Abstract
The genus Macrolepiota (Agaricales, Basidiomycota) is easy to recognize at the genus level because of big, fleshy basidiocarps with squamules covering the pileus; a single or double annulus; and big, thick-walled basidiospores with a germ pore. However, morphological identification is often unreliable in Macrolepiota due to similar morphological features among species. Due to the uncertainty of previous morphological identification in the genus Macrolepiota, it is necessary to re-examine Korean Macrolepiota using molecular data. We re-examined 34 Macrolepiota specimens collected from 2012 to 2018 in Korea using a reverse taxonomic approach, whereby species identification was first done based on the internal transcribed spacer (ITS) region analysis, followed by morphological confirmation. We identified the presence of four species: M. detersa, M. mastoidea, M. procera, and M. umbonata sp. nov. Two species (M. detersa and M. mastoidea) were previously unrecorded from Korea and M. umbonata is a new species. Detailed descriptions of all four species and taxonomic key are provided in this study. Macrolepiota procera and M. umbonata are distributed through the country, but M. detersa and M. mastoidea are distributed only in limited areas. According to our results, the combination of ITS locus and morphology proved to be a robust approach to evaluate the taxonomic status of Macrolepiota species in Korea. Additional surveys are needed to verify the species diversity and clarify their geographic distribution.
1. Introduction
The genus Macrolepiota, with a global distribution, is characterized by big, fleshy basidiocarps with squamules covering the pileus [Citation1]. Because of these noticeable morphological features, Macrolepiota species are easily detected in natural (grasslands, forest clearings) and man-made (gardens, lawns) open spaces. Globally, about 39 species of Macrolepiota have been recognized [Citation2–7]. The type species, M. procera (Scop.) Singer, is an edible mushroom cultivated and sold in some Asian countries [Citation8–10]. Unfortunately, there have been mushroom poisoning cases of mistakenly consuming toxic Chlorophyllum species which are visually similar to edible Macrolepiota species in Asia and North America [Citation11–15].
The taxonomy of Macrolepiota has been studied extensively in Europe and North America based on morphological features [Citation1,Citation16]. Singer [Citation1] characterized Macrolepiota to have a white-to-pink spore print, thick-walled big spores (above 10 µm in length) with a broad germ pore, absence of pleurocystidia, pileus scaly, and movable annulus. Macrolepiota was divided into two sections based on the presence or absence of clamp connections [Citation1]. Later the section Laevistipedes (Pázmány) Bon. was added [Citation17]. However, traditional classification and identification based only on morphology are unreliable because of the morphological disparities according to environmental conditions and developmental stage [Citation18,Citation19] or the morphological similarities of different species that originate from different geographical origin [Citation6]. Applying molecular data to study fungal taxonomy has been crucial in shedding light on the species diversity and phylogenetic relationships among Macrolepiota species.
Johnson [Citation20] suggested that Macrolepiota is not monophyletic based on DNA data from the internal transcribed spacer (ITS) region, the nuclear large subunit rDNA (nLSU), and the mitochondrial small subunit rDNA (mtSSU) region. Vellinga and colleagues established Macrolepiota sensu stricto after the section Laevistipedes was moved to the genus Chlorophyllum based on morphology (hymenidermal pileus covering, smooth stipe, basidiospore with lacking germ pore), ecology (wide distribution in the tropics or a preference for compost heaps [thermophilic or thermotolerant]), phylogeny based on ITS and nLSU [Citation21,Citation22]. Ge et al. [Citation3] proposed a new section, Volvatae Z. W. Ge, Zhu L. Yang & Vellinga, to accommodate the species with a volva within the genus Macrolepiota. Therefore, to date the genus Macrolepiota is partitioned into three sections: Macrolepiota, Macrosporae, and Volvatae. As a result, the genus Macrolepiota in the present sense is characterized by the combination of the following characters: pileal squamules of a trichodermal layer made up of long subcylindric elements, basidiospores with a germ pore, the presence of stipe squamules, and noticeable color bands in mature specimens [Citation2,Citation6,Citation21,Citation22].
