celesti-Grapow et al. Citation2006). Usually located on steep slopes unsuitable for residential development, these remnants of natural forests that have been enclosed within the urban sprawl harbour a number of rare and threatened species (Capotorti et al. Citation2013). One of the most valuable sites is the Insugherata nature reserve, which hosts residual mesophilous forests with Ilex aquifolium, a species that in Italy is usually found at higher altitudes in beech forests.
Parks and gardens are important refuges for urban biodiversity worldwide (Thompson et al. Citation2003; Goddard et al. Citation2009). In Rome, some of the largest public parks cover significant proportions of the urban area, as in the case of Villa Doria Pamphili and Villa Ada, which are the two largest (184 hectares and 180 hectares, respectively) and are among those sites with the highest plant species richness within the city (Ricotta et al. Citation2001). Besides, many of these parks still include areas that were uncultivated for centuries because they had been used as private hunting estates by royal and aristocratic families until they were bought by the municipality in the mid-20th century. Urban woods and parks play an important role in conserving what is one of the major characteristics of the flora of Rome, i.e. the occurrence of a remarkable woody component in the flora (Capotorti et al. Citation2013).
Other areas have been protected from high-intensity development because they are important sites of cultural heritage. This applies to the innumerable archaeological sites that are scattered throughout the urban mosaic and form a series of green patches connecting the centre to its hinterland; several studies have drawn attention to the role played by these areas in enhancing plant diversity in Rome (Celesti-Grapow & Blasi Citation1998; Celesti-Grapow et al. Citation2001b, Citation2006). Some of these sites cover relatively large portions of land in the inner city; for instance, the Colosseum – Palatine Hill– Roman Forum – Caracalla Baths complex covers an area of approximately 50 hectares in the very centre of the city. As they offer a wide variety of microhabitats for plant species, extensive archaeological areas are significant diversity hot spots in their own right (Ceschin & Caneva Citation2001; Celesti-Grapow et al. Citation2001a); at the same time, smaller sites are considered to improve connectivity by acting as corridors and by widening the size of other urban habitats (Ricotta et al. Citation2001). Whether it be on top of or among the ruins, archaeological remains host a wealth of flora rich in native species that originate in more southern surroundings and have higher temperature requirements (Celesti-Grapow et al. Citation2001b). This applies to many grasses from dry Mediterranean pastures that colonize the tops of walls and the dry sites among the ruins (e.g. Lamarckia aurea). Another common group of species consists of shrubs that originate in natural rocky habitats and thus grow on walls (e.g. Capparis spinosa), while several ferns and species with more humid requirements find shelter in moist, shady areas at the base of the ruins. Archaeological remains also offer shelter to taxa that have markedly declined in rural areas and that have become rare, or have even disappeared, in the surrounding region. Examples include an unusually large number of orchid species, which are particularly diverse and abundant among the ancient remains of Rome (Rossi Citation1989).
Ruderal flora
As they include rare species and habitat specialists that depend upon remnants of natural environments, it is the afore-mentioned fragments of natural vegetation located both within and outside the city boundaries that make the most valuable contribution to the plant diversity of metropolitan Rome. However, the most numerous group of recorded flora consists of species that grow entirely, or predominantly, in habitats markedly transformed by man. In Rome, the vast majority of this ruderal flora is made up of native species. They are associated with a very extensive spectrum of man-made environments that form as a result of urban development, such as roadside verges, abandoned allotments, disused railway yards and trampled areas. As these ruderal habitats contain a very high degree of species diversity, they are increasingly being acknowledged as a major source of floristic richness (Muratet et al. Citation2007) and as providers of important ecosystem functions in metropolitan areas (Secretariat of the CBD Citation2012).
Many of these widespread species are annuals and early successional cosmopolitan plants that possess traits that enable them to tolerate high levels of disturbance (McKinney Citation2002), such as trampling and mowing. These native weeds, which are in the European botanical literature traditionally referred to as “apophytes”, are considered to be either (1) species that have adapted to new selective pressures in artificial environments or (2) species from natural habitats that were pre-adapted to such environments and have therefore spread to anthropic sites (Baker Citation1974; Preston et al. Citation2004; Wittig Citation2004; Sukopp Citation2006). A remarkable case of range-expanding native species is Crepis bursifolia, an endemic plant (Pignatti Citation1982) that has, in recent decades, extended its originally restricted distribution by spreading into heavily disturbed habitats, thus becoming abundant in the most urbanized sites throughout Rome.
