Abstract
Azo dyes containing effluents from different industries pose threats to the environment. Though there are physico-chemical methods to treat such effluents, bioremediation is considered to be the best eco-compatible technique. In this communication, we discuss the decolorization potentiality of five azo dyes by Podoscypha elegans (G. Mey.) Pat., a macro-fungus, found growing on the leaf-litter layer of Bethuadahari Wildlife Sanctuary in West Bengal, India. The fungus exhibited high laccase and very low manganese peroxidase activities under different culture conditions. Decolorization of five high-molecular weight azo dyes, viz., Orange G, Congo Red, Direct Blue 15, Rose Bengal and Direct Yellow 27 by the fungus was found to be positive in all cases. Maximum and minimum mean decolorization percentages were recorded in Rose Bengal (70.41%) and Direct Blue 15 (24.8%), respectively. This is the first record of lignolytic study and dye decolorization by P. elegans.
Azo dyes represent up to 70% of dyestuffs used in textile and other industries [Citation1]. In textile dyeing process, 280,000 tons of unbound xenobiotic dyes are discharged annually as wastewater [Citation2]. Colored components are undesirable in water and most of the synthetic dyes being toxic, mutagenic, and carcinogenic and thus, can impart serious environmental hazard [Citation3]. The suspended dyes in water bodies influence the aquatic ecosystem [Citation4], pose public health risks due to bioaccumulation and cause soil contamination [Citation5]. In addition, the aromatic amines, which are the reduced intermediates of azo dyes, are more toxic than the dyes themselves [Citation6]. Due to cost effectiveness and eco-compatibility, bioremediation of such high-molecular weight xenobiotic dyes has come up as a promising alternative technology over the conventional physicochemical management practices [Citation7,Citation8].
In the last decade, some authors have argued the effectiveness of soil and water bioremediation potentialities of litter-decomposing fungi (LDF) over the widely studied white rot fungi (WRF) [Citation9,Citation10]. The LDFs naturally inhabit the soil-litter layers where they co-exist and compete with other microorganisms and possess similar lignolytic activity as that of the WRFs and can effectively utilize different high-molecular weight dyes from both soil and liquid conditions in vitro [Citation9,Citation11].
With this rationale, in this present study, we investigated the efficacy of Podoscypha elegans, a LDF, against five high-molecular weight azo dyes () used widely in textile industries. This is the first report of the lignolytic activity and dye decolourization potentiality of the fungus.
Table 1. Details of the different dyes.
Podoscypha elegans (G. Mey.) Pat. was collected from the litter bed of Bethuadahari Wildlife Sanctuary forest, Nadia and cultured in Potato Dextrose Agar supplemented with ampicillin 100 mgL−1, nystatin 50 mgL-1 and incubated at 28 ± 2 °C for 5–7 days. The fungus was identified previously by the present authors [Citation12].
Lignolytic activity was initially assayed in five growth media, viz. Kirk’s medium (KM) [Citation13], HNHC medium [Citation14], mineral salts medium (MSB) [Citation15], modified PDB (M-PDB) medium [Citation16], and low-nutrient PDB (LN-PDB) medium [Citation16]. Lignin peroxidase (LiP), manganese peroxidase (MnP) and laccase activities were measured spectrophotometrically using a spectrophotometer [Citation17–19]. Enzyme activity was expressed in nanokatals per milliliter (nkat/ml). The fungus was grown in flasks containing 100 ml of respective growth medium for 7, 14, 21 and 28 days at 28 ± 2 °C.
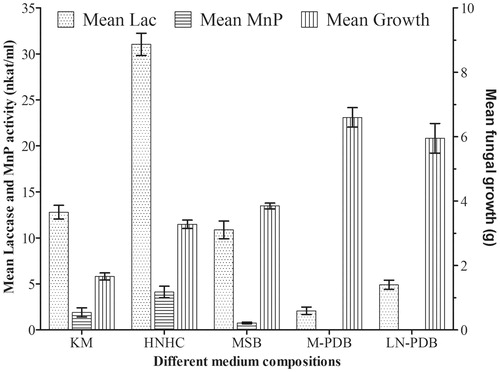
Laccase activity was recorded in all the media studied while low activity of MnP was recorded only in HNHC, KM and MSB. High fungal growth was recorded in nutrient rich M-PDB and LN-PDB, low and slow growth was observed in the other three low-nutrient starvation media (). HNHC was found to be the best inducer of laccase followed by KM and MSB. M-PDB and LN-PDB were found to be poor inducer of laccase activity. No LiP activity was recorded in any of the growth medium studied. Most lignin degrading LDFs produce two main extracellular ligninolytic enzymes, MnP, and laccase [Citation9] and LiP is not common in LDFs.