Since M. procera was first reported to Korea in 1940 (as Lepiota procera) [Citation23], four other species have been reported in Korea: M. alborubescens, M. molybdites, M. neomastoidea, and M. rhacodes [Citation24]. According to the most recent classification system [Citation22], M. procera is the only Korean species that should remain in the genus Macrolepiota, with the other four species being transferred to the genus Chlorophyllum. In previous Korean studies involving M. procera, identification was based solely on basidiocarp morphology. Molecular approaches have been useful in distinguishing morphologically similar species, discovering new species, and clarifying classification [Citation3–6]. For Macrolepiota several new species have been reported based on molecular data in North America [Citation21], China [Citation3], and Brazil [Citation6]. Due to the uncertainty of previous morphological identification in the genus Macrolepiota, it is necessary to re-examine Korean Macrolepiota using molecular data. In this study, we investigate the species diversity of Macrolepiota in Korea based on molecular data from the ITS locus. Additionally, we provide detailed morphological description of each species.
2. Materials and methods
2.1. Taxon sampling and molecular analysis
A total of 34 Macrolepiota specimens were used in this study. Specimens were collected throughout South Korea from 2012 to 2018 and kept in the Seoul National University Fungus Collection (SFC) and National Institute of Biological Resources (NIBR) ( and ). Due to limited distinguishing morphological features, we applied a reverse taxonomic approach, whereby species identification was first done based on DNA data, followed by morphological confirmation [Citation25].
Figure 1. Distribution maps of Macrolepiota species in Korea. (a); Macrolepiota detersa, (b); M. mastoidea, (c); M. procera, and (d); M. umbonata.
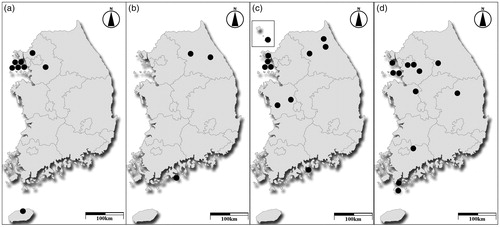
Table 1. Korean Macrolepiota species, collection information, and GenBank accession numbers used in this study.
Genomic DNA was extracted from basidiocarp tissue using a modified CTAB extraction protocol [Citation26]. The ITS region was amplified using the primers ITS1F and ITS4B [Citation27]. PCR amplifications were performed on a thermal cycler (C1000TM; Bio-Rad, Richmond, CA, USA) using the AccuPower PCR premix (Bioneer Co., Daejeon, Korea) following the protocol outlined in Park et al. [Citation28]. PCR products were visualized on a 1% agarose gel and purified using the Expin™ PCR Purification Kit (GeneAll Biotechnology, Seoul, Korea). Sanger sequencing was performed at Macrogen (Seoul, Korea) on an automated DNA sequencer (ABI PRISM 3730XL Analyzer; Applied Biosystems, Foster City, CA, USA) using the aforementioned PCR primers.
ITS sequences for each individual were assembled and proofread using MEGA version 5 [Citation29] and deposited in GenBank (accession numbers in ). All new sequences were aligned using Multiple Alignment Fast Fourier Transform (MAFFT ver. 7) [Citation30] with closely related ITS sequences of Macrolepiota obtained from GenBank. Alignments were checked, and ambiguous positions were adjusted manually. A maximum likelihood (ML) phylogenic analysis was performed in RAxML 8.0.2 [Citation31] implemented on the CIPRES Web portal [Citation32] using a GTRCAT model of sequence evolution and 1000 bootstrap replicates [Citation33]. Leucoagaricus barssii and L. meleagris were selected as outgroup based on a previous study [Citation3].
2.2. Morphological observations
Macromorphological features of studied materials were described based on fresh and dried specimens, comparing with published Macrolepiota data [Citation1,Citation3,Citation6,Citation21,Citation22,Citation34,Citation35]. All color names and alphanumeric codes followed the Methuen Handbook of Colour [Citation36]. Microscopic features were observed under a Nikon Eclipse 80i optical microscope (Nikon, Tokyo, Japan) at either 400× or 1000×. The amyloidity of basidiospores was observed using Melzer’s reagent [Citation37]. Other structures were observed in 3% aqueous KOH, Congo red solution, and cresyl blue.