The diversity of plants in metropolitan Rome is also due to a set of species that are, despite not being considered as non-native entities of the Italian flora, recorded spontaneously in the study area as cultivation escapees. The origin of some of these species is unknown, while others have been introduced as ornamentals from within the Italian territory, or even from the environs of the study area. On the basis of the information available, we labelled these species in Appendix 2 as escaped from cultivation. Many of these taxa, such as the dwarf palm (Chamaerops humilis), have been planted in the area ever since ancient Roman times (Saccardo Citation1909). Indeed, the Italian peninsula, which stretches out into the centre of the Mediterranean sea, has been a centre of intense exchange and colonization of introduced species as a result of long-distance human trade and migrations ever since pre-historic times (Saccardo Citation1909).
The occurrence of non-native plants introduced by early settlers, of species whose origins are found in the flora or the near surroundings, of cosmopolitan weeds found in urban settings worldwide and whose origins cannot easily be traced (Baker Citation1974), as well as of other taxa whose status as native or introduced is still unclear, suggests that the variety of biogeographic origins of the flora in the study area cannot simply be described by means of the alien/native dichotomy, nor by further categorizing non-native species into archaeophytes and neophytes. Indeed, the relative role of native and non-native plants in this flora, and more generally in the Mediterranean Basin, is a central, though complex, issue that has not yet been fully explored (Blondel & Aronson Citation1995).
Non-native flora
We will not focus on introduced species in this work, as such an investigation requires dedicated studies, particularly in view of their complex and unclear origin. However, on the basis of evidence from our field survey and from the large number of studies dedicated to such species, we will briefly discuss the role of such species in the biodiversity of the study area.
On the whole, the occurrence of the 228 species that have either deliberately or accidentally been introduced in Italy from other parts of the world appears to be yet another reason for the diversity of the flora of Rome. The presence of a high number of non-native plant species in urban areas is a renowned pattern that is typical of cities worldwide (Pyšek Citation1998; Kühn & Klotz Citation2006; McKinney Citation2006; Pyšek et al. Citation2010; Kowarik Citation2011). The two most common explanations for this pattern include higher levels of disturbance (Lososová et al. Citation2006) and high opportunities of dispersal (Kowarik Citation2003).
It is remarkable that almost all the non-native flora in the study area is, despite the intense and long-lasting disturbance and opportunities of dispersal, still confined to ruderal sites (Celesti-Grapow et al. Citation2001b), sometimes even to a separate temporal niche (Celesti-Grapow et al. Citation2003). There are some socio-economic risks associated with a few specific invasive species, which include the threat posed to the conservation of ancient ruins by the spread of Ailanthus altissima (Celesti-Grapow & Blasi Citation2004). However, while the spread of some invasive plants may be considered a threat to the conservation of biodiversity in some habitats in Italy, such as those found on small islands (Pretto et al. Citation2010, Citation2012), Alpine valleys (Siniscalco et al. Citation2011) and freshwater environments (Brundu et al. Citation2013), there is no evidence to indicate that the non-native flora negatively affects biodiversity in the area of Rome.
In conclusion, this study presents the state of the art of research on the vascular flora of Rome and provides the first comprehensive inventory of the flora of a large metropolitan area in southern Europe and the Mediterranean region. The information in this paper has revealed a remarkable species diversity of the flora of Rome and has outlined its main characteristics, which may be summarized as (1) the persistence of residuals of natural vegetation, including numerous taxa of high-conservation value, (2) the loss or decline of some species, particularly of native freshwater plants, (3) the remarkably high native species richness within the urban area, which includes many native woody species and (4) a rich ruderal flora, mainly composed of native species well adapted to human disturbance, along with a variety of taxa of non-native or uncertain origin. By providing an extensive set of data and an overview of its species diversity, this study establishes a general framework for guiding future research and for conserving biodiversity in metropolitan Rome.
SUPPLEMENTAL
Download MS Word (36.5 KB)Acknowledgements
We thank all the botanists from the Environmental Biology Department of the Sapienza University of Rome who have been involved in this project over the years, and in particular Mattia Azzella for providing unpublished data, and Giovanna Abbate, Sandro Bonacquisti, Laura Facioni, Duilio Iamonico and Mauro Iberite for their help on the treatment of critical groups. We also wish to thank Simona Ceschin and Giovanni Salerno for the useful information they provided.
