Abstract
Iris adriatica Trinajstić ex Mitić (Iridaceae L.) is a strictly endemic taxon from Croatia. It is a rhizomatous dwarf plant from the I. pumila complex with a distribution area limited to the Croatian part of the Mediterranean area, mainly central Dalmatia. The genus Iris is known for its richness in isoflavonoids which also play a significant role in chemotaxonomy and biological activity. Hence, in the current study, different plant batches of I. adriatica collected in early spring of 2016 were analysed for their phytochemical profiles and qualitatively compared. UHPLC-PDA-ESI-MS analyses of methanolic rhizome extracts were performed. Altogether, 36 compounds, representing isoflavonoids (including 6,7-methylendioxy derivatives), benzophenones and xanthones were found as aglycones or in glycosidically bound form to be the main constituent groups of I. adriatica rhizomes. Qualitative results were identical between different batches of plant material from collection sites in central Dalmatia, they differed only in quantity. For some phenolic compounds of I. adriatica, chemotaxonomic relevance was detected.
Introduction
Iris adriatica Trinajstić ex Mitić is an accepted name describing the strictly endemic taxon from Croatia (Mitić Citation2002; The international plant names index Citation2012; The Plant List Citation2013). The taxonomic position of I. adriatica has been clarified relatively lately (Mitić Citation2002). This diploid (2n = 16), rhizomatous dwarf plant, together with I. pseudopumila Tineo, I attica Boiss. et Heldr. and I. pumila L., belong to the so-called I. pumila complex (Mitra Citation1956; Randolph and Mitra Citation1959; Trinajstić et al. Citation1980; Mitić Citation2002) of the series Pumilae Lawr., section Iris, subgenus Iris (so called “bearded” or Pogon irises) (Lawrence Citation1953; Mathew Citation1981). The short stems (1–5 cm) are overtopped by narrow, gentle and often falcate leaves (3–10 cm), which are overtopped by relatively large and decorative solitary flowers. Greeny, slightly keeled, spathes, surrounded by scarious margins are slightly longer than or as long as the hypanthial tube which is 4 to 7 cm long. Tepals are usually of different shades of yellow and less frequent of violet or purple colour. The outer tepals have multicellular coloured hairs (so called “beard”). Plants bloom very early in the spring, in March and April. Its distribution area is limited to the Croatian part of the Mediterranean area – central Dalmatia, the surroundings of the cities of Drniš, Unešić, Zadar, Šibenik and Split, as well as the nearby islands (e.g. Vir, Čiovo, Brač, Kornati etc.). Populations of I. adriatica are generally small, and in the past few years, some of them have disappeared or decreased. For this reason, I. adriatica belongs to the NT (near threatened) IUCN category in the Croatian Red Book of Vascular Plants (Nikolić Citation2017). However, the plant is suitable for cultivation in greenhouses and in vitro (Vršek et al. Citation2004; Kereša et al. Citation2009), and therefore, besides an ornamental use, it has a good perspective to become a useful medical plant, as it was shown for some other Iris taxa (Kukula-Koch et al. Citation2015; Venditti et al. Citation2017).
Furthermore, phytochemical analysis of native populations of I. adriatica could also have possible taxonomic implications on different classification levels. Namely, chemical constituents are genetically controlled traits that are sometimes considered to have advantages over morphological ones in systematic research (Harborne and Turner Citation1984), and taxonomically useful phenolic variations have been established in the family Iridaceae (Harborne and Williams Citation2000), genus Iris (Wang et al. Citation2010), and especially in some other taxa of the subgenus Iris (Williams et al. Citation1997, Citation2000; Rusak et al. Citation2005; Venditti et al. Citation2017; Abdel-Mageed et al. Citation2018).
Isoflavones, flavones, xanthones and benzophenones have been discovered as major phenolic constituents in Iris spp. (Williams et al. Citation1997; Mizuno et al. Citation2012; Wei et al. Citation2012; Kaššák Citation2014; Xie et al. Citation2014; Kukula-Koch et al. Citation2015). Rhizomes are known from traditional usage and medicine, obviously because of their phenolic compounds (Kaššák Citation2012). Moreover, some recent surveys have revealed medically useful phenolic compounds in rhizomes of some other Iris taxa (Conforti et al. Citation2009; Rigano et al. Citation2009; Wei et al. Citation2012; Xie et al. Citation2014; Venditti et al. Citation2017). Recently, a number of isoflavones together with two benzophenones, a xanthone and the ubiquitous β-sitosterol have been isolated and identified from a sample of I. adriatica of undefined origin bought at an open market (Bukvički et al. Citation2017).
Hence, the detailed investigation of phenolic compounds from samples of I. adriatica collected at the wild habitats were chosen for three purposes: to provide the first results of phytochemical analysis of I. adriatica from different collection sites of its original habitats, to validate whether the established phenolic compounds have already been found earlier to have a useful phytotherapeutic potential and to provide new evidence for possible taxonomic implications of established phenolic compounds.
