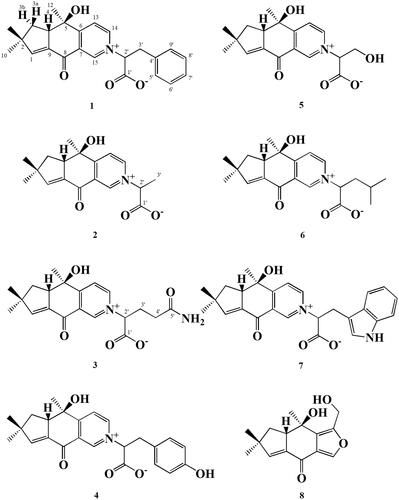
Abstract
In our ongoing search for new secondary metabolites from fungi, a basidiomycete fungus Irpex consors was selected for mycochemical investigation, and three new zwitterionic alkaloids (1-3) and five known compounds (4-8) were isolated from the culture broth (16 l) of I. consors. The culture filtrate was fractionated by a series of column chromatography including Diaion HP-20, silica gel, and Sephadex LH-20, Sep-Pak C18 cartridge, medium pressure liquid chromatography (MPLC), and high pressure liquid chromatography (HPLC) to yield eight compounds (1-8). The structures of the isolated compounds were elucidated by the interpretation of nuclear magnetic resonance (NMR) spectra and high-resolution mass spectrometry (HR-MS). Their antioxidant and antibacterial activities were examined. The zwitterionic structures of three new sesquiterpene alkaloids (1-3) were determined together with five known compounds identified as stereumamide E (4), stereumamide G (5), stereumamide H (6), stereumamide D (7), and sterostrein H (8). This is the first report of the zwitterionic alkaloids in the culture broth of I. consors. Three new zwitterionic alkaloids were named as consoramides A–C (1-3).
Mushrooms are a good source of functional foods and traditional therapeutic agents [Citation1]. They produce a wide range of biologically active compounds with unique chemical structures [Citation2–4]. The mushroom Irpex consors, belonging to the family Meruliacease, is distributed in India and East Asian countries such as Korea and Japan [Citation5]. Previous investigations of I. consors have reported that it possesses tricyclic sesquiterpene derivatives with anti-bacterial and anti-tumor activities [Citation6–8]. In our ongoing search for new secondary metabolites from fungal strains, three new zwitterionic alkaloids (1-3) together with five known compounds (4-8) were isolated from the culture broth of the fungus I. consors. Herein, we describe the isolation and structure determination of these compounds ().
Fungal strain Irpex consors was obtained from Rural Development Administration, Korea. The fungal strain I. consors was cultured on potato dextrose agar at 27 °C for two weeks. Small pieces of fresh mycelium were inoculated into 40 1-l flasks containing 400 ml of potato dextrose broth and cultured on a rotary shaker of 120 rpm at 27 °C for four weeks. The culture broth (about 16 l) was filtered to remove mycelia. The culture filtrate was fractionated by Diaion HP-20 column chromatography eluted with a mixture of methanol-water (30:70–100:0, v/v, stepwise), followed by silica gel column chromatography with stepwise chloroform-methanol (30:1–0:100, v/v) to afford four fractions (Fractions A-D). Fraction A was subjected to Sephadex LH-20 column chromatography, followed by medium pressure liquid chromatography (MPLC) to give two fractions A1 and A2. Fraction A1 was purified by Sep-Pak C18 cartridge eluted with 20% aqueous methanol to obtain two compounds 1 (4.3 mg) and 2 (12.9 mg). Fraction A2 was further separated by a Sep-Pak C18 cartridge eluted with 15% aqueous methanol to obtain compound 7 (4.5 mg). Fraction B was fractionated by Sephadex LH-20 column chromatography, followed by preparative reversed-phase high pressure liquid chromatography (HPLC) eluted with 18% aqueous methanol to yield two compounds 4 (9.3 mg) and 6 (9.5 mg). Fraction C was subjected to MPLC, followed by preparative reversed-phase HPLC eluted with 18% aqueous methanol to provide two compounds 5 (12.0 mg) and 8 (9.0 mg). Fraction D was separated by MPLC eluted with a gradient of increasing methanol (20–100%) in water, followed by preparative reversed-phase HPLC eluted with 27% aqueous methanol to obtain a compound 3 (4.8 mg).