Supplemental data
Supplemental data for this article (Appendix 1) is available online.
REFERENCES
- AbbateG, BonacquistiS, ScassellatiE. 2001. Il genere Rubus L. sez. Rubus (Rosaceae) in Italia centrale: Stato attuale delle conoscenze. Inform Bot Ital33: 481–487.
- AbbateG, BonacquistiS, ScassellatiE. 2002. Morphological study of three taxa of genus Rubus L. (Rosaceae), sect. Rubus, in western-central Italy. Plant Biosyst136: 321–330.
- AcostaA, StanisciA, ErcoleS, BlasiC. 2003. Sandy coastal landscape of the Lazio region (central Italy). Phytocoenologia33: 715–726.
- AnzaloneB. 1951. Flora e vegetazione dei muri di Roma. Ann Bot23: 393–497.
- AnzaloneB. 1952. Residui di vegetazione spontanea in Roma. 1° – I Monti Parioli. Nuovo Giorn Bot Ital59: 368–377.
- AnzaloneB. 1953. Residui di vegetazione spontanea in Roma. 2° – Monte Mario e i Monti della Farnesina. Ann Bot24: 1–29.
- AnzaloneB. 1978. Osservazioni sulla flora e vegetazione riparia lungo il fiume Tevere entro Roma. Lav Soc Ital Biogeog n.s.4: 1–19.
- AnzaloneB. 1986. La Flora vascolare spontanea delle rive del Tevere e suoi affluenti entro Roma. Ann Bot Suppl Studi sul territorio44: 1–46.
- AnzaloneB, IberiteM, LattanziE. 2010. La Flora vascolare del Lazio. Inform Bot Ital42: 187–317.
- AnzaloneB, LattanziE, LuccheseF. 1990. La flora della Tenuta di Castelporziano (Roma). Quad Accad Naz Lincei264: 133–218.
- ArdizzoneG, BelluscioA, MaioranoL. 2006. Long-term change in the structure of a Posidonia oceanica landscape and its reference for a monitoring plan. PSZNI Mar Ecol27: 299–309.
- AzzellaMM, IberiteM, FascettiS, RosatiL. 2013. Loss detection of aquatic habitats in Italian volcanic lakes using historical data. Plant Biosyst147: 521–524.
- BacchettaG, FarrisE, PontecorvoC. 2012. A new method to set conservation priorities in biodiversity hotspots. Plant Biosyst146: 638–648.
- BakerHG. 1974. The evolution of weeds. Annu Rev Ecol Syst5: 1–24.
- BanfiE, GalassoG. 1998. La flora spontanea della città di Milano alle soglie del terzo millennio e i suoi cambiamenti a partire dal 1700. Mem Soc Ital Sci Nat Mus Civ Stor Nat Milano27: 267–387.
- BartolucciF, De LorenzisA, CecereJG, editors. 2004. La flora vascolare. Quaderni dell'Oasi Castel di Guido. Vol. 1 LIPU.
- BeguinotA. 1899. La flora dei depositi alluvionali del Tevere entro Roma. Nota preventiva. Boll Soc Bot Ital : 222–229.
- BiancoPM, FanelliG, De LillisM. 2002. Flora and vegetation of Castel Fusano (Rome). Quad Bot Amb Appl13: 125–181.
- Blasi C, Capotorti G. 2005. Carta delle serie di vegetazione del territorio del Comune di Roma (1:50.000). Available: http://www.urbanistica.comune.roma.it. Accessed July 2013 20.
- BlasiC, CapotortiG, FrondoniR. 2005. Defining and mapping typological models at the landscape scale. Plant Biosyst139: 155–163.
- BlasiC, CapotortiG, MarcheseM, MartaM, BolognaMA, BombiP, et al. 2008a. Interdisciplinary research for the proposal of the urban biosphere reserve of Rome municipality. Plant Biosyst142: 305–312.
- BlasiC, CapotortiG, MartaM, MarcheseM. 2008b. An integrated approach to better define the concept and functions of Urban Biosphere Reserves. Plant Biosyst142: 324–330.
- BlasiC, DowgialloG, FollieriM, LuccheseF, MagriD, PignattiS, et al. 1995. La vegetazione naturale potenziale dell'area romana. Quad Accad Naz Lincei115: 423–457.
- BlasiC, FrondoniR. 2011. Modern perspectives for plant sociology: The case of eco-logical land classification and the ecoregions of Italy. Plant Biosyst145: 30–37.