Material and methods
Plant material
The Iris plants were collected at the beginning of April of 2016 on several different native localities of the species I. adriatica (Table S1, Supplementary data). Collections were made with the Permission of the Croatian Ministry of Environment and Nature Protection (Decision No. UP/1-612-07/15-48/23). Collecting of particular plant parts and their quantity depends on the fitness condition of the specific population. Different plant parts were separated and dried at room temperature for one month. Live voucher specimens are deposited within the Iris-collection of the Botanical Garden Zagreb (the codes presented in the Table S1), and voucher specimen no. 14341 in the herbarium collection ZA, as well.
Sample preparation
Air-dried samples (rhizomes) were extracted with methanol in a shaking water bath at 50 °C (0.5 g, 10 ml, 60 min), the solvent was evaporated to dryness. Samples for LC-MS analysis were prepared by dissolving the extracts in MeOH at a concentration of 5 mg/ml and centrifugation (5 min., 3000 U/min.).
In order to isolate selected compounds for structural confirmation by NMR, remaining amounts of rhizomes from all collection sites were combined (261 g) and extracted in a Soxhlet apparatus with methanol in two batches (4.5 h extraction time) with an extraction yield of 16.3%.
UHPLC-PDA-ESI-MS analysis
Analysis of extracts was carried out on a Dionex Ultimate 3000 RS LC system coupled to a LTQ XL linear ion-trap mass spectrometer equipped with an ESI ion source (all components Thermo Scientific). Separation was done on a Zorbax SB-C18 Rapid Resolution HD column, 100 × 2.1 mm, 1.8 μm particle size (Agilent). A gradient elution using 0.1% formic acid in water (A) and gradient grade acetonitrile (B) was performed, starting with 8% B, increasing to 45% B 0–9 min.; 9–13 min. 45% B to 100% B; 15–15.5 min. 100% B to 8% B, equilibrating for 8 min; flow rate 0.39 ml/min. Column temperature was 35 °C; injection volume 2 μl. PDA detection was performed in the 190 nm to 500 nm wavelength range. An estimate of quantitative relationships of major compounds was made on basis on PDA total scan chromatograms. The mass spectra were recorded in negative and positive ion mode in the m/z range of 50 to 2000 amu. Mass spectral conditions were set as follows: Source voltage 3.5 kV (ESI pos), 5.0 kV (ESI neg); capillary temperature 300 °C; source temperature 350 °C; sheath gas flow 65 arb (arbitrary units), auxiliary gas flow 15 arb.
Isolation of compounds
20.2 g of extract was separated by VLC on 200 g silica gel (Silicagel 60 (Merck), 0.04–0.063 mm) with gradient elution of different hexane – ethyl acetate – methanol ratios into 31 fractions. Compounds 11, 15, 19 and 24 were obtained from fractions F21–24 (eluted with MeOH – EtOAc 50:50, v/v), followed by semipreparative HPLC (Shimadzu CBM-20A controller, LC-20AT solvent delivery module, SIL-10AF autosampler, CTO-20AC column oven, SPD-M20A diode array detector, FRC-10A fraction collector) using a Luna C18(2) column, 250 × 10 mm, 10 μm (Phenomenex), water – acetonitrile 75:25 (v/v) isocratic; 4 ml/min; 25 °C column temperature; 200 μl injection volume. Compounds 31, 32, 34 and 36 were isolated from F9–10 (eluted with hexane – EtOAc 50:50, v/v) by CC on Sephadex LH-20 eluting with EtOAc – MeOH 9:1 (v/v), followed by semipreparative HPLC (Shimadzu system, see above) eluting with water – MeOH (35:65, v/v) (36) and water – MeOH (45:55, v/v) (31, 32, 34), respectively.
Results
Combining information of UV spectra and mass spectrometric detection, altogether 36 compounds, representing 22 isoflavones, 7 xanthones, 6 benzophenones and 1 acetophenone, could be identified in the methanolic extracts of I. adriatica rhizomes (see Table ). Representative chromatograms are presented in Figure and Figure S1 (Supplementary data), structures of compounds can be depicted from Figure . Comparison of samples of all accessions showed only differences in relative proportions of the identified compounds, which could be detected in all samples (see Figure S2, Supplementary data). Seven isoflavones (15, 19, 24, 31, 32, 34, 36) and irisxanthone (11) could be isolated and fully structure elucidated by additional NMR analysis, thereby confirming structural proposals deduced from UV spectral and mass spectrometric data and defining type and linkage of monosaccharides in isoflavone glycosides 15, 19 and 24, as well as irisxanthone (11).
Table 1. Compounds in rhizomes of Iris adriatica identified by UHPLC-PDA-ESI-MS analysis.
Figure 1. Representative UV chromatogram (320 nm) of the methanolic extract of Iris adriatica rhizome (Sample 1). For compound identities see Table , for experimental conditions refer to Materials and methods.
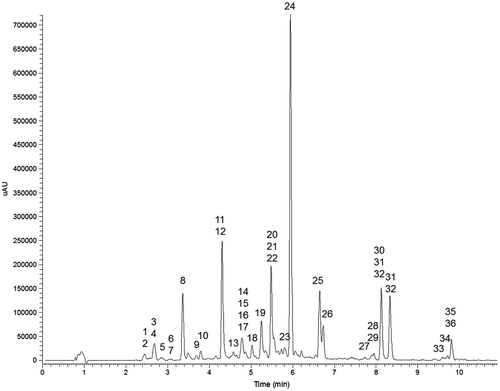
Figure 2. Structures of xanthones, benzophenone and isoflavone aglycones identified in Iris adriatica rhizomes.
