Abstract
Recently, Cryptococcus pseudolongus has been reported as a new pathogen of shiitake (Lentinula edodes). However, its pathological properties are not much known. To further understand its impact on the mushroom, we investigated the pathogen’s interactions with the mycelium of shiitake, histopathological properties, host range, and sensitivity to diverse antifungal agents. The strain C. pseudolongus DUCC 4014 inhibited the mycelial growth of L. edodes strain (cultivar Sanjo 701ho) and caused browning in the mycelia confronted with the yeast on PDA. Spray inoculation of the yeast caused an abnormal browning symptom on the cap and/or gills of three shiitake cultivars grown on sawdust media in vinyl bags. Scanning electron microscopic images of the abnormally browned parts of shiitake fruit body illustrated that mushroom tissues were loosed and dispersed in the middle and edge of the cap and the arrangement of basidiospores borne on basidia in the gills was disturbed compared to those of normal shiitake fruit body. Spray inoculation also led to developing abnormal browning on the harvested fruit body, indicating C. pseudolongus could be a problem during mushroom storage. But the yeast was not able to induce abnormal browning on mushrooms of Pleurotus ferulae, Pleurotus fostreatus, and Agaricus bisporus. But it induced browning only on button mushroom (A. bisporus) when they were inoculated after wounding. Tests with 16 kinds of fungicides revealed that the cell growth of C. pseudolongus could be inhibited by benzalkonium chloride at MIC 7 μg/ml and benomyl at MIC 3 μg/ml.
1. Introduction
The white rot basidiomycete Lentinula edodes forms an edible fruit body which is called oakwood mushroom or shiitake [Citation1]. As a native species distributed in warm and moist climates in East Asia, this mushroom has been cultivated and consumed as a staple food in many East Asian countries [Citation2]. It has attractive features such as meaty and savory flavor which makes it a favorable item in grocery stores and food markets. Regarding nutrient factors, it contains various nutrients such as crude protein, glycolipid, ash, carbohydrate, fiber, and amino acids [Citation3,Citation4]. Thus, it has been used in various mushroom foods, mushroom snacks, rich fermented milk, mushroom soup salads, and meat dishes. The cultivation of shiitake has become more stable with the development of sawdust-based media, and its commercial production has been increased. Now as the world’s leading cultivated edible mushroom its world’s supply reaches about 22% [Citation5]. Considering that 85% of the world’s total supply of cultivated edible mushrooms is from Lentinula, Pleurotus, Auricularia, Agaricus, and Flammulina, its production amount greatly is contributing to mushroom food supply.
Shiitake has also been broadly used as a medicinal material in traditional oriental medicine. Its consumption has been recommended for longevity and good health. Its medicinal and therapeutic value was introduced in the report of Jong and Birmingham [Citation6]. They indicated the β-D-glucan-based polymer lentinan acted as a host defense potentiator of T-cell activity, suggesting that the bioactive compound in shiitake is a potent antitumor compound that is now commercially available for clinical use. Along with the spotlighting researches of lentinan, researches have performed to search physiologically active ingredients for antitumor, lowering blood pressure and blood sugar, and antithrombosis [Citation7,Citation8]. In 2015, improved human immunity was found in a research that 52 young adults consumed oakwood mushroom regularly [Citation9].
Fungal diseases could reduce the quality and yields of shiitake [Citation10]. Sometimes fungal diseases threaten mushroom farming because they could destroy or damage seriously the mushroom from the spawn stage to the cultivation stages. Trichoderma, Diatrype, and Hypoxylon are problematic ascomycete genera during shiitake cultivation in Korea [Citation11]. Recently, several green mold species of Trichoderma and Gliocladium genera associated with mushroom fly damaged shiitake during cultivation on oak logs in the domestic environment [Citation12–14]. In a previous survey work of greenhouse diseases in shiitake mushroom, we found a new disease of brown rot caused by the basidiomycete yeast Cryptococcus pseudolongus [Citation15]. Typical symptoms of the disease included dark discoloration and its incidence was nearly 20% on two mushroom farms where sawdust media was used for cultivation. Thus, the pathogen’s emergence has brought concerns on shiitake production industry. For the development of disease management methods, the pathogen’s biology needs to be fully understood. However, biological information on the pathogen is very limited. Therefore, in this study, we further examined the pathological properties of the pathogen. For this aim, the pathogen’s interactions with the mycelium of shiitake, histopathological properties, host range, and sensitivity to diverse antifungal agents were investigated. Additionally, we examined whether other related Cryptococcus spp. could cause the brown rot disease.