- Blasi C, Lattanzi E, Tilia A, Celesti-Grapow L, Fortini P, Capotorti G. 2004. Carta delle emergenze floristico-vegetazionali del territorio del Comune di Roma (1:20,000). Available: http://www.urbanistica.comune.roma.it. Accessed Jul 2013 20.
- BlasiC, MichettiL. 2001. Carta del fitoclima dell'Area Romana. CD rom in Inform Bot Ital33(suppl. 1)
- BlasiC, ZavatteroL, MarignaniM, SmiragliaD, CopizR, RosatiL, et al. 2008c. The concept of land ecological network and its design using a land unit approach. Plant Biosyst142: 540–549.
- BlondelJ, AronsonJ. 1995. Biodiversity and ecosystem function in the Mediterranean Basin: Human and non-human determinants. In: DavisGW, RichardsonDM, editors. Mediterranean-type ecosystems: The function of biodiversity. Ecological studies 109.Berlin: Springer. pp. 43–119.
- BrunduG, AzzellaMM, BlasiC, CamardaI, IberiteM, Celesti-GrapowL. 2013. The silent invasion of Eichhornia crassipes (Mart.) Solms. in Italy. Plant Biosyst147. https://doi.org/http://dx.doi.org/10.1080/11263504.2013.861536.
- CacciatoA. 1952. La vegetazione antropocora dello scalo ferroviario ostiense di Roma. Nuovo Giorn Bot Ital59: 119–143.
- CadotteMW, MurrayBR, Lovett-DoustJ. 2006. Ecological patterns and biological invasions: Using regional species inventories in macroecology. Biol Invasions8: 809–821.
- CanevaG, PaciniA, Celesti-GrapowL, CeschinS. 2003. The Colosseum's use and state of abandonment analysed through its flora. Int Biodeterior Biodegrad51: 211–219.
- CapotortiG, Del VicoE, LattanziE, TiliaA, Celesti-GrapowL. 2013. Exploring biodiversity in a metropolitan area in the Mediterranean region: The urban and suburban flora of Rome (Italy). Plant Biosyst147: 174–185.
- CapotortiG, ZavatteroL, AnzellottiI, BurrascanoS, FrondoniR, MarchettiM, et al. 2012. Do National Parks play an active role in conserving the natural capital of Italy?Plant Biosyst146: 258–265.
- Celesti-GrapowL. 1995. Atlas of the flora of Rome [In Italian with English summary]. Rome: Comune di Roma. 222 p.
- Celesti-GrapowL, AlessandriniA, ArrigoniPV, AssiniS, BanfiE, BarniE, et al. 2010. Non-native flora of Italy: Species distribution and threats. Plant Biosyst144: 12–28.
- Celesti-GrapowL, AlessandriniA, ArrigoniPV, BanfiE, BernardoL, BovioM, et al. 2009. Inventory of the non-native flora of Italy. Plant Biosyst143: 386–430.
- Celesti-GrapowL, BlasiC. 1998. A comparison of the urban flora of different phytoclimatic regions in Italy. Global Ecol Biogeogr Lett7: 367–378.
- Celesti-GrapowL, BlasiC. 2004. The role of alien and native weeds in the deterioration of archaeological remains in Italy. Weed Technol18: 1508–1513.
- Celesti-GrapowL, CanevaG, PaciniA. 2001a. The Flora of Colosseum (Rome) [In Italian with English summary]. Webbia56: 321–342.
- Celesti-GrapowL, Di MarzioP, BlasiC. 2001b. The importance of alien and native species in the urban flora of Rome (Italy). In: BrunduG, BrockJ, CamardaI, ChildL, WadeM, editors. Plant invasions: Species ecology and ecosystem management. Leiden: Blackhuys. pp. 209–220.
- Celesti-GrapowL, Di MarzioP, BlasiC. 2003. Temporal niche separation of the alien flora of Rome (Italy). In: ChildL, BrockJH, BrunduG, PrachK, PyšekP, WadePM, editors., et al. Plant invasions. Leiden: Blackhuys. pp. 101–111.
- Celesti-GrapowL, FanelliG. 1993. The vanishing landscape of the Campagna Romana. Landscape Urban Plan24: 69–76.
- Celesti-GrapowL, PyšekP, JarošíkV, BlasiC. 2006. Determinants of native and alien species richness in the urban flora of Rome. Divers Distrib12: 490–501.