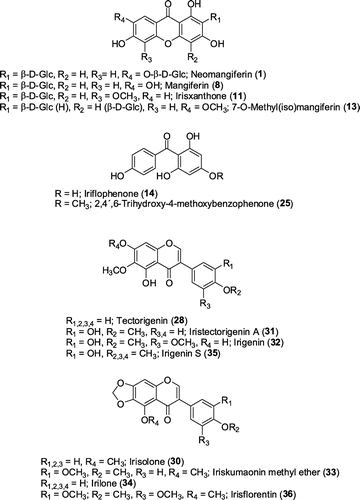
Structures of isoflavones
Description of mass spectral fragments of isoflavonoids due to retro-Diels-Alder reaction follows nomenclature of Ma et al. (Ma et al. Citation1997). Characteristic ring fissions are presented in Figure S3 (Supplementary data).
Compound 36 could be identified as irisflorentin (Arisawa et al. Citation1973a) which was deduced from the 1,3A+ fragment of m/z 195 and loss of 4 methyl groups and NMR data. Compound 35 was designated as irigenin S (Ibrahim et al. Citation2012) due to loss of 3 methyl groups and fragments 0,4B- at m/z 221 and 0,3B-+H at m/z 194. Irilone (34) (Rigano et al. Citation2007) was identified by its fragment 1,3A+ at m/z 181 and NMR spectral data. Loss of a hexose unit (−162 u) and identical aglycone fragments as in 34 led to the assignment of an irilone-O-hexoside for compound 26. The assignment of iriskumaonin methyl ether (33) (Pailer and Franke Citation1973) was based on loss of 3 methyl groups, loss of an oxymethylene fragment (−30 u) and loss of a 1,3B+-H (−161 u) unit. Irigenin (32) (Pailer and Franke Citation1973) showed consecutive loss of 3 methyl groups and representative 1,3A+ (m/z 183) and 1,3B+ (m/z 178) fragments. NMR data confirmed the structural proposal. Irigenin glycosides 17 and 22 were characterised as O-dihexoside (17) and O-hexoside (22) due to losses of −162 u and aglycone fragments matching with those of irigenin (32). According to mass spectral fragmentation compound 31 was identified as one of the isomers iristectorigenin A or B. Detailed analysis of HMBC correlations of H-6′ (7.28 ppm) to C-4′ (148.9 ppm) and methoxy protons at 3.78 ppm led to the unequivocal assignment of 31 to iristectorigenin A (Hanawa et al. Citation1991). Compounds 12, 15, 20 and 21 could be characterised as iristectorigenin A glycosides. In compound 15 the glycosidic moiety was shown to be a β-d-glucopyranosyl-(1→6)- β-d-glucopyranoside attached to the hydroxyl group at position 7 of the aglycone (Schütz et al. Citation2011). The consecutive losses of 3 hexosyl units (−162 u) indicated that compound 12 was a O-trihexoside of iristectorigenin A, which has not been described before in Iris spp. Similarly, an O-trihexoside of irisolone (16) could be found, which is a new report for Iris spp. In fact, the highest number of isoflavone glycosides in I. adriatica rhizomes was depicted as irisolone glycosides, their aglycone (30) (Dhar and Kalla Citation1972) could also be detected in free form. Glycosides 19 and 24 could be describe as irisolone-4′-O-[β-d-glucopyranosyl-(1→6)-β-d-glucopyranoside (19, Germanaism E) (Rigano et al. Citation2007) and irisolone-4′-O-β-d-glucopyranoside (24, Germanaism B) (Rigano et al. Citation2007) based on UV, ESI-MS and NMR data. Compounds 27 and 29 proved to be acylated hexosides of irisolone which was deduced from the loss of −338u (29) and −368u (27) in ESI- spectra and a consecutive loss of −176u (29) and –206u (27) as well as −162u (27, 29) in ESI+ mode (Cuyckens and Claeys Citation2004), assigning compound 27 tentatively to irisolone O-sinapoylhexoside and compound 29 to irisolone O-feruloylhexoside, respectively. Acylation with phenylpropanoic acids is also in accordance with the high retention times on reversed phase columns compared to corresponding dihexosides as well as an additional UV absorbance band at ca. 326 nm compared to irisolone 30. Aglycone 28 was identified as tectorigenin (Pailer and Franke Citation1973) due to loss of one methyl group in ESI- mode and a characteristic loss of a 1,3B+ fragment (−118 u) in MS3 spectrum of ESI+ mode. Its corresponding O-hexoside 18 showed a loss of a hexose unit (−162 u) and matching aglycone fragments.
Structures of xanthones
For xanthones, fragmentation pathways proposed by Yu et al. (Citation2013) and Schieber et al. (Citation2003) were used. In case of xanthones, UV spectra were useful for identifying this class of compounds due to 4 characteristic absorption bands at 230–245 nm, 250–265 nm, 305–330 nm and 340–400 nm (Harborne Citation1998).
