Abstract
In 2017, small, elliptical, brownish purple spots on spears and ferns of asparagus were found in fields of Gangwon-do. The isolated fungal species was identified as an ascomycete Stemphylium vesicarium based on morphological characteristics and molecular phylogenic analyses including nucleotide sequences of the internal transcribed spacer (ITS), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and cytochrome b (cytb). A pathogenicity test revealed that S. vesicarium was the causal agent of purple spot disease on asparagus. The occurrence of purple spots caused by S. vesicarium on asparagus is the first report in Korea.
Asparagus (Asparagus officinalis L.) in the family Asparagaceae is a spring perennial vegetable, which is rich in vitamins and minerals [Citation1]. Asparagus also contains anti-inflammatory nutrients and a variety of antioxidant nutrients, including vitamin C, beta-carotene, vitamin E, mineral zinc, manganese, and selenium [Citation2]. Therefore, asparagus has been used as a botanical drug for thousands of years [Citation3]. In recent years, beneficial effects of asparagus on health have been uncovered [Citation4,Citation5]. Its consumption as medicine and food is continuously increased [Citation6]. In 2016, asparagus has produced about 8.73 million tons per year in an area of 1.53 million ha worldwide. China produced about 7.68 million tons, accounting for about 88% of the total world production of asparagus, followed by Peru with 0.38 million tons and Mexico with 0.22 million tons [Citation7]. Asparagus is mainly growing in Gangwon province in Korea, but domestic consumption relies primarily on imports having a marginal domestic production.
Tender young shoots (spears) of asparagus are commonly eaten as a vegetable, emerging out in spring season from underground root system (crown). Asparagus lives for up to 20 years so that good soil preparation before planting and disease management after planting are unavoidable for sustainable cultivation. Fungal diseases of asparagus are reported in many parts of the world, which include anthracnose caused by Colletotrichum gloeosporioides [Citation8], leaf blight by Cercospora asparagi [Citation9], crown-root rot and spear spot by Fusarium oxysporum [Citation10], Fusarium proliferatum [Citation11], gray mold by Botrytis cinerea [Citation12], spear and crown spot by Phytophthora megasperma [Citation13], purple spot by Stemphylium botryosum [Citation14] and Stemphylium vesicarium [Citation15], rust by Puccinia asparagi [Citation16]. Fungal pathogens of asparagus reported in Korea are Cercospora asparagi, Phoma asparagi, F. oxysporum, F. oxysporum f. sp. asparagi, Colletotrichum sp. and B. cinerea [Citation17,Citation18]. Although the incidence and prevalence of fungal disease have increased in asparagus plants in Korea, detailed information for fungal disease, frequency, diagnosis, and management is currently absent together with lack of fungicides and biofungicides applicable to fungal diseases in asparagus.
Among fungal diseases of asparagus, purple spot caused by S. vesicarium becomes a severe problem. Asparagus purple spot develops on emerging spears in spring and occurs ferns in summer destroying stem, branches, and leaves, which result in reduction of the flow of carbohydrates to the roots and lowering next year yields [Citation19,Citation20]. Symptoms of asparagus purple spot are reported to be small (1–2 mm), elliptical, slightly sunken, and brownish purple spots that blemished the spears, damaging marketability during the harvest season [Citation21]. In the wet season during summer of 2017, severe symptoms of purple spots occurred on asparagus ferns in Chuncheon and Yanggu in Gangwon-do, South Korea. The disease appears as numerous, slightly sunken, purplish spots with brown centers occurring on asparagus ferns (). Infected tissues were taken to the laboratory, and isolated 24 strains. Surfaces of infected tissues were sterilized by 1% sodium hypochlorite (NaOCl) for 1 min, washed twice with sterilized distilled water, dried on sterilized filter paper, and placed into plates containing potato dextrose agar (PDA; BD Difco, Franklin Lakes, NJ) supplemented with 100 ppm ampicillin. The plates were then incubated at 25 °C for 5 days in a 16 h light of cool white and 8 h dark chamber, emerged fungal hyphae were transferred to new PDA plates. Single spore isolation was conducted for identification and storage of isolates at −70 °C in 20% glycerol for further study. A representative isolate, KNU1709YG was deposited (CFGR 2018-120-00001) at the Center for Fungal Genetic Resources (CFGR) at Seoul National University, Korea.