At least 20 basidiospores, 10 basidia, and 10 cheilocystidia were measured per specimen. In the descriptions of each species presented in this paper, the abbreviation [a/b/c] behind basidiospores and hymenial elements is as follows: a—number observed, b—number of basidiocarps, c—number of collection site. Dimensions for basidiospores and hymenial elements and are presented as minimum–maximum.
3. Results
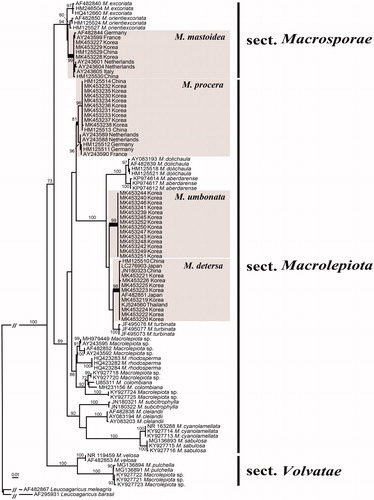
In this study, 34 new ITS sequences of Macrolepiota from Korea were generated and submitted to GenBank (). An additional 68 ITS sequences were retrieved from GenBank and used in constructing the alignment. The final ITS alignment was 696 bp long. The phylogenetic tree based on this ITS dataset separates Korean Macrolepiota specimens into four distinct taxa (). Three taxa were identified based on the clustering with previously reported species (M. detersa, M. mastoidea, and M. procera), while one was distinct from all recognized species and considered a new species. Macrolepiota detersa, M. procera, and the undescribed species (Macrolepiota sp.) belong to the section Macrolepiota, while M. mastoidea belongs to the section Macrosporae (). This is the first report of M. detersa and M. mastoidea in Korea. Macrolepiota spp. forms a distinct lineage with strong support (89%), as sister species to a clade including M. aberdarense, M. detersa, M. dolichaula, and M. turbinata (). Macrolepiota sp. showed sequence similarity of 93.9–94.1% to M. aberdarense, 96.2–96.5% to M. detersa, 94.7–94.9% to M. dolichaula, and 97.1–97.4% to M. turbinata.
Figure 2. Phylogeny of Macrolepiota species based on a maximum-likelihood analysis of the ITS region. Bootstrap values >70% are indicated. The scale bar indicates the number of expected nucleotide substitutions per site. Macrolepiota species found in Korea are denoted by the gray boxes.
The 34 specimens from Korea previously identified morphologically as M. procera have been confirmed as four different species using ITS data, and their detailed morphological characters are provided below.
4. Taxonomy
Macrolepiota detersa Z. W. Ge, Zhu. L. Yang & Vellinga in Fungal Diversity 45: 83 (2010) ( and ).
Figure 3. Basidiocarp (left column), pileal squamules (middle column), and annulus (right column) of Macrolepiota species. (a–d); M. detersa (bar = 5 cm), (e–h); M. mastoidea (bar = 2.5 cm), (i–l); M. procera (bar = 5 cm), and (m–p); M. umbonata (bar = 2.5 cm). Red arrow on the middle and right columns points to the pileal suamules and annulus, respectively.

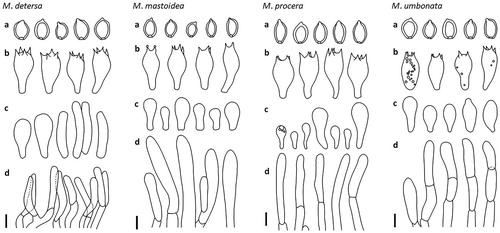
Figure 4. Microscopic features of the Macrolepiota detersa: M. mastoidea, M. procera, and M. umbonata. (a); basidiospores, (b); basidia, (c); cheilocystidia, and (d); hyphae of squamules on pileus. Scale bar = 10 mm.
Basidiocarp medium-sized to large. Pileus 9–15 cm in diam., ovoid to campanulate when young, becoming convex to applanate with age, whitish, covered with scattered, greyish orange (5B4) to brownish orange (6C6) floccus- or crust-like squamules which are easily detachable from the pileus; disc smooth, light brown (6D7). Lamellae free, crowded, white when young, white to pale cream colored when mature, thin, with lamellulae, sometimes with greyish brown spots on the lamellae. Stipe whitish, cylindrical to subcylindrical, 15.0–38.0 × 1.8–3.2 cm, slightly tapering upwards, with tiny brownish scales, hollow; base slightly bulbous. Annulus ascending, superior, about 4–6 cm below stipe apex, whitish, membranous, complex, with brownish squamules on the underside; movable when mature. Context white to whitish, spongy, unchanging when cut.