Compounds 11 and 13 proved to be xanthone-C-glycosides based on UV and mass spectral data. According to congruence with mass spectra of mangiferin 8 (Schieber et al. Citation2003; Yu et al. Citation2013) but mass fragments increased by 14u, 13 was assigned to 7-O-methylmangiferin or 7-O-methylisomangiferin (Fujita and Inoue Citation1981). Xanthones 4 and 7 revealed to be O-hexosides of either isomer due to loss of a hexose unit (−162 u). Based on mass spectral similarity but a higher UV absorption band II close to 280 nm, compound 11 was designated as irisxanthone (Arisawa et al. Citation1973b), in addition its O-hexoside compound 9 could be identified. The structural proposal of 11 was confirmed by NMR data of the isolated substance. MS2 spectrum of glycoside 1 (ESI- mode) was in congruence with that of neomangiferin (Yu et al. Citation2013), the 7-O-β-d-glucoside of mangiferin 8. So far, this xanthone was only found outside subgenus Iris, i.e. in I. dichotoma and I. domestica (Zhang et al. Citation2011; Wei et al. Citation2012).
Structures of benzophenones and acetophenones
Benzophenones showed fission of the C–C bond between central carbonyl group and one of the adjacent aromatic rings leading by proton migration to either substituted benzyl rings or bicyclic β-lactones, the latter were characterised by a further loss of CO2. According to that, compound 25 could be identified as 2,4′,6-trihydroxy-4-methoxybenzophenone (Roger et al. Citation2012). O-Glycosidically bound hexoses attached to aglycone 25 were found in compounds 6 and 10, the di-O-hexoside (6) eluting before the mono-O-hexoside (10). Compound 14 proved to be another benzophenone with an identical loss of the hydroxylated ring B, but showing ring A of 14 mass units less than compound 25. Hence, for compound 14 the structure of iriflophenone was proposed (Arisawa et al. Citation1973b). Glycosides 2 and 3 could be designated as hexosides of iriflophenone, with the hexose moiety attached to a hydroxyl (most likely in position 2) of the aglycone in case of 2, and a C-glycosidyl hexoside in case of compound 3. The latter showed a typical fragmentation of C-glycosidically bound hexoses in ESI- mode (Cuyckens and Claeys Citation2004; de Rijke et al. Citation2006). Tentative identification of compound 5 as 3-hydroxy-5-methoxyacetophenone or 4-hydroxy-3-methoxyacetophenone hexoside was deduced from fragmentation in ESI- mode due to losses of a hexose unit (−162 u), a methyl group (−15 u) and a further loss of CO (−28 u). 3-Hydroxy-5-methoxyacetophenone was identified before in I. germanica (Ibrahim et al. Citation2012).
Quantitative estimate of major compounds in I. adriatica rhizomes
Germanaism B (24) represented the major compound with 18.5–25.6% of total peak area (based on PDA total scan chromatograms) in extracts of samples 1–5 and 7–9. On the contrary, in sample 6 compounds 30, 31 and 32 summed up to 50.4% of total peak area, and the relative proportion of compound 24 was only 12.1%. Also germanaism E (19, 0.4%) and irigenin-O-hexoside (22, 0.9%) showed exceptionally low levels in sample 6, compared to all other samples in which these compounds were present in proportions of 1.4–5.0% (19) and 2.0–3.0% (22), respectively. The major xanthones were mangiferin (8, 1.7–5.3%) and irisxanthone (11, 3.6–5.6%, in a mixture with 12). Among benzophenones, compound 25 (2,4′,6-trihydroxy-4-methoxybenzophenone) was dominating (2.7–6.3%). Table S2 summarises a quantitative estimate of major phenolic compounds in the analysed samples.
Discussion
This is the first LC-PDA-MS analysis performed on taxa of the I. pumila complex in order to get a more detailed insight into the pattern of phenolic compounds (xanthones, benzophenones, isoflavones) in the native rhizomes of Croatian stenoendemic taxon I. adriatica. Complete or partial phenolic metabolite profiles of Iris spp. have been published before, and some researched species belong to the subgenus Iris, e.g. I. albicans Lange (Abdel-Mageed et al.Citation2018), I. germanica L. (Schütz et al. Citation2011), I. hookeriana (Dar et al. Citation2016), I. marsica (Venditti et al. Citation2017), I. nigricans (Huwaitat et al. Citation2013) and I. pseudopumila (Rigano et al. Citation2007; Conforti et al. Citation2009).