Figure 1. Purple spot caused by Stemphylium vesicarium on asparagus. (A) Symptoms of purple spot of asparagus ferns in field; (B,C) Signs of the causal fungus of purple spot on asparagus fern and spear by artificial inoculation; (D) Control of non-inoculation; (E,F) Fungal mycelial colonies on PDA and V8 juice agar plates (left, front view; right, back view); (G–I), Conidiophores; and (J) Morphological characteristics of conidia. Scale bars = 10 µm.

To determine pathogenicity of a representative isolate, KNU1709YG, we performed artificial inoculation on spears and ferns of asparagus. In brief, Conidia of KNU1709YG were harvested from PDA culture plates grown for 7 days. Spears and ferns of asparagus grown in Yanggu were sterilized with 1% NaOCl for 2 min and washed twice with sterilized distilled water. Conidia suspension (1 × 105 conidia/mL) was sprayed on asparagus spears and placed in a humid box at 25 °C. The control was sprayed the distilled water. As a result, tan-to-brown sunken and elliptical early common lesions appeared in the ferns and spears after 7 days (). The symptom was identical to that caused by S. vesicarium, and the fungus was subsequently re-isolated from the lesions. However, no symptom was observed in the control (). This experiment was repeated three times and the same results were obtained.
When the fungus was cultured at room temperature under white fluorescent light with 16 h photoperiod, colors of mycelial colonies appeared light brown and dark green after 7 days on PDA and V8 medium, respectively (,F)). The mycelial growth rate was 6 cm on both PDA and V8 medium for a week. Hyphae were pale brown in color, and 4–7 um in width. Conidiophore was swelling and developed dark-brown conidia (). Conidia were oblong or broadly oval, 22–38 × 13–18 µm in size, having inequilaterally, 1–6 transverse septa and 1–3 longitudinal septa per transverse sector (). The length of the fungal tissues was measured using a Carl Zeiss Axio Imager A2 microscope (Carl Zeiss Microscope Division, Oberkochen, Germany). Morphological characteristics were summarized in . As a result, it was found that KNU1709YG was morphologically identical to the fungus S. vesicarium [Citation22]. The genus Stemphylium was first reported in 1833 [Citation23] with Stemphylium botryosum (Teleomorph: Pleospora tarda) as the type species. Stemphylium is a dematiaceous hyphomycete, which can be distinguished from other hyphomycetes forming phaeodictyo spores based on the percurrent rejuvenation of its conidiophores, and apically swollen conidiogenous cells [Citation24]. Identification of Stemphylium species has relied on morphological characters such as variation in conidium, conidiophore, and ascospore morphology. However, accurate identification of species in Stemphylium is difficult due to overlapped morphological characters. Furthermore, the sexual morph Pleospora of Stemphylium is known to be polyphyletic [Citation25]. Therefore, species identification based exclusively on morphological data was not feasible. Combined morphological and molecular data are necessary for unambiguous identification of species in Stemphylium. Recently, a multi-gene phylogenic analysis of Stemphylium species reveals new species [Citation24].
Table 1. Morphological characteristics of Stemphilium vesicarium isolated in this study.
For a precise species identification, morphological observation of KNU1709YG was combined with molecular analysis. First, genomic DNA of KNU1709YG was extracted using the quick DNA methods [Citation26,Citation27]. Sequence 18s rRNA of internal transcribed spacer (ITS) was amplified using primers ITS1 and ITS4 [Citation28]. Sequence of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was amplified using primers GPD1 and GPD2 [Citation29]. Conditions for PCR amplification were described by Graf et al. [Citation29]. The PCR products were purified according to the MEGA quick-spin total fragment DNA purification kit (iNtRON Bio Technology, Daejeon, South Korea) and sequenced with the same primers. The resulting sequences (GenBank accession numbers MK073013 and MK105974) were compared to all available fungal sequence data in the NCBI-GenBank database using the BLAST search tool [Citation30]. Compared with the sequences of S. vesicarium of GenBank, the analyzed sequence showed 100% homology with the GAPDH sequence (GenBank accession No. KU850710, KU850723, and KU850735), 99% with the ITS sequences (GenBank accession No. KU850563, KU850576, and KU850588). The multi-locus sequences were aligned with closely related strains by ClustalW [Citation31]. The phylogenetic tree was constructed using the neighbor-joining method with 1000 bootstrap replicates by MEGA 7 [Citation32] (). The multi-gene phylogenic tree using two genes (ITS and GAPDH) was very similar to that previously done by Woudenberg et al. [Citation24], suggesting that the isolate KNU1709YG may be S. vesicarium ().