Basidiospores [40/2/2] 15.0–18.4 × 10.2–12.4 µm, Q = 1.32–1.66, ellipsoid to ovoid, thick-walled, smooth, hyaline, dextrinoid, congophilous, metachromatic in cresyl blue, a disconnection in the episporium on the rounded apex with germ pore, covered with a hyalinous cap in KOH; apiculus about 0.8–1.1 µm long. Basidia [20/2/2] 29.6–37.3 × 12.5–15.0 µm, clavate, thin-walled, hyaline, 4-spored, sometimes 2-spored. Cheilocystidia [20/2/2] 21.2–41.6 × 6.6–13.1 µm, cylindrical to pyriform, sometimes broadly clavate, hyaline, thin-walled. Pleurocystidia absent. Squamules on pileus a palisade of vertically arranged subcylindric, clampless hyphae (20.2–42.2 µm in length, 5.8–13.1 µm in diam.), sometimes shortly septate, rarely branched, with terminal elements slightly tapering toward the tip, with yellowish to brownish vacuolar pigment, slightly thick-walled. Clamp connections very common at the base of basidia and cheilocystidia.
Habitat: Solitary to scattered on ground beside roads or edge of deciduous forest.
Comment: Macrolepiota detersa, compared to other species in the section Macrolepiota, has the most easily detachable pileus squamules. The presence of cylindrical cheilocystidia are distinguishable character of this species.
Macrolepiota mastoidea (Fr.: Fr.) Singer in Lilloa 22: 417. 1951 (“1949”) ( and ).
Basidiocarp medium-sized to large. Pileus 7–15 cm in diam., ovoid to campanulate when young, becoming convex to applanate when mature, with a papilla at disc, white to greyish white, covered with reddish grey (7B3) to light brown (7D4) scaly squamules, which are at first smooth and entire, then gradually break up into irregular patches, and become minute and sparse toward margin; margin appendiculate with the fibrillose veil when young; disc smooth, reddish brown (8E4). Lamellae free, crowded, white to greyish white, with lamellulae. Stipe cylindrical to subcylindrical, 9–25 × 0.8–2.0 cm, tapering upwards, whitish, covered with tiny furfuraceous cream to pale brown squamules; base slightly enlarged or bulbous. Annulus ascending, superior, about 3–5 cm below stipe apex, simple, whitish, membranous. Context whitish, not changing color when cut.
Basidiospores [40/2/2] 11.2–14.4 × 8.0–10.4 µm, Q = 1.31–1.67, ellipsoid to ovoid, thick-walled, smooth, hyaline, dextrinoid, congophilous, metachromatic in cresyl blue, a disconnection in the episporium on the rounded apex with germ pore, covered with a hyalinous cap in KOH; apiculus 1.2–1.4 µm long. Basidia [28/2/2] 33.0–39.7 × 11.6–13.8 µm, clavate, thin-walled, hyaline, rarely with small granules and bubble-like contents, 4-spored. Cheilocystidia [20/2/2] 15.6–25.0 × 6.1–11.3 µm, clavate, hyaline, thin-walled, in bunches forming a sterile edge. Pleurocystidia absent. Squamules on pileus a palisade of cylindric to subcylindric, clampless hyphae (36.9–71.9 µm in length, 8.2–10.9 µm in diam.), with terminal elements slightly tapering toward the tip, with yellowish to brownish vacuolar pigment, slightly thick-walled. Clamp connections occasionally observed at the base of basidia, not observed at the base of cheilocystidia.
Habitat: Solitary or scattered on ground in open meadows or edge of forest.
Comment: Macrolepiota mastoidea is only a species belonging to the section Macrosporae in Korea. This species is easily distinguished from other species in Korea by simple annulus and absence of clamp connection at the base of cheilocystidia.
Macrolepiota procera (Scop.: Fr.) Singer in Papers Mich. Acad. Sci., Arts Letters 32: 141. 1948 (“1946”) ( and ).