Isoflavones were the main group of phenolic compounds in I. adriatica rhizomes of both native and planted collections. Similar to the report of Bukvički et al. (Citation2017), likewise in our research isoflavonoids showing a 6,7-methylendioxy group were dominating in terms of number (compounds 16, 19, 23, 24, 26, 27, 29, 30, 33, 34). Among them six glycosides of irisolone (30), including the major compound in all extracts except that of sample 6 (cf. Table S1 and Table S2), i.e. compound 24, could be identified. In sample 6, collected at Brnjica-Pokrovnik, iristectorigenin A (31) and irigenin (32) dominated. This population was the only one growing within the Festuco-Koelerietum splendentis Horvatić 1963 association (an extensive open calcareous meadow), whilst the rest of the researched populations belong to the Stipo-Salvietum officinalis Horvatić 1985 association (hills, limited rocky pastures, endangered by the succession of garrigue). If further research proves that our finding for this population of I. adriatica is of chemotaxonomic value, this could point to a more distinct position of this particular population, which might be a specific ecotype of the typical species. The occurrence of methoxylated isoflavones with and without 6,7-methylendioxy structural element is in accordance with the findings for rhizomes of I. pseudopumila Tineo (Rigano et al. Citation2007), belonging to the I. pumila complex. However, in contrast to the report of Rigano et al. (Citation2007), in none of our investigated I. adriatica rhizomes flavones and flavonol derivatives like isoscutellarein and kaempferol glycosides could be detected. This finding would be of chemotaxonomic value as well, especially in case of such closely related taxa, often mistakenly mixed in the past (Randolph and Mitra Citation1959; Webb and Chater Citation1980; Mitić Citation2002). Though, this difference in flavonoid composition between the closely related taxa should be considered with care, as the flavonoid composition of the aerial parts of plants of the subgenus Iris with typical occurrence of C-glycosyl flavones as reported by others (Williams et al. Citation1997; Conforti et al. Citation2009) and according to our preliminary investigations of a few leave samples (data not shown here), can be quite different from rhizomes. Also, in reports of Bukvički et al. (Citation2017) no flavones/flavonols as constituents of I. adriatica rhizomes were mentioned. Hence, careful separation of leaves and rhizomes before analysis seems to be essential. On the other hand, the absence of 7-O-methyltectorigenin derivatives in I. adriatica compared to I. pseudopumila rhizomes (Rigano et al. Citation2007, Citation2009) should also be considered as taxonomical marker and investigated further.
During our study of I. adriatica for the first time for representatives of the subgenus Iris, two isoflavone triglycosides were described. In addition, acylated irisolone glycosides (27, 29) have never been reported before in the genus Iris, and are potential candidates of chemotaxonomic importance on taxonomic levels higher than species level. Yet, they were present only in low amounts which makes them difficult to isolate and get their detailed structures. Actually, isoflavone glycosides with cinnamic acids attached to the glycosidic moiety are rare and exemplified only by a few reports from Juglans sigillata (Hu et al. Citation2017), Pterocarpus santalinus and Pterocarpus marsupium (Veitch Citation2007). Since mentioned plants are phylogenetically very distant from the genus Iris, these results require further investigation of isoflavone glycosides within the genus Iris and family Iridaceae, as well.
Mangiferin (8) and irisxanthone (11) are the dominating xanthones in I. adriatica rhizomes. The occurance of 8, isomangiferin and methylated derivatives thereof has also been described for Italian taxa of the subgenus Iris including I. pseudopumila (Williams et al. Citation1997).
According to our studies, benzophenones are represented in I. adriatica by iriflophenone (14) and 2,4′,6-trihydroxy-4-methoxybenzophenone (25) not only as aglycons, but also in their glycosidic form, including a possible C-glycosyl derivative of 14. This is a new finding for taxa of the I. pumila complex, however, the 2-O-β-d-glucopyranoside of 25 has been reported for I. germanica (subgenus Iris, series Iris) (Xie et al. Citation2014).
The inter- and intra-population morphological, karyological and molecular variations within the subgenus Iris are sometimes difficult to detect (Williams et al. Citation2000; Mitić et al. Citation2001; Wilson Citation2011), and in such cases phytochemical features might help in providing additional taxonomical information. However, for more precise conclusion about the role of chemical markers in the taxonomy of the I. adriatica and its closely related taxa, further phytochemical analysis should be carried out.
Biological activity of some of the established phenolic compounds from the rhizomes of I. adriatica was already tested and proved in some other Iris taxa (Kaššák Citation2012). Compared to activities reported for I. germanica rhizomes, it seems that a number of isoflavones which are also present in I. adriatica could be active as cancer chemopreventors (Wollenweber et al. Citation2003). The methanolic extract of rhizomes of I. pseudopumila showed significant anti-inflammatory activity based on inhibition of LPS induced NO production in RAW 264.7 macrophages (Conforti et al. Citation2009), IC50 values of 67.5 and 55.1 μM were determined for compounds 19 and 22. Similarly, inhibition of NO release was also reported for 2,4′,6-trihydroxy-4-methoxybenzophenone (25) (Pan et al. Citation2016) which additionally showed DPPH radical scavenging effects (Pan et al. Citation2016) as well as induction of apoptosis in HT-29 colon carcinoma cells (Lay et al. Citation2014). Mangiferin (8), the main xanthone in I. adriatica rhizomes, revealed several activities related to antidiabetic effects, like inhibiting endothelial insulin resistance (Xu et al. Citation2017) or α-glucosidase inhibition (Vo et al. Citation2017) as well as anti-inflammatory and antidepressant action (Cao et al. Citation2017). Irilone (34) was shown to possess α-amylase inhibitory effects which would support antidiabetic action (Ibrahim et al. Citation2017). The same compound (34) could also play a role as phytoestrogen as it was contributing to the oestrogenic activity of red clover extracts ((Lutter et al. Citation2014). Hence, there is remarkable potential of I. adriatica constitutents to be explored further for antiinflammatory, chemopreventive, antidiabetic and phytoestrogenic effects.