- CeschinS, CancellieriL, SalernoG, ToscanoS, CanevaG. 2012. Contributo alla conoscenza floristica della Campagna Romana: Il Vallone di Ponte Lupo (Roma). Inform Bot Ital44: 81–95.
- CeschinS, CanevaG. 2001. Contributo alla conoscenza della Flora dell'area archeologica del Palatino (Roma). Inform Bot Ital33: 391–406.
- CeschinS, SalernoG, BisceglieS, KumbaricA. 2010. Temporal floristic variations as indicator of environmental changes in the Tiber River in Rome. Aqua Ecol44: 93–100.
- ChronopoulosG, ChristodoulakisD. 1996. Contribution to the urban ecology of Greece: The flora of the city of Patras and the surrounding area. Bot Helv106: 159–176.
- ChronopoulosG, ChristodoulakisD. 2006. Contribution to the urban ecology of Greece: The flora of the city of Alexandroupolis (NE Greece) and its vicinity. Fresenius Environ Bull15: 1455–1466.
- ContiF, AbbateG, AlessandriniA, BlasiC, editors. 2005. An annotated Checklist of the Italian Vascular Flora. Rome: Palombi. p. 420.
- ContiF, ManziA, PedrottiF. 1997. Liste Rosse Regionali delle Piante d'Italia. Camerino: WWF and Societá Botanica Italiana. p.139.
- CorneliniP, PetrellaP. 1994. La flora della stazione di Roma Ostiense: Variazioni e confronti con il censimento di Cacciato (1952). Ann Bot Suppl Studi sul territorio52: 457–478.
- CroceA, NazzaroR, La ValvaV. 2012. Evidence of dramatic biodiversity loss in a wet biotope calls for urgent conservation strategies. Plant Biosyst146: 827–834.
- DanaED. 2002. Flora y vegetación urbanícolas de la ciudad de Almería: Características taxómicas, biogeográficas, fitocenológicas y ecológicas de las especies vegetales no ornamentales de la ciudad. Almería: Instituto de Estudios Almerienses.
- De NataleA, La ValvaV. 2000. The Urban Flora of Naples [In Italian with English summary]. Webbia54: 271–375.
- DearbornDC, KarkS. 2009. Motivations for conserving urban biodiversity. Conserv Biol24: 432–440.
- Dehnen-SchmutzK. 2004. Alien species reflecting history: Medieval castles in Germany. Divers Distrib10: 147–151.
- Dehnen-SchmutzK, TouzaJ, PerringsC, WilliamsonM. 2007. The horticultural trade and ornamental plant invasions in Britain. Conserv Biol.21: 224–231.
- DolanRW, MooreME, StephensJD. 2011. Documenting effects of urbanization on flora using herbarium records. J Ecol99: 1055–1062.
- FanelliG. 2002. Analisi fitosociologica dell'area metropolitana di Roma. Braun-Blanquetia27: 1–269.
- FanelliG, De SanctisM, Serafini SauliA. 2012. The rediscovery of Trifolium latinum (Fabaceae) in Rome one century after its apparent disappearance from Italy. [In Italian with English summary]. Inform Bot Ital44: 337–339.
- FrondoniR, MolloB, CapotortiG. 2011. A landscape analysis of land cover change in the Municipality of Rome (Italy): Spatio-temporal characteristics and ecological implications of land cover transitions from 1954 to 2001. Landscape Urban Plan100: 117–128.
- FuentesN, PauchardA, SánchezP, EsquivelJ, MarticorenaA. 2013. A new comprehensive database of alien plant species in Chile based on herbarium records. Biol Invasions15: 847–858.
- Fuentes-BazanS, UotilaP, BorschT. 2012. A novel phylogeny-based generic classification for Chenopodium sensu lato, and a tribal rearrangement of Chenopodioideae (Chenopodiaceae). Willdenowia42: 5–24.
- FunicielloR, GiordanoG, editors. 2008. Note illustrative della carta geologica d'Italia alla scala 1:50.000, foglio 374.Roma.
- GiulianiC, Maleci BiniL. 2012. Glandular trichomes as further differential characters between Stachys subgenus Betonica (L.) Bhattacharjee and Stachys subgenus Stachys. Plant Biosyst146: 1–8.
- GoddardMA, DougillAJ, BentonTG. 2009. Scaling up from gardens: Biodiversity conservation in urban environments. Trends Ecol Evol25: 90–98.