2. Materials and methods
2.1. Fungal strains and mushroom
The brown rot pathogen Cryptococcus pseudolongus DUCC 4104 [Citation15] and three other Cryptococcus yeast species, C. albicosimilis (currently Naganishia albicosimilis CBS 7711), C. albidus (currently Naganishia albida CBS 142), C. humicola (currently Vanrija humicola CBS 571) were used in the present study. These Crytococcus strains were maintained on yeast malt agar (YMA) (Thermo Fisher Scientific, Seoul, Korea) at 25 °C for 14 days. Since these strains are morphologically similar, the identity of these species was further confirmed by sequencing the 28S rDNA sequences [Citation16]. For the preparation of fungal inoculum, each strain was grown in yeast malt broth (YMB) (Thermo Fisher Scientific, Seoul, Korea) for 3 days at a shaker at 25 °C and its cell concentration was adjusted to 106 cells/ml. The cell number was counted using a hemocytometer by an optical microscope (Axioskop40, Carl Zeiss, Göttingen, Germany). Three shiitake strains of Sanjo 701ho, Youjiro, and Chamaram cultivars were maintained on PDA at 25 °C for 15 days. Cultivation of these mushrooms was undertaken on the oak sawdust substrate as described by Noh et al. [Citation17].
2.2. Interactions between C. pseudolongus and shiitake mycelium
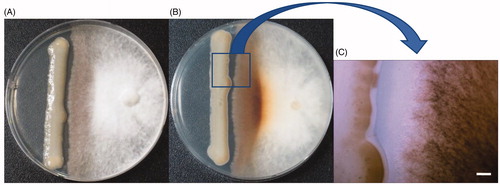
An agar plug (5 mm diameter) of Sanjo 701ho strain grown on PDA was inoculated on the right side of a PDA plate and grown at 25 °C for 7 days. YMA grown C. pseudolongus DUCC 4014 cells were inoculated with a sterile loop by streaking linearly on the left side of the PDA plate with the 7 days-grown Sanjo 701ho strain. This dual culture was incubated at 25 °C for 7 days in darkness. The presence of mycelial inhibition by C. pseudolongus DUCC 4014 was monitored 2 days intervals. The production of brown pigment in the mycelium of Sanjo 701ho was recorded by visual observation.
2.3. Inoculation test of C. pseudolongus DUCC 4014 to shiitake cultivars and other mushrooms
The fruit bodies of three shiitake strains of Sanjo 701ho, Youjiro, and Chamaram cultivars grown on plastic vinyl bags filled with oak sawdust media were inoculated by C. pseudolongus DUCC 4014 cell suspension (1 × 106 cells/ml) using a sprayer and the wound and drop method described in a previous study [Citation15]. Sterile distilled water was sprayed as a control inoculation. The yeast inoculated shiitake fruit bodies were incubated at 25 °C for 7 days in humid condition. The browning of the cap and gill in the fruit body was recorded by visual observation. The pathogen was reisolated from the browned lesions.
Inoculation test of C. pseudolongus DUCC 4014 was extended to the fruit bodies of three other mushroom species including button mushroom (Agaricus bisporus, cultivar 201ho) and oyster mushrooms (Pleurotus ferulae cultivar Mushmaru, Pleurotus ostreatus cultivar Suhan 1ho). These mushrooms were cultivated in two local commercial farms until their fruit bodies developed enough for selling in food markets. Freshly developed fruit bodies in media bags were transferred to our laboratory and directly used for the inoculation test. Wound inoculation applied to the gills of P. ferulae and the caps of A. bisporus and P. ostreatus. These inoculated fruit bodies were incubated at 25 °C for 7 days in a humid condition and their browning in the cap and gill of the fruit body was recorded by visual observation.