Conclusions
To conclude, I. adriatica proved to be a rich source of phenolic compounds of great pharmacological and taxonomical value. Although it is an endemic species, it is also easy to grow both in greenhouses and in vitro (Vršek et al. Citation2004; Kereša et al. Citation2009), and therefore is suitable for further exploration and utilisation for both scopes – pharmaceutical and chemotaxonomical.
Supplemental data
Supplemental data for this article can be accessed https://doi.org/10.1080/11263504.2018.1478906
Disclosure statement
The authors declare that they are not in a potential conflict of interest.
TPLB_A_1478906_Supplemental Material
Download PDF (547.1 KB)Acknowledgements
The authors are grateful to several colleagues who have been assisting us in the organisation of the field work (Milenko Milović, Miroslav Mitić, Radnić family, Dalibor Vladović and Nediljko Ževrnja) and to Elvira Knauder for assistance in phytochemical work. The Croatian Ministry of Environment and Nature Protection is acknowledged for giving us the permission to collect I. adriatica plant material (Decision No. UP/1-612-07/15-48/23).
References
- Abdel-Mageed WM, Al-Wahaibi LH, Al-Saleem MSM, Gouda YG, Abdel-Kader MS, Ibraheim ZZ. 2018. Phytochemical and chemotaxonomic study on Iris albicans Lange. Biochem Syst Ecol. 76: 32–34.10.1016/j.bse.2017.11.007
- Arisawa M, Morita N, Kondo Y, Takemoto T. 1973a. Constituents of Iris genus plants. IV. Constituents of Iris florentina. 2. Chem Pharm Bull. 21(10): 2323–2328.10.1248/cpb.21.2323
- Arisawa M, Morita N, Kondo Y, Takemoto T. 1973b. Constituents of Iris florentina. 3. Structure of irisxanthone, a new C-glycosylxanthone. Chem Pharm Bull. 21(11): 2562–2565.10.1248/cpb.21.2562
- Bukvički D, Novaković M, Ab Ghani N, Marin PD, Asakawa Y. 2017. Secondary metabolites from endemic species Iris adriatica Trinajstić ex Mitić (Iridaceae). Nat Prod Res. 1–4: doi:10.1080/14786419.2017.1402309
- Cao C, Su M, Zhou F. 2017. Mangiferin inhibits hippocampal NLRP3 inflammasome and exerts antidepressant effects in a chronic mild stress mice model. Behav Pharmacol. 28(5): 356–364.10.1097/FBP.0000000000000305
- Conforti F, Rigano D, Menichini F, Loizzo MR, Senatore F. 2009. Protection against neurodegenerative diseases of Iris pseudopumila extracts and their constituents. Fitoterapia. 80: 62–67.10.1016/j.fitote.2008.10.005
- Cuyckens F, Claeys M. 2004. Mass spectrometry in the structural analysis of flavonoids. J Mass Spectrom. 39(1): 1–15.10.1002/(ISSN)1096-9888
- Dar BA, Lone SH, Shah WA, Bhat KA. 2016. LC-MS guided isolation of bioactive principles from Iris hookeriana and bioevaluation of isolates for antimicrobial and antioxidant activities. Drug Res. 66(8): 427–431.
- Dhar KL, Kalla AK. 1972. Isoflavones of Iris kumaonensis and I. germanica. Phytochemistry. 11(10): 3097–3098.10.1016/0031-9422(72)80125-0
- Fujita M, Inoue T. 1981. Studies on the constituents of Iris florentina L.I. Isolation of C-glucosylxanthones from the underground parts and their biosynthesis. Yakugaku Zasshi 101(12): 1118–1123.10.1248/yakushi1947.101.12_1118
- Hanawa F, Tahara S, Mizutani J. 1991. Isoflavonoids produced by Iris pseudacorus leaves treated with cupric chloride. Phytochemistry. 30(1): 157–163.10.1016/0031-9422(91)84117-B
- Harborne JB. 1998. Phytochemical methods. A guide to modern techniques of plant analysis. 3rd ed. London: Chapman & Hall.
- Harborne JB, Turner BL. 1984. Plant chemosystematics. St Louis: Academic Press.
- Harborne JB, Williams CA. 2000. The phytochemical richness of the Iridaceae and its systematic significance. Ann Bot. 58: 43–50.
- Hu L, Wang K, Li G, Zhang R, Luo Y, Si C, Wang J. 2017. Isolation and structural elucidation of heartwood extractives of Juglans sigillata. Holzforschung. 71(10): 785–791.
- Huwaitat S, Al-Khateeb E, Finjan S. 2013. Isolation and identification of some phytochemical compounds from different parts of Iris nigricans. Eur Sci J 9(6): 32–37.
- Ibrahim SRM, Mohamed GA, Al-Musayeib NM. 2012. New constituents from the rhizomes of Egyptian Iris germanica L. Molecules. 17: 2587–2598.10.3390/molecules17032587
- Ibrahim SRM, Mohamed GA, Zayed MF, Ross SA. 2017. 8-Hydroxyirilone 5-methyl ether and 8-hydroxyirilone, new antioxidant and α-amylase inhibitors isoflavonoids from Iris germanica rhizomes. Bioorg Chem. 70: 192–198.10.1016/j.bioorg.2016.12.010
- Kaššák P. 2012. Secondary metabolites of the choosen genus Iris species. Acta Uni Agric Silvic Mendel Brunensis 60(8): 269–280.