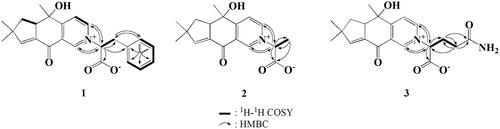
Compound 1 was obtained as a brown powder with a specific rotation value of −92.8° (c = 0.1, 24.5 °C, methanol). Its molecular formula was established as C24H25NO4 by a high-resolution fast atom bombardment (FAB)-mass measurement (m/z 392.1849 [M + H]+, Δ −1.2 mmu). The 1H NMR spectrum of 1 revealed the presence of a substituted benzene moiety at δ 7.18 (× 2), 7.14, and 7.09 (× 2), a 3,4-disubstituted pyridine moiety at δ 9.05, 8.99, and 8.27, a olefinic methine at δ 6.93, two methines at δ 5.59 and 3.69, two methylenes at δ 3.88/3.47 and 2.10/1.97, and three methyls at δ 1.28, 1.24, and 1.19 (). In the 13C NMR spectrum, twenty-four carbons including one ketone carbon at δ 181.1, one carbonyl carbon at δ 171.0, nine sp2 methine carbons at δ 155.7, 148.6, 145.7, 130.1 (× 2), 129.9 (× 2), 128.6, and 125.0, four sp2 quaternary carbons at δ 171.4, 137.4, 137.3, and 131.6, one oxygenated quaternary carbon at δ 74.1, two methine carbons at δ 78.8 and 53.8, one quaternary carbon at δ 47.0, two methylene carbons at δ 40.7 and 40.6, and three methyl carbons at δ 28.6, 27.6, and 25.2 were evident (). In the 1H-1H correlated spectroscopy (COSY) spectrum, correlations between H-3 and H-4 and between H-13 and H-14 were observed, and the long-range correlations from H-1 to C-8 and C-9, from H-3 to C-5 and C-9, from H-10 and H-11 to C-1, C-2, and C-3, from H-12 to C-4, C-5, and C-6, from H-13 to C-5 and C-7, from H-14 to C-6 and C-15, and from H-15 to C-6 and C-8 established the presence of sterostrein Q moiety. The long-range correlations from H-2′ to C-1′, C-3′ and C-4′, from H-8′ to C-4′, and from H-9′ to C-3′ and C-7′ as well as 1H-1H COSY correlations of H-5′/H-6′/H-7′/H-8′/H-9′ and H-2′/H-3′ revealed the presence of a phenylalanine moiety. Finally, the long-range correlations from H-14 and H-15 to C-2′ and from H-2′ to C-14 and C-15 indicated that the phenylalanine moiety was connected to sterostrein Q via carbon-nitrogen bond (). The partial relative stereochemistry of 1 was established by the NOESY correlations. The cross peaks of H-4/H-3a and H-4/H-11 indicated the same face, while those of H-10/3b and H-3b/H-12 confirmed the other face. Therefore, the structure of 1 was determined as a new zwitterionic alkaloid and named consoramide A.
Table 1. 1H and 13C NMR data of compounds 1-3 in methanol-d4.
Compound 2 was purified as a yellow oil with specific rotation of −107.2° (c = 1.0, 25.0 °C, methanol) and exhibited UV maxima (log ε) at 203 (3.34) and 238 (3.61) nm. Its molecular formula was determined to be C18H21NO4 by the high-resolution FAB-mass measurement (m/z 316.1531 [M + H]+, Δ −1.8 mmu). The 1 D NMR spectra of 2 revealed that the hydroxymethyl group in 5 was replaced by a methyl group (). The long-range correlations from H-3′ to C-1′ and C-2′ as well as 1H-1H COSY correlations between H-2´and H-3′ supported the presence of an alanine moiety in 2 (). Therefore, compound 2 was determined to be a new zwitterionic alkaloid and named consoramide B.