2.4. Microscopic analyses by a stereo light microscope and SEM
Further observation of tissues in the wound inoculated parts in the three shiitake cultivars was performed after cross-sectioning of the cap parts with a sterile blade. The presence of browning in the inoculated tissues was observed by a stereomicroscope (SZ61, Olympus, USA) and microscopic images were documented. The C. pseudolongus DUCC 4014 inoculated and non-inoculated (control) fruit bodies were also subject to scanning electron microscopy (SEM) to observe comparatively the microstructures of the surface layer and the edge of the mushroom cap and the basidia and basidiospores developed and aligned on gills. For SEM image analysis, the subject samples were prefixed with 1% osmium tetroxide, prepared as described by Kim et al. [Citation12], and observed by SEM (S-4300 Hitachi, Tokyo, Japan).
2.5. Pathogenicity test to shiitake with other Cryptococcus species taxonomically related with C. pseudolongus
Three Cryptococcus species, C. albicosimilis (CBS 7711), C. albicus (CBS 142), C. humicola (CBS 571), were shaking-cultured in SMB at 25 °C for 3 days. Their cell suspensions were prepared to the concentration of 1 × 106 cells/mL. Shiitake cultivar Chamaram was used for the inoculation host. The caps and gills of the fruit bodies of Chamaram were inoculated with the prepared cell suspension of the three Cryptococcus species using the spray and wounding methods as in section 2.3. After incubation at 25 °C for 7 days in a humid condition, the development of the browning symptom on the cap and gills was observed by naked eyes and a stereomicroscope.
2.6. C. Pseudolongus growth inhibitory assay by antifungal agents
To select antifungal agents and minimum inhibitory concentration (MIC) that inhibit the growth of C. pseudolongus, tebuconazole, benzimidazole, difenoconazole, hexaconazole, tricyclazole, fluquinoconazole, fludioxonil, fenarimol, dichloran, fluxilazole, acetic acid, sodium hypochlorite, Ace, Daisen-M, benzalkonium chloride, and benomyl were tested. The concentrations of these antifungal agents in YMA were adjusted to 10 µg/ml, 100 µg/ml, and 1000 µg/ml, respectively. 10 µl of C. pseudolongus suspension at five different concentration (1 × 103 cells/ml, 1 × 104 cells/ml, 1 × 105 cells/ml, 1 × 106 cells/ml, and 1 × 107 cells/ml) was spotted onto the YMA which contains each antifungal agent at three different concentrations. An agent that inhibited the cell growth of C. pseudolongus was selected as an antifungal agent that can be used for prevention. To get the MIC of the selected antifungal agent, its concentration was evaluated at 3 µg/ml, 5 µg/ml, 7 µg/ml, and 9 µg/ml. Along with the antifungal activity test, the selected antifungal agents were tested on MEA to check their potential to inhibit the growth of the mycelium of Chamaram strain.
3. Results
3.1. Interactions between C. pseudolongus and shiitake mycelium
Since the yeast pathogen C. pseudolongus DUCC 4014 causes brown rot on shiitake fruit body [Citation15], it is assumed that it may cause inhibition and/or browning of shiitake mycelia on media plate. But this assumption has not been proved. Thus, to confirm this assumption, the yeast was dual cultured on PDA with Sanjo 701ho, one of the popular shiitake cultivars used for commercial production in Korea. The shiitake mycelia did not grow over the yeast colony, indicating that its growth was retarded by C. pseudolongus. Also, brown pigmentation appeared on the shiitake mycelia which was confronted with the pathogen (). The degree of brown pigmentation in the shiitake mycelia was thicker in the bottom side of the media than the top side of the media (). Interestingly, browning also appeared on C. pseudolongus cells which were confronted with the mushroom mycelia (). These results suggest that there are interactions between the yeast pathogen and the shiitake strain. From the results of , we could conclude that C. pseudolongus owns the ability to deter the growth of shiitake mycelia.
3.2. Inoculation test of C. pseudolongus DUCC 4014 to shiitake cultivars and other mushrooms
To obtain information on the range of host that C. pseudolongus could cause the abnormal browning symptom, an inoculation test performed against both shiitake cultivars and other mushroom species.