- Kaššák P. 2014. Screening of the chemical content of several Limniris group Irises. J Pharmacogn Phytochem 3(2): 11–14.
- Kereša S, Mihovilović A, Ćurković-Perica M, Mitić B, Barić M, Vršek I, Marchetti S. 2009. In vitro regeneration of Croatian endemic species Iris adriatica Trinajstić ex Mitić. Acta Biol Cracov 51(2): 7–12.
- Kukula-Koch W, Sieniawska E, Widelski J, Urjin O, Głowniak P, Skalicka-Woźniak K. 2015. Major secondary metabolites of Iris spp. Phytochem Rev. 14(1): 51–80.10.1007/s11101-013-9333-1
- Lawrence GHM. 1953. A reclassification of the genus Iris. Gent Herb. 8: 346–371.
- Lay MM, Karsani SA, Abd M, Sri N. 2014. Induction of apoptosis of 2,4′,6-trihydroxybenzophenone in HT-29 colon carcinoma cell line. Bio Med Res Int. 468157/1-468157/13, 13 pp. doi: 10.1155/2014/468157
- Lutter S, Schmalbach K, Esch HL, Lehmann L. 2014. The isoflavone irilone contributes to the estrogenic potential of dietary supplements containing red clover. Arch Toxicol. 88(2): 309–321.10.1007/s00204-013-1114-5
- Ma YL, Li QM, Van den Heuvel H, Claeys M. 1997. Characterization of flavone and flavonol aglycons by collision-induced dissociation tandem mass spectrometry. Rapid Commun Mass Spectrom. 11(12): 1357–1364.10.1002/(ISSN)1097-0231
- Mathew B. 1981. The Iris. London: B. T. Batsford Ltd..
- Mitić B, Nikolić T, Liber Z. 2001. Morphological and karyological relationships within Alpine – dinaaric populations of the Genus Iris L., Pallidae series (A. Kern.) Trinajstić (Iridaceae). Acta Soc Bot Pol. 70(3):221–227.
- Mitić B. 2002. Iris adriatica (Iridaceae), a new species from Dalmatia (Croatia). Phyton (Horn, Austria). 42(2):305–313.
- Mitra J. 1956. Karyotype analysis of bearded Iris. Bot Gaz. 117: 265–293.10.1086/335916
- Mizuno T, Yabuya T, Sasaki N, Iwashina T. 2012. Phenolic compounds, including Novel C-glycosylflavone, from the flowers of the tall bearded Iris cultivar “Victoria Falls”. Nat Prod Comm. 7(12): 1591–1594.
- Nikolić T, editor. 2017. Flora croatica database. [Online]. Croatia: Department of Biology, Faculty of Science, University of Zagreb. [Accessed 2017 Nov 21]. http://hirc.botanic.hr/fcd
- Pailer M, Franke F. 1973. Constituents of Iris germanica. Monatsh Chem. 104(5): 1394–1408.10.1007/BF00910057
- Pan J, Yi X, Wang Y, Chen G, He X. 2016. Benzophenones from Mango leaves exhibit α-glucosidase and NO inhibitory activities. J Agric Food Chem. 64(40): 7475–7480.10.1021/acs.jafc.6b02404
- Randolph LF, Mitra J. 1959. Karyotypes of Iris pumila and related species. Am J Bot. 46: 93–102.10.1002/ajb2.1959.46.issue-2
- Rigano D, Conforti F, Formisano C, Menichini F, Senatore F. 2009. Comparative free radical scavenging potential and cytotoxicity of different extracts from Iris pseudopumila Tineo flowers and rhizomes. Nat Prod Res. 23(1): 17–25.10.1080/14786410701740237
- Rigano D, Formisano C, Grassia A, Grassia G, Perrone A, Piacente S, Vuotto ML, Senatore F. 2007. Antioxidant flavonoids and isoflavonoids from rhizomes of Iris pseudopumila. Planta Med. 73: 93–96.
- de Rijke E, Out P, Niessen WMA, Ariese F, Gooijer C, Brinkman UAT. 2006. Analytical separation and detection methods for flavonoids. J Chromatogr A 1112(1–2): 31–63.10.1016/j.chroma.2006.01.019
- Roger B, Jeannot V, Fernandez X, Cerantola S, Chahboun J. 2012. Characterisation and quantification of flavonoids in Iris germanica L. and Iris pallida Lam. resinoids from Morocco. Phytochem Anal. 23(5): 450–455.10.1002/pca.v23.5
- Rusak G, Mitić B, Liber Z. 2005. Leaf flavonoid patterns in the Croatian endemic species Iris illyrica Tomm. and I. pseudopallida Trinajstić (Iridaceae). Period Biol. 107(1):45–49.