Compound 3 was obtained as a yellow powder with the specific rotation of −89.2° (c = 1.0, 25.0 °C, methanol) and showed UV maxima (log ε) at 202.0 (3.37) nm. Its molecular formula was established as C20H24N2O5 by the high-resolution FAB-mass measurement (m/z 373.1745 [M + H]+, Δ −1.9 mmu). The NMR spectra revealed that 3 was consisted of sterostrein Q and glutamine (). The glutamine moiety was determined by the 1H-1H COSY correlations and the long-range correlations from H-2′ to C-1′ and C-3′ and from H-3′ and H-4′ to C-5′ (). Thus, compound 3 was determined to be a new zwitterionic alkaloid and named consoramide C. The configuration of all amino acid moieties in 1-3 was tentatively deduced as L-form, because the L-amino acids are abundant in nature literature [Citation9,Citation11].
Compounds 4-8 were identified as stereumamide E (4), stereumamide G (5), stereumamide H (6), stereumamide D (7), and sterostrein H (8), respectively, by the comparison of their spectroscopic data with the literatures previously reported [Citation9–11].
The antioxidant activities of these compounds (1-8) were evaluated by the ABTS (2,2′-azinobis[3-ethylbenzothiazoline-6-sulonate]) and DPPH (1,1-diphenyl-2-picrylhydrazyl) radical-scavenging assays [Citation12]. All compounds (1-8) displayed no radical scavenging activity up to 200 µM. In the present study, all compounds exhibited no antibacterial activity up to 50 µg/disk against Bacillus subtilis, Staphylococcus aureus, Staphylococcus epidermidis, Propionibacterium acnes, and Escherichia coli.
Supplementary information
The online version of this article (https://doi.org/10.1038/s41429-017-00XX-X) contains supplementary material, which is available to authorized users.
Supplemental Material
Download MS Word (759.7 KB)Acknowledgements
The authors thank Ms. Ji-Young Oh, Center for University-wide Research Facilities (CURF) at Jeonbuk National University, for NMR measurement.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Zhang JJ, Li Y, Zhou T, et al. Bioactivities and health benefits of mushrooms mainly from China. Molecules. 2016;21(7):938–954.
- Zjawiony JK. Biologically active compounds from Aphyllophorales (Polypore) fungi. J Nat Prod. 2004;67(2):300–310.
- Lee IK, Yun BS. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance. J Antibiot. 2011;64(5):349–359.
- Ríos JL, Andújar I, Recio MC, et al. Lanostanoids from fungi: a group of potential anticancer compounds. J Nat Prod. 2012;75(11):2016–2044.
- Ko KS, Jung HS. Phylogenetic re-evaluation of Trametes consors based on mitochondrial small subunit ribosomal DNA sequences. FEMS Microbiol Lett. 1999;170(1):181–186.
- Wang GYS, Abrell LM, Avelar A, et al. New hirsutane based sesquiterpenes from salt water cultures of a marine sponge-derived fungus and the terrestrial fungus Coriolus consors. Tetrahedron. 1998;54(26):7335–7342.
- Takeuchi T, Ishizuka M, Umezawa H. Chemical modification of coriolin B. J Antibiot. 1977;30:59–65.
- Takeuchi T, Takahashi S, Iinuma H, et al. Diketocoriolin B, an active derivative of coriolin B produced by Coriolus consors. J Antibiot. 1971;24(9):631–635.
- Duan YG, Feng J, Bai N, et al. Four novel antibacterial sesquiterpene-α-amino acid quaternary ammonium hybrid from the mycelium of mushroom Stereum hirsutum. Fitoterapia. 2018;128:213–217.
- Isaka M, Srisanoh U, Sappan M, et al. Sterostreins F-O, illudalanes and norilludalanes from cultures of the Basidiomycete Stereum ostrea BCC 22955. Phytochemistry. 2012;79:116–120.
- Hu QY, Duan YC, Pu XJ, et al. Stereumamides E-H, four new minor quaternary ammonium hybrids from Stereum hirsutum. Nat Prod Res. 2020. DOI:https://doi.org/10.1080/14786419.2020.1779266
- Lee IK, Jung JY, Kim YS, et al. p-Terphenyls from the fruiting bodies of Paxillus curtisii and their antioxidant properties. Bioorg Med Chem. 2009;17(13):4674–4680.