Figure 2. The phylogenetic tree based on combined of ITS and GAPDH gene sequences of Stemphylium vesicarium and other Stemphylium spp. DNA sequences from the GenBank were aligned using the ClustalW program in MEGA 7.0 and constructed using the neighbor-joining method with 1000 bootstrap replicates. The scale bar indicates the number of differences in nucleotide substitutions per sequences.
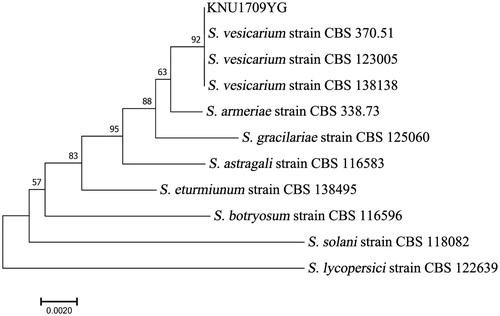
Table 2. Species and sequences used for phylogenetic analyses.
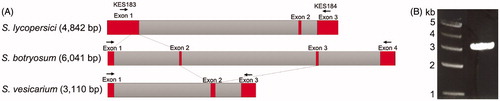
Cytochrome b gene in mitochondrial DNA is commonly used to determine phylogenetic relationships between species due to difference in structure and sequence [Citation33–35]. Two causal agents of the asparagus purple spots, S. vesicarium and S. botryosum, are differentiated with the intron-exon structure of the cytb gene, in which many isolates initially classified as S. botryosum are also identified as S. vericarium in Germany [Citation29]. In Korea, there is a short report that S. botryosum caused the purple spot of asparagus, which suggests that examination of exact identification and abundance of the two species S. botryosum and S. vericariuim in asparagus would be necessary with collections of more isolates in a future study. Stemphylium lycopersici, also known for causing leaf spot in crops [Citation36,Citation37] was revealed to be differentiated from S. vesicarium and S. botryosum, based on the structure of the cytb gene [Citation38] (). By analyzing the sequence and structure of the cytb gene (3 kb) of the isolate KNU1709YG that was amplified with a primer set of KES183 and KES184, the isolate KNU1709YG was revealed to form a clade with S. vesicarium (). Taken together with symptoms, pathogenicity by artificial inoculation, morphology and molecular analysis, we were able to confirm that the isolate KNU1709YG was S. vesicarium and causal agent of purple spot of asparagus. Although purple spot on asparagus caused by S. vesicarium was reported in other countries including Germany and Australia [Citation29,Citation39], the occurrence of asparagus purple spots caused by S. vesicarium in Korea is the first report as we know. Further analyses including pathogenicity, control measures, and genetics of S. vesicarium population will provide an integrated strategy for the management of asparagus purple spot.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Group AP. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc. 2009;161:105–121.
- Veganrus [Internet]. California: Gio; [updated 2013 Nov 14; cited 2018 Sep 27]. Available from: http://veganrus.com/health-benefits-of-asparagus/
- Diwanay S, Chitre D, Patwardhan B. Immunoprotection by botanical drugs in cancer chemotherapy. J Ethnopharmacol. 2004;90:49–55.
- Chin C, Garrison S, Ho C, et al. Functional elements from asparagus for human health. Acta Hortic. 2002;589:233–242.
- Yuan Z, Liu XS. Health function and utilization of asparagus. Food Res Dev. 2008;8:48.
- Wang M, Tadmor Y, Wu QL, et al. Quantification of protodioscin and rutin in asparagus shoots by LC/MS and HPLC methods. J Agric Food Chem. 2003;51:6132–6136.
- Food and Agriculture Organization of the United Nations [Internet]. Rome: FAOSTAT; [cited 2018 Aug 11]. Available from: http://www.fao.org/
- Cheah L, Horlock C, Davis R. Field survey to assess spread of new asparagus diseases in Queensland. N Z Plant Prot. 2003;56:106–108.
- Cooperman C, Jenkins S. Conditions influencing growth and sporulation of Cercospora asparagi and Cercospora blight development in asparagus. Phytopathology. 1986;76:617–622.
- He C, Wolyn D. Potential role for salicylic acid in induced resistance of asparagus roots to Fusarium oxysporum f. sp. asparagi. Plant Pathol. 2005;54:227–232.
- Logrieco A, Doko B, Moretti A, et al. Occurrence of fumonisin B1 and B2 in Fusarium proliferatum infected asparagus plants. J Agric Food Chem. 1998;46:5201–5204.