- HahsAK, McDonnellMJ, McCarthyMA, VeskPA, CorlettRT, NortonBA, et al. 2009. A global synthesis of plant extinction rates in urban areas. Ecol Lett12: 1165–1173.
- IamonicoD. 2008. Sulla presenza di alcune entità del genere Amaranthus L. (Amaranthaceae) nel Lazio. Inform Bot Ital40: 23–26.
- IamonicoD. 2009. First record of Amaranthuspowellii subsp. powellii (Amaranthaceae) in Lazio region (central Italy) with taxonomical, morphological, corologycal and ecological notes. Acta Bot Malacit34: 221–226.
- IamonicoD. 2012. Amaranthuspowellii S. Watson subsp. cacciatoi comb. & stat. nov. (Amaranthaceae). Nord J Bot30: 12–16.
- IamonicoD, LorenzettiR. 2008. La Flora vascolare della Riserva Naturale di Monte Mario (Roma): Studio di base ed analisi comparativa con la flora di altre aree verdi di Roma. Riv Piemont Stor Nat29: 141–168.
- JarošíkV, KonvičkaM, PyšekP, KadlecT, BenešJ. 2011. Conservation in a city: Do the same principles apply to different taxa?Biol Conserv144: 490–499.
- KowarikI. 2003. Human agency in biological invasions: Secondary releases foster naturalisation and population expansion of alien plant species. Biol Invasions5: 293–312.
- KowarikI. 2005. Urban ornamentals escaped from cultivation. In: GresselJ, editor. Crop ferality and volunteerism. Boca Raton: CRC Press. pp. 97–121.
- KowarikI. 2011. Novel urban ecosystems, biodiversity, and conservation. Environ Pollut159: 1974–1983.
- KowarikI, von der LippeM, CierjacksA. 2013. Prevalence of alien versus native species of woody plants in Berlin differs between habitats and at different scales. Preslia85: 113–132.
- KrigasN, KokkiniS. 2004. A survey of the alien vascular flora of the urban and suburban area of Thessaloniki, N Greece. Willdenowia34: 81–99.
- KühnI, BrandlR, KlotzS. 2004. The flora of German cities is naturally species rich. Evol Ecol Res6: 749–764.
- KühnI, KlotzS. 2006. Urbanization and homogeneization – comparing the floras of urban and rural areas in Germany. Biol Conserv127: 292–300.
- La SorteF, McKinneyML, PyšekP, KlotzS, RapsonGL, Celesti-GrapowL, et al. 2008. Distance decay of similarity among European urban floras: The impact of anthropogenic activities on β diversity. Glob Ecol Biogeogr17: 363–371.
- LambdonPW, PyšekP, BasnouC, DelipetrouP, EsslF, HejdaM, et al. 2008. Alien flora of Europe: Species diversity, temporal trends, geographical pattern and research needs. Preslia80: 101–149.
- LattanziE, ScoppolaA, TiliaA. 2003. Apport à la connaissance des espèces du genre Rosa L. dans le Latium (Italie centrale). Bocconea16: 723–730.
- LattanziE, TiliaA. 2002. Il genere Rosa L. nel Lazio: Studio preliminare. Inform Bot Ital33: 524–528.
- LattanziE, TiliaA. 2004a. Area archeologica di Ostia Antica: Analisi floristica preliminare e relativa valutazione della pericolosità. 40° Congresso della Società Italiana di Fitosociologia, February 19–21 2004, Roma. Roma: Dipartimento di Biologia, Università degli Studi “Roma Tre”, p 19.
- LattanziE, TiliaA. 2004b. Tre entità del genere Rosa L. nuove per il Lazio. Inform Bot Ital36: 165–167.
- LattanziE, TiliaA, BlasiC. 2005. Il contributo dell'indagine floristica nelle analisi territoriali. Inform Bot Ital37: 340–341.
- LososováZ, ChytrýM, KühnI, HájekO, HorákováV, PyšekP, et al. 2006. Patterns of plant traits in annual vegetation of man-made habitats in central Europe. Perspect Plant Ecol8: 69–81.
- LososováZ, ChytrýM, TichýL, DanihelkaJ, FajmonK, HájekO, et al. 2012a. Biotic homogenization of Central European urban floras depends on residence time of alien species and habitat types. Biol Conserv145: 179–184.
- LososováZ, ChytrýM, TichýL, DanihelkaJ, FajmonK, HájekO, et al. 2012b. Native and alien floras in urban habitats: A comparison across 32 cities of central Europe. Global Ecol Biogeogr21: 545–555.