Basidiocarp medium-sized to large. Pileus 10– 21 cm in diam., ovoid when young, becoming convex to plano-convex with age, with an umbo at disc, white to greyish white, covered with brownish grey (5C2), light brown (6D4) to dark brown (7F5) thin squamules; disc smooth, dark brown (6F5 to 7F5); covering easily peelable, thin squamules which are irregularly arranged toward margin on the greyish brown speckled with whitish background. Lamellae free, crowded, thin, white when young, pale cream colored when mature, with lamellulae. Stipe whitish, subcylindrical, 16.5–28.0 × 1.2–2.0 cm, tapering upwards, at base enlarged or bulbous, covered with brown to dark brown speckled squamules, sometimes hollow. Annulus superior, about 4–5 cm below stipe apex, upper side white, underside grayish brown, membranous, complex, moveable when mature. Context spongy, whitish at the pileus, grayish red to grayish brown at the stipe.
Basidiospores [40/2/2] 12.6–16.8 × 7.8–10.2 µm, Q = 1.35–1.70, ellipsoid to ovoid, thick-walled, smooth, hyaline, dextrinoid, congophilous, metachromatic in cresyl blue, a disconnection in the episporium on the rounded apex with germ pore, covered with a hyalinous cap in KOH; apiculus not distinctive. Basidia [33/2/2] 34.5–40.3 × 14.7–17.2 µm, clavate, thin-walled, hyaline, 4-spored. Cheilocystidia [25/2/2] 11.5–35.5 × 5.0–14.5 µm, clavate to subclavate, hyaline, thin-walled, massed forming a sterile edge. Pleurocystidia absent. Squamules on pileus a palisade of subcylindric to cylindric, slightly thick-walled, clampless hyphae which are 8–13.5 µm in diam., seldom branched, with terminal elements slightly attenuate toward the tip, with yellowish vacuolar pigment, slightly thick-walled. Clamp connections common both of basidia base and cheilocystidia base.
Habitat: Solitary or scattered on ground in open meadows or edge of forest.
Comments: Macrolepiota procera can be distinguished from species in the same section by thin squamules on pileus. In addition, the background of the pileus surface is darker greyish color than other Macrolepiota species distributed in Korea.
Macrolepiota umbonata H. J. Cho, H. Lee & Y.W. Lim, sp. nov ( and ).
MycoBank: MB829835
Diagnosis: Macrolepiota umbonata is characterized by a large basidiocarp, big and knob-like umbo at disc, long and brownish fragmented stipe, superior and movable annulus, and squamules on pileus composed of subcylindrical, clampless yellowish brown hyphae.
Type:—SOUTH KOREA. Incheon-si, Ongjin-gun, Yeongheungdo island, 72 m elev., N37°16'12′′ E126°27'35′′, Nam Kyu Kim, Jae Young Park, 9 September 2016, SFC20160909-16 (Holotype, SFC!)
Basidiocarp large-sized. Pileus 12– 25 cm in diam., white to whitish, convex to plano-convex, sometimes applanate with slightly reflexed margin, umbonate to knobbed at disc, covered with brownish orange (5C3) to light brown (6D5) floccus-like squamules, which become sparse toward margin, revealing a white flesh between them; margin occasionally crenulate, the fibrillose veil when young; disc smooth, dark brown (6F8). Lamellae free, crowded, white when young, cream to greyish cream colored when mature, sometimes orange brown to greyish brown tinge, thin, with lamellulae. Stipe whitish, subcylindrical, 15–40 × 1–3 cm, slightly tapering upwards, orange brown to greyish brown fragmented bands, hollow; base club shaped to bulbous, 3.5–4.5 cm wide. Annulus ascending, superior, about 3–4 cm below stipe apex, whitish, membranous, slightly complex, with brownish patchy squamules on the underside; movable when mature. Context white to whitish, spongy, unchanging color when cut, but sometimes at edge of stipe with pinkish tinge.