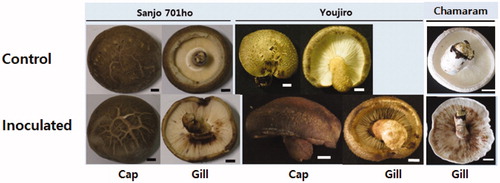
Regarding shiitake cultivars, inoculation tests were undertaken to two domestic cultivars, Sanjo 701ho and Chamaram, which have been cultivated for more than ten years after their breeding, and one foreign cultivar, Youjiro, which has also been cultivated for more than ten years after its introduction. There was no change in the fruit bodies of sterile water sprayed fruit bodies of the three shiitake cultivars (control). But the spray inoculation of the C. pseudolongus DUCC 4140 cell suspension induced the development of the browning symptom on the gills of the three tested shiitake cultivars (). Browning was more apparent on the gills of Chamaram than those of Sanjo 701ho and Youjiro. Compared to the gills, browning was not apparent on the surface of caps. Compared to the control, there was just a little bit darkening in the caps of Sanjo 701ho and Youjiro () and Chamaram (data not shown). The inoculated C. pseudolongus was reisolated from the browned lesions of the three shiitake cultivars. These results demonstrate that C. pseudolongus can cause discoloration on fruit bodies of the three shiitake cultivars. Consequently, we assume C. pseudolongus is not likely to restrict its effect to a certain cultivar shiitake. Spray inoculation also led to developing abnormal browning on the harvested fruit body, indicating C. pseudolongus could be a problem during mushroom storage (Data not shown).
Figure 2. Inoculation test of Cryptococcus pseudolongus DUCC 4014 to three shiitake cultivars using a spray method. Sterile distilled water was used for the inoculum of the control test. The cell concentration of 1 × 106 cells/ml was used for the inoculation. Scales: 10 mm.
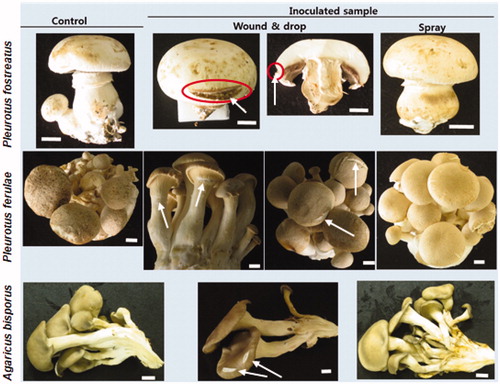
The cell suspension spray of the C. pseudolongus DUCC4140 did not cause the browning symptom on the fruit bodies of three mushroom species A. bisporus, P. ferulae and P. ostreatus (). Thus, wound inoculation was tried. No browning symptom was shown on the fruit bodies of P. ferulae and P. ostreatus (). In the fruit bodies of A. bisporus, browning was observed but it was confined to the scarred lesion. The inoculated C. pseudolongus was reisolated from the scarred lesion.
Figure 3. Inoculation test of Cryptococcus pseudolongus DUCC4014 to three different mushrooms (Agaricus bisporus, Pleurotus ferulae, Pleurotus ostreatus) using spray and wound & drop methods. The cell concentration of 1 × 106 cells/ml was used as inoculum. White arrows indicate the wounded position on the cap of the mushroom fruit bodies. Red circles indicate browning of the cut-inoculated area of the mushroom cap. Scales: 10 mm.
3.3. Microscopic analyses by a stereo light microscope and SEM
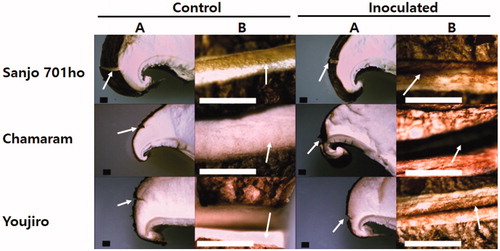
Because there is no information on the structural properties of the abnormal browning symptom developed on the tissues of shiitake fruit bodies, we further analyzed using microscopes. Stereo microscopic images of the C. pseudolongus DUCC 4014 inoculated parts on the cap of three different shiitake cultivars were shown in . The tissues on the cap cut with a sterile blade did not show an apparent color change in all three shiitake cultivars (control). Besides, a drop of sterile water also did not induce any change. Light reflection of the cut area was shown brightly. This result indicates wounding itself is not likely to induce a remarkable response from the shiitake fruit bodies. While spotting a drop of the C. pseudolongus DUCC 4014 cell suspension to the wounded area led to browning across the tissues on the cap in all three shiitake cultivars. These obvious changes are compared between the control and inoculated fruiting bodies. C. pseudolongus certainly interacts with the mycelial cells in the fruit bodies of shiitake host as it did with shiitake mycelia in .