- MartiniF. 2006. La flora vascolare spontanea della città di Trieste (Italia nordorientale). Webbia61: 57–94.
- McKinneyML. 2001. Effects of human population, area, and time on non-native plant and fish diversity in the United States. Biol Conserv100: 243–252.
- McKinneyML. 2002. Urbanization, biodiversity and conservation. Bioscience52: 883–890.
- McKinneyML. 2006. Urbanization as a major cause of biotic homogenization. Biol Conserv127: 247–260.
- MontelucciG. 1953–54. Investigazioni botaniche nel Lazio; V – Flora e vegetazione della valle dell'Inferno a Roma (Monte Mario). Ann Bot24: 1–167.
- MuratetA, MachonN, JiguetF, MoretJ, PorcherE. 2007. The role of urban structures in the distribution of wasteland flora in the greater Paris area, France. Ecosystems10: 661–671.
- MyersN, MittermeierRA, MittermeierCG, da FonsecaGAB, KentJ. 2000. Biodiversity hotspots for conservation priorities. Nature403: 853–858.
- PalmerGC, FitzsimonsJA, AntosMJ, WhiteJG. 2008. Determinants of native avian richness in suburban remnant vegetation: Implications for conservation planning. Biol Conserv141: 2329–2341.
- PanaroliD. 1643. Jatrologismi Sive Medicae Observationes Quibus Additus est in Fine Plantarum Amphitheatralium Catalogus. Rome: Typis Dominici Marciani. p. 15.
- PapiniA, SimeoneMC, BellarosaR, SpadaF, SchironeB. 2011. Quercus macranthera Fisch. & Mey. ex Hohen. and Quercus iberica M. Bieb.: Taxonomic definition and systematic relationships with European oaks inferred from nuclear internal transcribed spacer (ITS) data. Plant Biosyst145: 37–49.
- PignattiS. 1982. Flora d'Italia. Bologna: Edagricole.
- PoldiniL, VitaliM, GanisP. 2011. Riparian Salix alba: Scrubs of the Po lowland (N-Italy) from an European perspective. Plant Biosyst145: 132–147.
- PrestonCD, PearmanDA, HallAR. 2004. Archaeophytes in Britain. Bot J Linn Soc145: 257–294.
- PrestonCD, SheailJ, ArmitagePD, Davy-BowkerJ. 2003. The long-term impact of urbanisation on aquatic plants: Cambridge and the River Cam. Sci Total Environ314–316: 67–87.
- PrettoF, Celesti-GrapowL, CarliE, BlasiC. 2010. Influence of past land use and current human disturbance on non-native plant species on small Italian islands. Plant Ecol210: 225–239.
- PrettoF, Celesti-GrapowL, CarliE, BrunduG, BlasiC. 2012. Determinants of non-native plant species richness and composition across small Mediterranean islands. Biol Invasions14: 2559–2572.
- PyšekP. 1998. Alien and native species in Central European urban floras: A quantitative comparison. J Biogeogr25: 155–163.
- PyšekP, BacherS, ChytrýM, JarošíkV, WildJ, Celesti-GrapowL, et al. 2010. Contrasting patterns in the invasions of European terrestrial and freshwater habitats by alien plants, insects and vertebrates. Global Ecol Biogeogr19: 317–331.
- PyšekP, RichardsonDM, RejmánekM, WebsterGL, WilliamsonM, KirschnerJ. 2004. Alien plants in checklist and floras: Towards better communication between taxonomists and ecologists. Taxon53: 131–143.
- RichardsonDM, PyšekP. 2012. Naturalization of introduced plants: Ecological drivers of biogeographical patterns. New Phytol196: 383–396.
- RicottaC, Celesti-GrapowL, AvenaGC, BlasiC. 2001. Topological analysis of the spatial distribution of plant species richness across the city of Rome (Italy) with the echelon approach. Landscape Urban Plan57: 69–76.
- RicottaC, Di NepiM, GugliettaD, Celesti-GrapowL. 2008a. Exploring taxonomic filtering in urban environments. J Veg Sci19: 229–238.
- RicottaC, GodefroidS, Celesti-GrapowL. 2008b. Common species have lower taxonomic diversity. Evidence from the urban floras of Brussels and Rome. Divers Distrib14: 530–537.