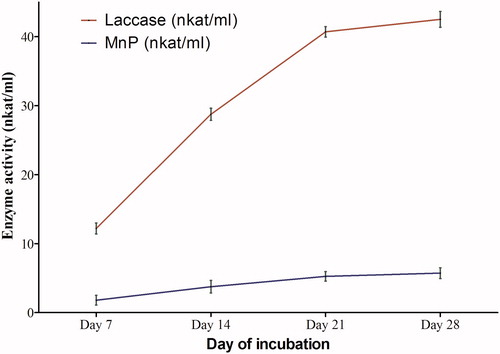
Podoscypha elegans showed significant laccase activity (31.04 nkat/ml), but a low MnP activity (4.13 nkat/ml) activity in HNHC (). Maximum laccase activity in HNHC medium was recorded at 28 days. However, it was observed that though there was a steep increase in the rate of laccase production up to 21 days, the rate of increase decreased after 21 days, possibly because of accumulation of other fungal metabolites in the medium.
Since HNHC medium showed the best result of laccase induction, the different parameters (pH, temperature, rotation speed) and effect of different concentrations of veratryl alcohol (VA) were standardized using this growth medium. A perusal of the results indicated that all the four cultural parameters had significant effect on the production of laccase (). Podoscypha elegans produced maximum laccase at a slightly acidic pH 6.5, though significant activity was recorded at a pH range of 5.5–7.5 (). Fungal laccases typically exhibit pH optima in the acidic pH range [Citation20]. Not only the rate of oxidation but also the reaction products can differ according to pH as pH may affect abiotic follow-up reactions of primary radicals formed by laccase. The stability of fungal laccases is generally higher at acidic pH [Citation21], although exceptions exist [Citation22]. Laccase stable at elevated pH (8–8.5) is of special importance in bleaching of pulp [Citation23]. In view of this, the laccase activity of P. elegans should further be examined in alkaline pH range.
Figure 3. Effect of different parameters on laccase activity in HNHC medium. (A) pH. (B) Temperature. (C) Rotation speed. (D) VA concentrations.
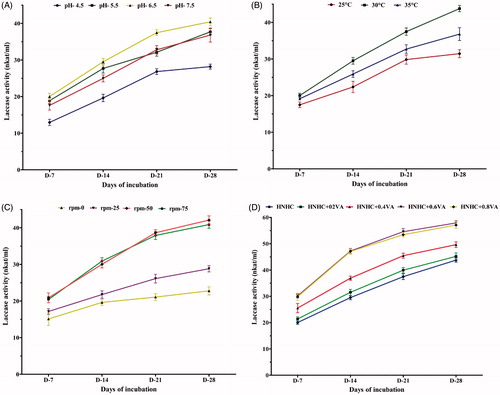
Laccase activity was observed in the 25–35 °C temperature range, the most effective being around 30 °C ().Temperature profiles of laccase activity are not different from other extracellular ligninolytic enzymes with optima between 50° and 70 °C [Citation20]. Few enzymes with optima below 35 °C have been described, e.g., the laccase from Ganoderma lucidum has its highest activity at 25 °C [Citation24]. Rotation speed was another factor that determined the production of laccase under cultural conditions. Aeration and its circulation () played an important role in boosting laccase activity in comparison to the activity in still cultures. The optimum enzyme activity was noted at rotation speed range of 50–75 rpm.
VA has been reported as a laccase inducer in ascomycete Botryosphaeria sp. [Citation25] and in Trametes versicolor [Citation26], while it was found to have no effect on laccase production in Penicillium chrysogenum, [Citation27]. VA was a positive inducer of laccase production by P. elegans (). All concentrations, from 0.2 to 0.8 mM, showed enhancement of laccase activity. However, 0.6 and 0.8 mM VA showed the best results.
Based on these results, the best condition for laccase production was found to be HNHC supplemented with 0.6 mM VA at pH 6.5 and incubation at 30 ± 2 °C at 50 rpm. This condition was thus selected for dye decolorization studies.