Figure 4. Stereo microscopic images of the Cryptococcus pseudolongus DUCC 4014 inoculated parts on the cap of three different shiitake cultivars. (A) Image of a part of the dissected mushroom cap with a wounded area; (B) Enlarged image in the cut-wounded area with a blade. Arrows in the A column indicate the wounded position on the cap of the mushroom fruit bodies. Arrows in the B column indicate inside of the cut-wounded area with a blade. Inoculation was performed after wounding with a blade cut. A drop of cells (1 × 106 cells/ml) was inoculated onto the wounded area. Scales: 1 mm.
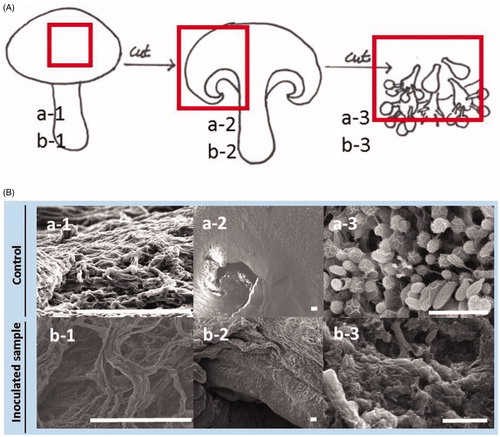
To understand these changes at the level of microstructures, the surface of the cap, the edge part of the cap, and gill in the fruit bodies were observed from both the C. pseudolongus DUCC 4014 and water (control) inoculated tissues by SEM. Compactly netted mycelial structures are shown on the surface of the cap in the fruit body of control (). But the netted mycelia are not compact and degraded in the C. pseudolongus inoculated fruit body. The edge tissues in the dissected cap are smooth and normal in the control fruit body. On the other hand, they are wrinkled and loosed in the yeast pathogen inoculated fruit body. Basidia and basidiospores are arranged well in the control fruit body. But they are ruptured and dispersed in the C. pseudolongus inoculated fruit body. These results indicate that the abnormal browning symptom caused by the C. pseudolongus involves changes in microstructures of cap and gills in the shiitake fruit body.
Figure 5. Scanning electron microscopic images of shiitake fruit body (Chamaram cultivar) inoculated by sterile water (a1–a3) and Cryptococcus pseudolongus DUCC 4014 (b1–b3) using a spray method. (A) Mimetic diagram of sampling position on the fruit body for SEM image analysis. Specimens of red boxed area in the control and inoculated fruit bodies were used for SEM observation, respectively; (B) SEM images of mushroom fruit bodies inoculated with sterile water (control, top pictures) and C. pseudolongus (bottom pictures). Scales: 100 μm (a-1, a-2, b-1, b-2) and 10 μm (a-3, b-3).
3.4. Pathogenicity test to shiitake with other Cryptococcus species taxonomically related with C. pseudolongus
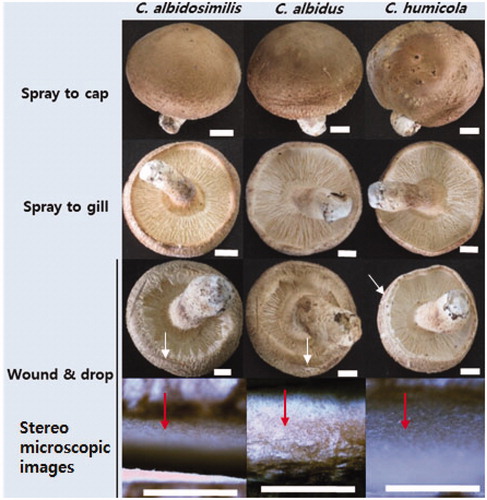
So far, it has not been known yet that whether the occurrence of an abnormal browning symptom and rot in the shiitake fruit body can be triggered not only by C. pesudolongus but also by other related species. Thus, we tried to inoculate the shiitake fruit body of Chamaram cultivar using three Cryptococcus species including C. albicosimilis, C. albidus, and C. humicola. No typical browning symptom was observed from all the fruit bodies that were inoculated by the cell suspension of three Cryptococcus species using a spray method (). Even with wound inoculation, three Cryptococcus species could not generate a browning symptom in the shiitake fruit body.