Basidiospores [60/3/3] 11.8–16.6 × 9.2–12.0 µm, Q = 1.17–1.50, broadly ellipsoid to ellipsoid, thick-walled, smooth, hyaline, dextrinoid, congophilous, metachromatic in cresyl blue, a disconnection in the episporium on the rounded apex with germ pore, covered with a hyalinous cap in KOH; apiculus not distinctive, about 0.8–1 µm long. Basidia [42/3/3] 26.0–35.1 × 12.3–15.6 µm, clavate, thin-walled, hyaline, sometimes with small granules and bubble-like contents, 4-spored. Cheilocystidia [30/3/3] 12.6–35.2 × 5.3–13.8 µm, mostly clavate to subclavate, occasionally obtusely fusiform, hyaline, thin walled, in bunches forming a sterile edge. Pleurocystidia absent. Squamules on pileus a palisade of cylindric to subcylindric, clampless hyphae (7–31 µm in length, 6–11 µm in diam.), rarely branched, terminal elements slightly tapering toward the tip, yellowish to brownish vacuolar pigment, slightly thick-walled. Clamp connections occasionally observed at the base of basidia, common at the base of cheilocystidia.
Habitat: Solitary or scattered on ground in open meadows or edge of forest.
Etymology: “umbonata” means umbonate pileus which refers to the big and knob-like umbo of this species.
Additional studied material: SOUTH KOREA. Seoul, Gwanak-gu, Seoul National University, 101 m elev., N37°27′27′′ E126°56′58′′, Hyun Lee, Young Woon Lim, 3 September 2012, SFC20120903-06 (Paratype SFC!); SOUTH KOREA. Gyeongsangbuk-do, Yecheon-gun, Hakgasan Natural Recreation Forest, 367 m elev., N36°40′31′′ E128°35′31′′, Hyun Lee, Won Ju Kim, 17 September 2013, SFC20130917-H01 (Paratype SFC!); SOUTH KOREA. Gyeonggi-do, Gwangju-si, Taehwasan University Forest, 159 m elev., N37°18′42′′ E127°18'41′′, Hyun Lee, Hae Jin Cho, 19 August 2015, SFC20150819-27 (Paratype SFC!); SOUTH KOREA. Incheon-si, Ongjin-gun, Jangbongdo island, 105 m elev., N37°31'57′′ E126°21′05′′, Nam Kyu Kim, Jae Young Park, 6 September 2016, SFC20160906-05 (Paratype, SFC!); Ibid., Yeongheungdo island, 81 m elev., N37°15′26′′ E126°28′09′′, Nam Kyu Kim, Jae Young Park, 9 September 2016, SFC20160909-01 (Paratype, SFC!).
Comment: Macrolepiota umbonata belongs to the Macrolepiota section Macrolepiota and is morphologically similar to M. detersa, M. dolichaula, and M. procera. Macrolepiota detersa is distinguished from M. umbonata by its small basidiocarp (9–15 cm) and cylindrical cheilocystidia. Macrolepiota umbonata differs from M. dolichaula by having a simple annulus [Citation3]. Pileus squamule is a character that distinguishes M. umbonata from M. procera: the former has floccus-like squamules while the latter has thin pileus squamules.
Key to the recognized species of Macrolepiota from Korea
Annulus simple; clamp connections lack at the base of cheilocystidia M. mastoidea
Annulus complex; Clamp connections present at the base of basidia and cheilocystidia 2
Squamules on the pileus thin and greyish brown M. procera
Squamules on the pileus floccus and light brown to dark brown 3
Cheilocystidia subclavate to clavate M. umbonata sp. nov.
Cheilocystidia manily cyilindrical to pyriform M. detersa
5. Discussion
Macrolepiota procera is easily recognized in the field due to its large basidiocarp and conspicuous pileal squamules. For this reason, there have been little attempts to clarify identity in Korea using microscopic features or sequence analysis. Surprisingly, results of our ITS analysis of 34 specimens previously identified morphologically as M. procera revealed that there are three additional species in Korea besides M. procera. Two species, M. detersa and M. mastoidea, are new records to Korea. Macrolepiota sp. is morphologically similar to other species in the section Macrolepiota, but clearly differentiated from them based on phylogenetic analysis of the ITS locus. We named this species as M. umbonata because it is characterized by umbonate to knobbed pileus center.