To test the decolorization potential, HNHC was selected and the different factors, viz, pH, temperature, rotation speed, and different concentrations of VA, a laccase inducer, were optimized. Decolorization test of five high-molecular weight dyes () was carried out in 100 ml of HNHC supplemented with 0.6 mM VA (pH 6.5) and the dyes. Three concentrations (0.5%, 1.0%, and 1.5%) of each dye were tested. Each treatment was inoculated with the fungus and incubated in a shaker incubator (50 rpm) for 28 days at 28 ± 2 °C keeping three replications and uninoculated control sets. Absorbance was measured in spectrophotometer at 7 days interval and the decolorization percentage was determined using the formula:
[Where A (ini) =Absorbance of un-inoculated dye; A (fin) = Final absorbance of dye after inoculation and incubation.]
Two-way ANOVA was performed to test the significance of combination effect of days of incubation and dye concentrations on decolorization, using IBM SPSS Statistics 23. Significance was determined at 5% level.
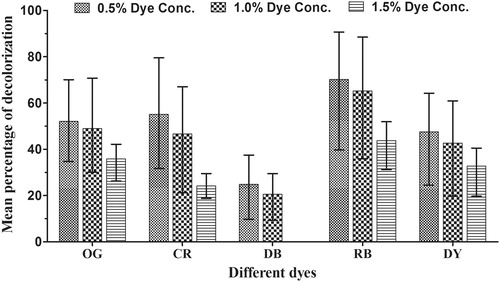
The results indicated that the different dyes were utilized by P. elegans at different rates (). The concentration of dye in the medium was crucial for decolourization by the fungus. Thus, the maximum decolorization was observed in the 0.5% concentration treatment for all the dyes (). Increase in concentration affected the process of dye utilization by the fungus. The rate of decolourization was low at the concentration of 1.5% in all dyes and no decolourization was observed where there was poor fungal growth as in Direct Blue 15 (DB).
Rose Bengal (RB) showed the maximum decolorization and the mean decolourization percentages were 70.41, 65.21, and 43.78 at 0.5, 1.0, and 1.5% dye concentrations, respectively. The lowest decolourization (24.8 and 20.52% at 0.5 and 1.0%, respectively) was recorded in DB. No decolorization was recorded in 1.5% experimental sets supplemented with DB indicating that the dye concentration was too high.
The maximum utilization of all the dyes were found between 0 to 21 days. At 0.5% concentration of Congo Red (CR) and Orange G (OG), maximum utilization by the fungus occurred within 14 to 21 days of incubation whereas, while Direct Yellow (DY) utilization was maximum between 7 to 14 days at concentration of 1.5%. These results indicate the fungus utilized the different dyes at different rates depending on their concentration in the culture medium. Both the independent effects of incubation period and initial dye concentration on decolorization were statistically significant (p < .05). The interaction of incubation period and initial dye concentration on decolorization was also significant (p = .001).
Thus, the present study establishes that LDFs with their lignolytic enzyme resource are capable of replacing their wood rotting kin in soil remediation programs. This is the first report of P. elegans, a litter decomposing macro fungus, exhibiting significant laccase activity and efficiently decolorizing high-molecular weight azo dyes under cultural conditions. Moreover, the laccase showed significant activity at higher pH. Thus, soil litter being a natural habitat, P. elegans can be used in studies of dye contaminated soil bioremediation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Reference
- Wang ZW, Liang JS, Liang Y. Decolorization of reactive black 5 by a newly isolated bacterium Bacillus sp. YZU1. Int Biodeter Biodegr. 2013;76:41–48.
- Jin XC, Liu GQ, Xu ZH, et al. Decolorization of a dye industry effluent by Aspergillus fumigatus XC6. Appl Microbiol Biotechnol. 2007;74:239–243.
- Leechart P, Nakbanpote W, Thiravetyan P. Application of ‘waste’ wood-shaving bottom ash for adsorption of azo reactive dye. J Environ Sci. 2009;90:912–920.
- Gupta VK, Jain R, Mittal A, et al. Photochemical degradation of the hazardous dye safranin-T using TiO2 catalyst. J Colloid Interface Sci. 2007;309:464–469.
- Sriram N, Reetha D, Saranraj P. Biological degradation of reactive dyes by using bacteria isolated from dye effluent contaminated soil. Middle-East J Sci Res. 2013;17:1695–1700.
- Gomi N, Yoshida S, Matsumoto K, et al. Degradation of the synthetic dye amaranth by the fungus Bjerkandera adusta Dec 1: inference of the degradation pathway from an analysis of decolorized products. Biodegradation. 2011;22:1239–1245.