- Schieber A, Berardini N, Carle R. 2003. Identification of flavonol and xanthone glycosides from mango (Mangifera indica L. Cv. “Tommy Atkins”) peels by high-performance liquid chromatography-electrospray ionization mass spectrometry. J Agric Food Chem. 51(17): 5006–5011.10.1021/jf030218f
- Schütz C, Quitschau M, Hamburger M, Potterat O. 2011. Profiling of isoflavonoids in Iris germanica rhizome extracts by microprobe NMR and HPLC-PDA-MS analysis. Fitoterapia. 82(7): 1021–1026.10.1016/j.fitote.2011.06.005
- The International Plant Names Index. 2012. Published on the Internet. Kew: Royal Botanic Gardens Kew, Harvard University Herbaria, and Australian National Herbarium. [Accessed 2017 March 8]. http://www.ipni.org/
- The Plant List. 2013. Version 1.1. Published on the Internet. Kew: Royal Botanic Gardens Kew, Missouri Botanical Garden. [Accessed 2017 March 8]. http://www.theplantlist.org/
- Trinajstić I, Papeš D, Lovašen-Eberhardt Ž, Baćani Lj. 1980. Biosistematska i kariološka istraživanja roda Iris L. (Iridaceae) u Jugoslaviji [Biosystematic and karyological researches of the Genus Iris L. (Iridaceae) in Yugoslavia]. In: Abstracts of the Fourth Symposium of the Yugoslav Biosystematicians 1980; Đerdap (YU). p. 25. Croatian.
- Veitch NC. 2007. Isoflavonoids of the leguminosae. Nat Prod Rep. 24(2): 417–464.10.1039/b511238a
- Vo THT, Nguyen TD, Nguyen QH, Ushakova NA. 2017. Extraction of mangiferin from the leaves of the mango tree Mangifera indica and evaluation of its biological activity in terms of blockade of α-glucosidase. Pharm Chem J. 51(9): 806–810.10.1007/s11094-017-1697-x
- Venditti A, Frezza C, Rai R, Sciubba F, Di Cecco M, Ciaschetti G, Serafini M, Bianco A. 2017. Isoflavones and other compounds from the roots of Iris marsica I. Ricci ee Colas. Collected from Majella National Park, Italy. Med Chem 7(2): 787–794.
- Vršek I, Mitić B, Bujan M, Čoga L, Milović M, Richter M. 2004. Iris adriatica Trinajstić ex Mitić, potencijalna biljka pogodna za uzgoj kao lončanica [Iris adriatica Trinajstić ex Mitić, a potential plant suitable for growing in pots]. In: Mitić B, Šoštarić R, editors. 1st Croatian Botanical Symposium with international participation 2004. Book of Abstracts ot the 1st Croatian Botanical Symposium with international participation, September 30–October 2; Zagreb (Croatia): Croatian Botanical Society, p. 173–174. Croatian.
- Wang H, Cui Y, Zhao C. 2010. Flavonoids of the genus Iris (Iridaceae). Minirev Med Chem. 10: 643–661.10.2174/138955710791384027
- Webb DA, Chater AO. 1980. Iris L. In: Tutin TG, Heywood VH, Burges NA, Moore DM, Webb DA, editors. Flora Europaea, vol. 5. Cambridge (UK): University Press. pp. 87–92.
- Wei Y, Shu P, Hong J, Qin M. 2012. Qualitative and quantitative evaluation of phenolic compounds in iris dichotoma Pall. Phytochem Anal. 23(3): 197–207.10.1002/pca.v23.3
- Williams CA, Harborne JB, Colasante M. 1997. Flavonoid and xanthone patterns in bearded Iris species and pathway of chemical evolutions in the genus. Biochem Syst Ecol 25: 309–325.10.1016/S0305-1978(97)00008-2
- Williams CA, Harborne JB, Colasante M. 2000. The pathway of chemical evolution in bearded Iris species based on flavonoid and xanthone patterns. Ann Bot. 1(2): 51–58.
- Wilson CA. 2011. Subgeneric classification in Iris re-examined using chloroplast sequence data. Taxon. 60(1): 27–35.
- Wollenweber E, Stevens JF, Klimo K, Knauft J, Frank N, Gerhäuser C. 2003. Cancer chemopreventive in vitro activities of isoflavones isolated from Iris germanica. Planta Med. 69: 15–20.10.1055/s-2003-37030
- Xie G, Zhu Y, Shu P, Qin X, Wu G, Wang Q, Qin M. 2014. Phenolic metabolite profiles and antioxidants assay of three Iridaceae medicinal plants for traditional Chinese medicine “She-gan” by on-line HPLC–DAD coupled with chemiluminescence (CL) and ESI-Q-TOF-MS/MS. J Pharm Biomed Anal. 98(Supplement C):40–51. 10.1016/j.jpba.2014.05.008
- Xu X, Chen Y, Song J, Hou F, Ma X, Liu B, Huang F. 2017. Mangiferin suppresses endoplasmic reticulum stress in perivascular adipose tissue and prevents insulin resistance in the endothelium. Eur J Nutr. 57:1563–1575. doi: 10.1007/s00394-017-1441-z
- Yu Q, Qi J, Yu H, Chen L, Kou J, Liu S, Yu B. 2013. Qualitative and quantitative analysis of phenolic compounds in the leaves of Aquilaria sinensis using liquid chromatography-mass spectrometry. Phytochem Anal. 24(4): 349–356.10.1002/pca.v24.4
- Zhang Y, Wang Q, Qi L, Qin X, Qin M. 2011. Characterization and determination of the major constituents in Belamcandae Rhizoma by HPLC-DAD-ESI-MSn. J Pharm Biomed Anal. 56(2): 304–314.10.1016/j.jpba.2011.05.040