Figure 6. Inoculation test using different Cryptococcus spp. to shiitake fruit bodies (Chamaram cultivar). Wound and spray methods were used for inoculation using the inoculum concentration of 1 × 106 cells/ml. White arrows indicate the wounded position on the cap of the mushroom fruit bodies. Red arrows indicate inside of the cut-wounded area with a blade. Scales: 10 mm for fruit body and 1 mm for the stereo microscopic images.
3.5. C. Pseudolongus growth inhibitory assay by antifungal agents
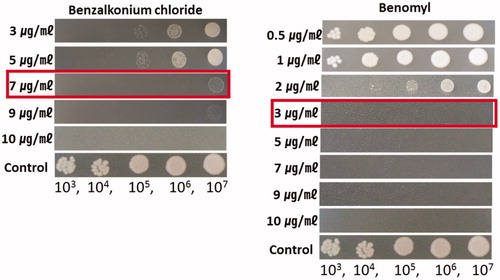
Antifungal agents capable of inhibiting the growth of fungi were used in the experiment. Sixteen antifungal drugs including benomyl were tested to test whether they could inhibit the growth of C. pseudolongus. The growth of C. pseudolongus was inhibited by difenozonaole, flusilazole, and mancozeb at the concentration of 1000 µg/ml and by acetic acid at the concentration of 10,000 µg/ml (). But benzalkonium chloride and benomyl inhibited the growth of C. pseudolongus at all concentrations used in the test. Therefore, we tried to determine the minimum concentration that can inhibit the growth of C. pseudolongus by these two antifungal agents. When they were tested at the concentration of 3 µg/ml, 5 µg/ml, 7 µg/ml, and 9 µg/ml, the MIC of benzalkonium chloride was 7 µg/ml and that of benomyl was 3 µg/ml, respectively (). The mycelial growth of two domestic cultivars, Chamaram and Sanjo 701ho was not affected on PDA containing benomyl or benzalkonium chloride at a concentration of 10 µg/ml (data not shown).
Figure 7. The effect of sixteen kinds of fungicides at three different concentrations on the cell growth of Cryptococcus pseudolongus DUCC 4014 on YMA. The tested C. pseudolongus cell concentration was adjusted from 1 × 103 cells/ml to 1 × 107 cells/ml, respectively.
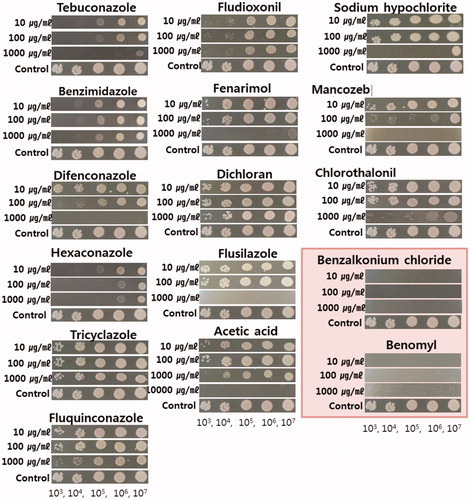
Figure 8. Minimum inhibition concentration of benzalkonium chloride and benomyl to Cryptococcus pseudolongus DUCC 4014 on YMA. The concentration of the fungicides was made at 3 μg/ml to 10 μg/ml (benzalkonium chloride) and 0.5 μg/ml to 10 μg/ml (benomyl). C. pseudolongus cell concentration was adjusted from 1 × 103 cells/ml to 1 × 107 cells/ml.
4. Discussion
Fungi is always a concerning matter in the commercial production of shiitake mushrooms. Problems caused by fungi could occur from the mushroom culture preparation to spawn making to the cultivation process and storage period after harvesting. Because fungal contaminants could inhibit the mycelial growth and fruitbody development of shiitake, resulting in economic losses. Several filamentous fungi have been known to cause diseases or damages to shiitake fruit bodies [Citation18–20]. However, abnormal browning of shiitake fruit bodies, especially in the gill, is not typical symptoms of the shiitake diseases caused by filamentous fungi. Abnormal browning symptom in shiitake was only reported from the bacteria Ewingella americana in Japan [21,146] before the report of brown rot caused by the basidiomycete yeast Crytococcus pseudolongus [Citation15]. Inoculation test of this bacterial pathogen on fruit body, gill, and mycelium showed its ability to cause discoloration and mycelial growth inhibition in oyster mushrooms, Pleurotus ostreatus, and Pleurotus eryngii. On the contrary, the pathological ability of C. pseudolongus has not been much studied.