- Romanazzi G, Feliziani E. Botrytis cinereal (gray mold). Postharvest decay. Amsterdam (Netherlands): Elsevier; 2014. p. 131–146.
- Falloon PG, Falloon L, Benson B, et al. Effect of Phytophthora megasperma var. sojae on yield of Asparagus officinalis. Plant Dis. 1986;70:15–19.
- Leuprecht B. Stemphylium botryosum Wallr. on asparagus. Gesunde Pflanz. 1990;42:187–191.
- Lacy M. Purple spot: a new disease of young asparagus spears caused by Stemphylium vesicarium. Plant Dis. 1982;66:1198–1200.
- Johnson DA. Two components of slow-rusting in asparagus infected with Puccinia asparagi. Phytopathology. 1986;76:208–211.
- Han K, Kim B, Park J, et al. First confirmed report of Cercospora blight of asparagus caused by Cercospora asparagi in Korea. Plant Dis. 2013;97:428–428.
- The Korean Society of Plant Pathology. List of plant diseases in Korea. 5th ed. Seoul (Korea): Korean Society of Plant Pathology; 2009.
- Elena K. Asparagus diseases. Eur J Plant Sci Biotechnol. 2007;1:76–83.
- Hausbeck M, Hartwell J, Byrne J. Epidemiology of Stemphylium leaf spot and purple spot in no-till asparagus. Acta Hortic. 1999;479:205–210.
- Elmer WH. The economically important diseases of asparagus in the United States. Plant Health Prog. 2001;2:1.
- Simmons EG. Perfect states of Stemphylium. Mycologia. 1969;61:1–26.
- Wallroth K. Flora cryptogamica Germaniae. Pars II. Fl Crypt Germ Sect. 1833;2:208–219.
- Woudenberg J, Hanse B, Van Leeuwen G, et al. Stemphylium revisited. Stud Mycol. 2017;87:77–103.
- Simmons E. Perfect states of Stemphylium. II. Sydowia. 1985;38:284–293.
- Chi MH, Park SY, Lee YH. A quick and safe method for fungal DNA extraction. Plant Pathol J. 2009;25:108–111.
- Han JH, Lee HM, Shin JH, et al. Role of the MoYAK1 protein kinase gene in Magnaporthe oryzae development and pathogenicity. Environ Microbiol. 2015;17:4672–4689.
- White TJ, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. Vol. 18. Cambridge (MA): Academic Press Inc;1990. p. 315–322.
- Graf S, Bohlen-Janssen H, Miessner S, et al. Differentiation of Stemphylium vesicarium from Stemphylium botryosum as causal agent of the purple spot disease on asparagus in Germany. Eur J Plant Pathol. 2016;144:411–418.
- Altschul SF, Gish W, Miller W, et al. Basic local alignment search tool. J Mol Biol. 1990;215:403–410.
- Thompson JD, Gibson TJ, Higgins DG. Multiple sequence alignment using ClustalW and ClustalX. Curr Protoc Bioinformatics. 2003;0:2.3.1–2.3.22.
- Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874.
- De Mandal S, Chhakchhuak L, Gurusubramanian G, et al. Mitochondrial markers for identification and phylogenetic studies in insects–a review. DNA Barcodes. 2014;2:1.
- Galtier N, Nabholz B, Glémin S, et al. Mitochondrial DNA as a marker of molecular diversity: a reappraisal. Mol Ecol. 2009;18:4541–4550.
- Yokoyama K, Biswas SK, Miyaji M, et al. Identification and phylogenetic relationship of the most common pathogenic Candida species inferred from mitochondrial cytochrome b gene sequences. J Clin Microbiol. 2000;38:4503–4510.
- Min J, Kim B, Cho K, et al. Grey leaf spot caused by Stemphylium lycopersici on tomato plants. Plant Pathol J. 1995;11:282–284.
- Tomioka K, Sato T. Fruit rot of sweet pepper caused by Stemphylium lycopersici in Japan. J Gen Plant Pathol. 2011;77:342–344.
- Franco MEE, López SMY, Medina R, et al. The mitochondrial genome of the plant-pathogenic fungus Stemphylium lycopersici uncovers a dynamic structure due to repetitive and mobile elements. PLoS One. 2017;12:e0185545.
- Suheri H, Price T. Stemphylium leaf blight of garlic (Allium sativum) in Australia. Austral Plant Pathol. 2000;29:192–199.