- Kolekar YM, Nemade HN, Markad VL, et al. Decolorization and biodegradation of azo dye, reactive blue 59 by aerobic granules. Bioresour Technol. 2012;104:818–822.
- Sannino F, Nuzzo A, Ventorino V, et al. Effective degradation of organic pollutants in aqueous media by microbial strains isolated from soil of a contaminated industrial site. Chem Biol Technol Agric. 2016;3:2.
- Steffen KT, Hofrichter M, Hatakka A. Mineralisation of 14C-labelled synthetic lignin and ligninolytic enzyme activities of litter-decomposing basidiomycetous fungi. Appl Microbiol Biotechnol. 2000;54:819–825.
- Steffen KT, Schubert S, Tuomela M, et al. Enhancement of bioconversion of high-molecular mass polycyclic aromatic hydrocarbons in contaminated non-sterile soil by litter-decomposing fungi. Biodegradation. 2007;18:359–369.
- Liers C, Pecyna MJ, Kellner H, et al. Substrate oxidation by dye-decolorizing peroxidases (DyPs) from wood-and litter-degrading agaricomycetes compared to other fungal and plant heme-peroxidases. Appl Microbiol Biotechnol. 2013;97:5839–5849.
- Pramanik S, Chaudhuri S. Macrofungal diversity in the forest litter of Nadia District, West Bengal, India. Afr J Microbiol Res. 2017;11:927–944.
- Kirk TK, Schultz E, Connors WJ, et al. Influence of culture parameters on lignin metabolism by Phanerochaete chrysosporium. Arch Microbiol. 1978;117:277–285.
- Katagiri N, Tsutsumi Y, Nishida T. Correlation of brightening with cumulative enzyme activity related to lignin biodegradation during biobleaching of kraft pulp by white rot fungi in the solid-state fermentation system. Appl Environ Microbiol. 1995;61:617–622.
- Arora DS, Gill PK. Comparison of two assay procedures for lignin peroxidase. Enzyme Microb Technol. 2001;28:602–605.
- Sun SJ, Liu JZ, Hu KH, et al. The level of secreted laccase activity in the edible fungi and their growing cycles are closely related. Curr Microbiol. 2011;62:871–875.
- Tien M, Kirk TK. Lignin peroxidase of Phanerochaete chrysosporium. Methods Enzymol. 1988;161:238–249.
- Glenn JK, Gold MH. Purification and characterization of an extracellular Mn(II)-dependent peroxidase from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys. 1985;242:329–341.
- Rasera K, Ferla J, Dillon AJP, et al. Immobilization of laccase from Pleurotus sajor-caju in polyamide membranes. Desalination. 2009;245:657–661.
- Baldrian P. Fungal laccases - occurrence and properties. FEMS Microbiol Rev. 2005;30:215–242.
- Leonowicz A, Edgehill RU, Bollag JM. The effect of pH on the transformation of syringic and vanillic acids by the laccases of Rhizoctonia praticola and Trametes versicolor. Arch Microbiol. 1884;137:89–96.
- Mayer AM. Polyphenol oxidases in plants-recent progress. Phytochemistry. 1986;26:11–20.
- Sharma A, Thakur VV, Shrivastava A, et al. Xylanase and laccase based enzymatic kraft pulp bleaching reduces adsorbable organic halogen (AOX) in bleach effluents: a pilot scale study. Bioresour Technol. 2014;169:96–102.
- Ko EM, Leem YE, Choi H. Purification and characterization of laccase isozymes from the white-rot basidiomycete Ganoderma lucidum. Appl Microbiol Biotechnol. 2001;57:98–102.
- Dekker RF, Barbosa AM. The effects of aeration and veratryl alcohol on the production of two laccases by the ascomycete Botryosphaeria sp. Enzyme Microb Technol. 2001;28:81–88.
- Saraiva JA, Tavares APM, Xavier AMRB. Effect of the inducers veratryl alcohol, xylidine, and ligninosulphonates on activity and thermal stability and inactivation kinetics of laccase from Trametes versicolor. Appl Biochem Biotechnol. 2012;167:685–693.
- Kumar VV, Kirupha SD, Periyaraman P, et al. Screening and induction of laccase activity in fungal species and its application in dye decolorization. Afr J Microbiol Res. 2011;5:1261–1267.