In this study, the confrontation assay of dual cultures demonstrated that C. pseudolongus could inhibit and induce browning in the mycelia of shiitake (). This result agreed with the results of E. americana bacteria inoculation test to the mycelium of Lentinua edodes [Citation21]. The results of the inoculation test of C. pseudolongus to the cap and gills of the shiitake fruit bodies also showed that the development of the browning symptom is common in three different shiitake cultivars. Stereomicroscopic and SEM image analyses on the tissues of the browning symptom developed by the inoculation tests provided evidence of the physical degradation and rupturing of mycelial tissues accompanying the browning symptom. These findings support the rotting ability of C. pseudolongus and complement its identity as the causal agent of the brown rot disease in shiitake [Citation15]. Thus, we conclude that C. pseudolongus possesses the ability to cause discoloration and mycelial growth inhibition in shiitake mushrooms. However, the Crytococcus yeast did not show its ability to cause discoloration and mycelial growth inhibition in two oyster mushroom species, Pleurotus ostreatus, and P. ferulae (). And it could not cause browning on the gill of white button mushroom fruit body. These results of the inoculation tests by the C. pseudolongus to shiitake fruit bodies coincide with those of E. americana bacteria. But the results of the inoculation tests by the C. pseudolongus to Pleurotus spp. did not coincide with those of E. americana bacteria. This inconsistency may come from that the mechanism of discoloration and mycelial inhibition is different between C. pseudolongus yeast and E. americana bacteria. But further study is needed to elucidate the unconformity. These results suggest that C. pseudolongus may not be a problem in the cultivation of oyster mushrooms. But it might be a problem for white button mushroom when there is a scar or wound in the process of the mushroom handling because it caused discoloration on the wounded tissues of white button mushroom by the wound inoculation test (). In that case, C. pseudolongus may result in the reduction of commodity value by discoloring the white-colored fruit bodies which are not favored by consumers.
Regarding shiitake cultivation, our study reveals that the C. pseudolongus pathogen may cause a problem not only in fruit body development but also in the culture and spawn preparation and during mushroom storage. A question remains whether the abnormal browning symptom in shiitake is C. pesudolongus specific because there have been no trials to find other Crytococcus yeast species which might also cause problems to shiitake. This question was partially answered by the extended inoculation test with three other Crytococcus yeast species. C. albidosimilis, C. albidus, and C. humicola showed that they cannot have the ability to generate the abnormal browning in the fruit bodies of shiitake. These results suggest that the abnormal browning symptom in shiitake is C. pesudolongus specific. Consequently, we screened 16 kinds of antifungal agents only with C. pseudolongus as an effort to develop the management of the abnormal browning pathogen. Among the tested agents, difenozonaole, flusilazole, and mancozeb inhibited the growth of C. pseudolongus at the concentration of 1000 µg/ml. But great inhibition effect was shown by benzalkonium chloride and benomyl inhibited at all concentrations used in the test. These results mean that benomyl or benzalkonium can be used for the control of C. pseudolongus without drug damage on shiitake. Overall, the results of our study could be applied to the management of disease control in the process of shiitake production. So far, pathological researches for Crytococcus yeasts have been conducted on only a few species that are known to be pathogenic to animals and humans [Citation22]. Of all the pathogenic Crytococcus species, the most studied species is C. neoformans which causes cryptococcosis [Citation23]. We are certain that the pathological properties of C. pseudolongus studied in this work surely add new information to the biology of pathogenic Cryptococcus species.
Disclosure statement
The authors declare that they have no potential conflicts of interest.
Additional information
Funding
References
- Stamets P. Growing gourmet and medicinal mushrooms. 3rd ed. Berkeley (CA): Ten Speed Press; 2000. p. 260.
- Wasser S. Shiitake (Lentinula edodes). In: Coates PM, Blackman M, Cragg GM, White JD, Moss J, Levine MA, editors. Encyclopedia of dietary supplements. Boca Raton (FL): CRC Press; 2004. p. 653–664.
- Chang ST. World production of cultivated edible and medicinal mushrooms in 1997 with emphasis on Lentinula edodes in China. Int J Med Mushr. 1999;1(4):291–300.
- Mattila P, Salo-Väänänen P, Könkö K, et al. Basic composition and amino acid contents of mushrooms cultivated in Finland. J Agric Food Chem. 2002;50(22):6419–6422.