The four Korean Macrolepiota species are members of two sections: Macrolepiota and Macrosporae. The section Macrolepiota is characterized by the pileus that forms big plate-like squamules, a complex annulus, stipe usually two to three times the pileus diameter with fine brown squamules, relatively big ovoid-ellipsoid basidiospores (usually 14– 20 µm), common presence of clamp connections at the base of the cheilocystidia and basidia, and mainly broadly clavate cheilocystidia [Citation1,Citation3,Citation17]. Macrolepiota detersa, M. procera, and M. umbonata are included in the section Macrolepiota and share common characters of this section. However, M. detersa primarily has cylindrical cheilosystidia, with clavate cheilocystidia occurring occasionally. The section Macrosporae is characterized by a smooth stipe, simple annulus, rare clamp connections, furfuraceous fine squamules composed of a single layer with rarely branched, pale brownish, and thin-walled cylindrical hyphae [Citation1,Citation3]. Macrolepiota mastoidea, in the section Macrosporae, has a simple annulus, tiny furfuraceous cream to pale brown squamules, smaller basidiospore 11.2–14.4 × 8.0–10.4, and no clamp connection at the base of cheilocystidia. However, clamp connections at the base of basidia are occasionally observed.
Macrolepiota procera and M. mastoidea are known as European species [Citation38,Citation39] and distributed worldwide. European and Asian populations are similar based on basidiocarp morphology, but differ slightly genetically. For both species, Korean samples show a closer relationship to other Asian samples compared to European samples (). Similarly, Ge et al. [Citation3] found that Chinese M. procera differ European samples in the phylogeny, but exhibit no distinct morphological characters. Since M. detersa was reported as a new species from China [Citation3], it has also been discovered to occur in Korea, Japan, and Thailand. Therefore, M. detersa should be regarded as a species restricted to Asia.
In conclusion, we confirmed the presence of four Macrolepiota species in Korea using a reverse taxonomic approach. Macrolepiota procera and M. umbonata are distributed widely through the country, but M. detersa and M. mastoidea are distributed only in limited areas (). These results were obtained from a limited number of specimens collected in a short period of time. Additional surveys are needed to verify the species diversity and clarify their geographic distribution.
Acknowledgements
The authors thank the two anonymous reviewers for their valuable and detail comments.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Singer R. The Agaricales in modern taxonomy. 4th ed. Koenigstein: Koeltz Scientific Books; 1986.
- Kirk PM, Cannon PF, Minter DW, et al. Dictionary of the fungi. 10th ed. Wallingford: CABI; 2008.
- Ge ZW, Zhu LY, Vellinga EC. The genus Macrolepiota (Agaricaceae, Basidiomycota) in China. Fungal Divers. 2010;45(1):81–98.
- Lebel T, Syme A. Sequestrate species of Agaricus and Macrolepiota from Australia: new species and combinations and their position in a calibrated phylogeny. Mycologia. 2012;104(2):496–520.
- Otieno DO. Macrolepiota aberdarense, a new edible mushroom from Kenya. Curr Res Environ Appl Mycol J Fungal Bio. 2018;8:247–253.
- Fazolino EP, Suaza Blandón SC, Alves–Silva G, et al. Taxonomy and phylogeny of Macrolepiota: two new species from Brazil. Mycologia. 2018;110(5):930–940.
- Ge ZW, Chen ZH, Yang ZL. _Macrolepiota subcitrophylla sp. nov., a new species with yellowish lamellae from southwest China. Mycoscience. 2012;53(4):284–289.
- Ding ZQ, Huang SZ. Characteristics and high–yield culture technique of Macrolepiota procea. Edible Fungi. 2003;4:33. Chinese.
- Kwon H, Thatithatgoon S. Mushroom growing in Northern Thailand. In: Mushroom Growers’ Handbook 1: Oyster Mushroom Cultivation. Seoul, Korea: MushWorld; 2004.
- Shim SM, Oh YH, Lee KR, et al. The characteristics of cultural conditions for the mycelial growth of Macrolepiota procera. Mycobiology. 2005;33(1):15–18.
- Bon M, Vallée L, Jacob M. Une nouvelle lépiote toxique: Macrolepiota venenata Bon sp. nov. Documents mycologiques. 1979;9:13–21.
- Yokoyama K, Yamaji D. Poisoning by Lepiota neomastoidea. Trans Mycol Soc Jpn. 1981;22:255–258.
- Mazzolai I. Intossicazioni da Macrolepiota venenata Jacob ex Bon. Riv Micol. 1989;32:264–265.