- RicottaC, La SorteFA, PyšekP, RapsonGL, Celesti-GrapowL, ThompsonK. 2009. Phyloecology of urban alien floras. J Ecol97: 1243–1251.
- RicottaC, La SorteFA, PyšekP, RapsonGL, Celesti-GrapowL, ThompsonK. 2012. Phylogenetic beta diversity of native and alien species in European urban floras. Global Ecol Biogeogr21: 751–759.
- RossiW. 1989. Native orchids in the main archeological sites of Rome. Braun-Blanquetia3: 269–270.
- SaccardoPA. 1909. Cronologia della flora Italiana. Padova: Tipografia del Seminario.
- SalernoG, CeschinS, CutiniM. 2007. Contributo alla conoscenza floristica della Campagna Romana: L'area archeologica di Gabi-Castiglione (Roma). Inform Bot Ital39: 167–180.
- ScoppolaA, LattanziE. 2012. Viola section Melanium (Violaceae) in Italy. New data on morphology of Viola tricolor-Group. Webbia67: 47–64.
- ScoppolaA, MagriniS, CelestiniM. 2011. Lupinus sect. Albus (Fabaceae): Taxonomic criticism and conservation value. The case of wild populations in central Italy. Plant Biosyst145: 514–526.
- Secretariat of the CBD. 2012. Cities and biodiversity outlook. A global assessment of the links between action and policy: Urbanization, biodiversity, and ecosystem services, Montreal, 64 pp.
- SelviF, GreuterW. 2012. Proposal to conserve the name Myosotis sicula against M. gussonei (Boraginaceae). Taxon61: 467–468.
- ShaminéeJHJ, JanssenJAM, HennekensSM, OzingaWA. 2011. Large vegetation databases and information systems: New instruments for ecological research, nature conservation, and policy making. Plant Biosyst145: 85–90.
- SharrockS. 2011. GSPC, Global strategy for plant conservation. A guide to the GSPC. All the targets, objectives and facts. Richmond: Botanic Gardens Conservation International.
- SiniscalcoC, BarniE, BacaroG. 2011. Non-native species distribution along the elevation gradient in the western Italian Alps. Plant Biosyst145: 150–158.
- SiniscalcoC, MontacchiniF. 1994. Prodromo della flora urbica torinese. Allionia32: 137–161.
- SukoppH. 2002. On the early history of urban ecology in Europe. Preslia74: 373–393.
- SukoppH. 2004. Human-caused impact on preserved vegetation. Landscape Urban Plan68: 347–355.
- SukoppH. 2006. Apophytes in the flora of Central Europe. Pol Bot Stud22: 473–485.
- ThompsonK, AustinKC, SmithRM, WarrenPH, AngoldPG, GastonK. 2003. Urban domestic gardens (I): Putting small-scale plant diversity in contex. J Veg Sci14: 71–78.
- TroíaA, AzzellaMM. 2013. Isoëtes sabatina (Isoëtaceae, Lycopodiophyta), a new aquatic species from Central Italy. Plant Biosyst. http://www.tandfonline.com/doi/full/10.1080/11263504.2013.782902.
- TutinTG, BurgesNA, ChaterAO, EdmondsonJR, HeywoodWH, MooreDM, et al. 1993. Flora Europaea. Vol. 1. 2nd ed., Cambridge: University Press.
- TutinTG, HeywoodVH, BurgesNA, ValentineDH, WaltersSM, WebbDA. 1964–1980. Flora Europaea. 5 vols. Cambridge: University Press.
- ViscosiV, FortiniP, SliceDE, LoyA, BlasiC. 2009. Geometric morphometric analyses of leaf variation in four oak species of the subgenus Quercus (Fagaceae). Plant Biosyst143: 575–587.
- WaniaA, KühnI, KlotzS. 2006. Plant richness patterns in agricultural and urban landscapes in Central Germany – spatial gradients of species richness. Landscape Urban Plan75: 97–110.
- WernerP. 2011. The ecology of urban areas and their functions for species diversity. Landscape Ecol Eng7: 231–240.
- WittigR. 2004. The origin and development of the urban flora of Central Europe. Urban Ecosyst7: 323–339.
- ZerbeS, MaurerU, SchmitzS, SukoppH. 2003. Biodiversity in Berlin and its potential for nature conservation. Landscape Urban Plan62: 139–148.
- ZidornC. 2012. Leontodon and Scorzoneroides (Asteraceae, Cichorieae) in Italy. Plant Biosyst146: 41–51.