- Royse DJ, Baars JJP, Tan Q. Current overview of mushroom production in the world: technology and applications. In: Zied DC, Pardo-Giménez A, editors. Edible and medicinal mushrooms: technology and applications. Hoboken (NJ): Wiley-Blackwell; 2017. p. 5–13.
- Jong SC, Birmingham JM. Medicinal and therapeutic value of the shiitake mushroom. Adv Appl Microbiol. 1993;28:153–184.
- Ohnuma N, Amemiya K, Kakuda R, et al. Sterol constituents from two edible mushrooms, Lentinula edodes and Tricholoma matsutake. Chem Pharm Bull (Tokyo). 2000;48(5):749–751.
- Yaoita Y, Amemiya K, Ohnuma H, et al. Sterol constituents from five edible mushrooms. Chem Pharm Bull. 1998;46(6):944–950.
- Xiaoshuang D, Stanilka JM, Rowe CA, et al. Consuming Lentinula edodes (shiitake) mushrooms daily improves human immunity: a randomized dietary intervention in healthy young adults. J Am Coll Nutr. 2015;34(6):478–487.
- Ahn GR, Kwon HW, Ko HK, et al. Unrecorded fungal species isolated from greenhouses used for shiitake cultivation in Korea. Kor J Mycol. 2016;44(1):8–15.
- Bak WC, Kwon H. Biology and control of pests and diseases in shiitake log cultivation. Chapter 5. Pest and disease management of shiitake. In Mushroom Grower’s hand book 2. Shiitake cultivation. Seoul: Mush World; 2005. p. 152–161.
- Kim JY, Yun YH, Hyun MW, et al. Identification and characterization of Gliocladium viride isolated from mushroom fly-infested oak log beds used for shiitake cultivation. Mycobiology. 2010;38(1):7–12.
- Kim JY, Kwon HW, Yun YH, et al. Identification and characterization of Trichoderma species damaging shiitake mushroom bed-logs infested by Camptomyia pest. J Microbiol Biotechnol. 2016;26(5):909–917.
- Kim JY, Kwon HW, Tang L, et al. Identification and characterization of Trichoderma citrinoviride isolated from mushroom fly-infested oak log beds used for shiitake cultivation. Plant Pathol J. 2012;28(2):219–219.
- Kwon HW, Yun YH, Kim SH, et al. First report of brown rot caused by Cryptococcus pseudolongus on fruiting body of Shiitake (Lentinula edodes) in Korea. Pl Dis. 2016;100(5):1013–1013.
- Putignani L, Paglia MG, Bordi E, et al. Identification of clinically relevant yeast species by DNA sequence analysis of the D2 variable region of the 25-28S rRNA gene. Mycoses. 2008;51(3):209–227.
- Noh J, Ko H, Park H. Selection of parental strain on the sawdust cultivation and mycelial growth and cultural characteristics of Lentinula edodes hybrid strains. J Mushroom. 2015;13(1):41–49.
- Badham ER. Growth and competition between Lentinus edodes and Trichoderma harzianum on sawdust media. Mycologia. 1991;83(4):455–463.
- Gea FJ, Navarro MJ, Suz LM. First report of cobweb disease caused by Cladobotryum dendroides on shiitake mushroom (Lentinula edodes) in Spain. Pl Dis. 2018;102(5):1030.
- Miyazaki K. Through studies on harmful microorganisms to mushroom cultivation# points of countermeasure against harmful microorganisms and influences of global warming on mushroom cultivation. Mushroom Sci Biotechnol. 2018;26:10–17. (In Japanese with English summary)
- Arima S, Nanaumi T, Shinohara H, et al. Bacterial brown rot, a new disease of shiitake (Lentinula edodes) caused by Ewingella americana. Mushroom Sci Biotechnol. 2010;18:139–144. (In Japanese with English summary)
- Kido N, Makimura K, Kamegaya C, et al. Long-term surveillance and treatment of subclinical cryptococcosis and nasal colonization by Cryptococcus neoformans and C. gattii species complex in captive koalas (Phascolarctes cinereus). Med Mycol. 2012;50(3):291–298.
- Perfect JR, Casadevall A. Cryptococcosis. Infect Dis Clin North Am. 2002;16(4):837–874.