- Lehmann PF, Khazan U. Mushroom poisoning by Chlorophyllum molybdites in the Midwest United States. Mycopathologia. 1992;118(1):3–13.
- Kim SY, Baek YH, Han SY, et al. Mushroom poisoning by Macrolepiota neomastoidea. Kor J Gastroenterol. 2018;71(2):94–97.
- Candusso M, Lanzoni G. Fungi Europaei 4. Lepiota sl. Saronno: Giovanna Biella; 1990.
- Bon M. European flora of large mushrooms 3. Lepiotaceae (translated and edited by F. Medjebeur–Thrun, WU Thrun). IHW–Verlag: Eching; 1996. German.
- Jennings DH. Morphological plasticity in fungi. Symp Soc Exp Biol. 1986;40:329–346.
- Kües U, Navarro-Gonzalez M. How do Agaricomycetes shape their fruiting bodies? 1. Morphological aspects of development. Fungal Biol Rev. 2015;29(2):63–97.
- Johnson J. Phylogenetic relationships within Lepiota sensu lato based on morphological and molecular data. Mycologia. 1999;91(3):443–458.
- Vellinga EC, Rogier PJ, Bruns TD. Phylogeny and taxonomy of Macrolepiota (Agaricaceae). Mycologia. 2003;95(3):442–456.
- Vellinga EC. Chlorophyllum and Macrolepiota (Agaricaceae) in Australia. Aust Systematic Bot. 2003;16(3):361–370.
- Kaburagi Y. Korea forest experiment station. Korean and Manchurian practical manual of forest. Tokyo: Yokendo; 1940.
- Lee YS, Lim YW, Kim JJ, et al. Korean Society of Mycology. National list of species of Korea: Basidiomycota. Incheon: National Institute of Biological Resources; 2015.
- Markmann M, Tautz D. Reverse taxonomy: an approach towards determining the diversity of meiobenthic organisms based on ribosomal RNA signature sequences. Philos Trans R Soc Lond, B, Biol Sci. 2005;360(1462):1917–1924.
- Rogers SO, Bendich AJ. Extraction of total cellular DNA from plants, algae and fungi In: Gelvin SB, Schilperoort RA, editors. Plant molecular biology manual. Boston (MA): Kluwer Academic Publishers; 1994. p. 183–190.
- Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes‐application to the identification of mycorrhizae and rusts. Mol Ecol. 1993;2(2):113–118.
- Park MS, Fong JJ, Lee H, et al. Delimitation of Russula subgenus Amoenula in Korea using three molecular markers. Mycobiology. 2013;41(4):191–201.
- Tamura K, Peterson D, Peterson N, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739.
- Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–780.
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post–analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–1313.
- Miller MA, Pfeiffer W, T S. Creating the CIPRES science gateway for inference of large phylogenetic trees. SC10 workshop on gateway computing environments (GCE10); Nov 13–19; New Orleans (LA): IEEE Computer Society; 2010. p. 1–8.
- Stamatakis A. Phylogenetic models of rate heterogeneity: a high performance computing perspective. In Proceedings 20th IEEE International Parallel & Distributed Processing Symposium; 2006. Apr 25–29; Rhodes Island: IEEE; 2006. p. 278.
- Breitenbach J, Kränzlin F. Fungi of Switzerland, Vol. 4. Agarics 2nd part. Lucerne: Verlag Mykologia; 1995.
- Vellinga EC. Macrolepiota In: Noordeloos ME, Kuyper TW, Vellinga EC, editors. Flora agaricina neerlandica, Vol. 5. Lisse, Abingdon, Exton (PA), Tokyo: A.A. Balkema Publishers; 2001. p. 64–73.
- Kornerup A, Wanscher JH. Methuen handbook of colour. 3rd ed. London: Eyre Methuen Ltd.; 1978.
- Largent D, Johnson D, Watling R. How to identify fungi to genus III: microscopic features. CA: Mad River Press; 1977.
- Scopoli JA. Flora carniolica (in Latin). 2nd ed. Vienna: K.P. Krause; 1772.
- Singer R. New and interesting species of Basidiomycetes. II. Paper Michigan Acad Sci. 1948;32:103–150.
