Abstract
Itajahya rosea was found growing in association with Leucaena leucocephala plants at Savitribai Phule Pune University campus in India. The species identity was confirmed by phylogenetic analysis based on ITS and LSU regions of rDNA, wherein, our fugus was placed along with I. rosea in phylogenetic tree. It represents first record of I. rosea from India. Frequent visitation by Drosophila species on I. rosea fruiting body particularly on gleba was observed. The Drosophila got attracted to the detached gleba under the laboratory conditions and even sometimes, they prefer to sit over the gleba as compare to their food banana. It suggested that I. rosea gleba or pseudostipe produces some compounds for attraction and feeding behavior of Drosophila species. Therefore, we characterized the volatile attractants produced by gleba and pseudostipe of I. rosea by GC-MS analysis. Nineteen compounds were identified from gleba while nine compounds were recovered from the pseudostipe. Out of them, blends of three abundant odor producing volatile compounds were reported namely, Hexadecane, Pentadecane and Nonadecane, which are responsible for attraction of Drosophila toward the gleba. Three fatty acids namely 9,12-octadecadienoic acid (Z,Z), hexadecanoic acid and benzoic acid ethyl ester produced are served as an appetitive signal through olfactory response of Drosophila, so the flies were feed on the gleba. Two pheromones’ compounds, heneicosane and (+)-(5S,9S)-5,9-dimethylpentadecane, were also reported in pseudostipe and gleba, respectively, which play a role in Drosophila for breeding. Our study highlights an intriguing chemical ecology of fungus–Drosophila interaction.
1. Introduction
Phalloids (commonly known as “stinkhorns”) are currently classified in family Phallaceae, order Phallales E. Fisch., subclass Phallomycetidae [Citation1]. First, Itajahya was reported by Moller in 1895 from Brazil. Later on, one more report of the phalloid from Bolivia [Citation2]. Spegazzini listed a fungus from Argentina under the genus name Alboffiella [Citation3], for which Fries and others were certain that it was synonymous to Itajahya. Therefore, Itajahya now is treated as a subgenus Phallus which include four species, with I. galericulata as type species [Citation4,Citation5]. These fungi exhibit very rare occurrence, thus, making this genus the least known members of the family Phallaceae. The main feature that distinguishes Itajahya from other taxa of this family is the presence of a structure termed the “calyptra” located at the apex of the gleba [Citation6–8]. Based on the morphological characters like flat calyptra at apex, Phallus roseus was originally described from Egypt [Citation9]. This is considered the taxonomic placement of Ph. roseus, a species assigned to the genera Itajahya or Phallus [Citation4,Citation5]. DNA sequencing and phylogenetic analyses of Ph. rosea and demonstrated that it does not cluster with other species of the genus Phallus, so it was, therefore, separated from Phallus and accepted as a member of the genus Itajahya [Citation10]. Itajahya rosea is a rare species found not only in semidesertic habitats, known from Egypt, Morocco, Ghana, Israel, Pakistan, and southern France, but it also occurs in South America, from rich soils in Brazil and South Africa (Pretoria). There has not been any valid publication describing the occurrence and phylogenetic placement of I. rosea from India.
The stinkhorn fungi produce gelatinous, variously colored gleba with unpleasant smell similar to that of a rotten meat, which attract insects and play a role in basidiospore dispersal through these insects [Citation11,Citation12]. Seven different species of Drosophila were proved to breed in stinkhorn particularly reported on Ph. impudicus [Citation13]. It has been an interesting aspect to find out what chemicals are produced by these fungi to attract the insects. Total 59 compounds were reported from Ph. impudicus at different developmental stages of basidiomata [Citation14]. One drosophilid species reported to feed the spores of the Ganoderma applanatum have found to maintain germination capacity that have passed through the intestines, which suggests that this drosophilid acts as a spore dispersal agent [Citation15]. Frequent visitation by Drosophila species on I. rosea fruiting body particularly on gleba was observed, and further the fungus also produces an unpleasant odor. However, chemical nature of these volatile attractants of I. rosea remains uncharacterized.
Therefore, the present paper deals with the correct identification, and phylogenetic placement of I. rosea from India and to investigate the identification of odorant compounds produced by both gleba and pseudostipe, and their function in Drosophila attraction.
2. Materials and methods
2.1. Specimen collection and macroscopic analysis
The eggs and basidiome of I. rosea were collected from Savitribai Phule Pune University Campus, India near root system of Leucaena leucocephala. The morphology of fresh basidiomata size, shape and colors was recorded. Microscopic characteristics of basidiomata were determined by vertical axis of the egg and basidiomata. Average spore size was done by measuring length and breadth of 25 spores.
2.2. Molecular identification and phylogeny
DNA extraction: The DNA was extracted from Basidioma with some modification [Citation16]. The amplification of complete ITS and partial LSU regions was performed using primer ITS1/ITS4 and LROR/LR7 [Citation17,Citation18]. The PCR reaction mixture consisted of DNA template 2 μl (10–20 ng), 5 μl Taq DNA polymerase buffer (10×) (Sigma-Aldrich, Mumbai, India), dNTP 1 μl (200 μM) (Sigma-Aldrich, Mumbai, India), 1 μl of 10 pmol primers and the final volume made up by MiliQ H2O (Sterile Ultra-Pure Water, Sigma-Aldrich, Mumbai, India). Amplification was done by using Mastercycler (Eppendorf, Hamburg, Germany) as follows: first step was 95 °C for 5 min, 1 min at 95 °C with repetition of 30 cycles, annealing temperature was for 30 s at 55 °C (for ITS rDNA) and 30 s at 52 °C (for D1D2 region of LSU rDNA), extension was for 1 min at 72 °C (for ITS) and 2 min at 72 °C (for LSU), and a final extension step having 7 min at 72 °C. The PCR products were purified with FavorPrep PCR Purification Kit (Favorgen Biotech Corp., Macomb, MI) and sequenced by using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Bedford, MA). The generated sequences were then submitted in the NCBI GenBank. The DNA sequences were subjected to BLASTn search and identified according to the closely related isolates that were available in the database (www.ncbi.nlm.nih.gov/geo). The 23 and 26 sequences of ITS and LSU, respectively, representing different allied species were aligned by using MUSCLE (see ). The phylogenetic tree was constructed based on the neighbor-joining method with Trappea darkeri as an outgroup species by using MEGA7 software package [Citation19–21].
Table 1. The ITS and LSU sequences and their GenBank accession numbers used for phylogenetic analysis.
2.3. In vitro behavioral studies of Drosophila melanogaster
An experiment was performed to check whether fruit flies reared in lab get attracted to the mucoid gleba of I. rosea and whether they prefer this mucoid gleba over a fermented Banana (Musa paradisiaca), for which a clean transparent plastic box was taken ensuring that the fly should have sufficient amount of air to respire and space to spread out for at least 24 h. An equal amount of mucoid substrate from I. rosea gleba and smashed 2-day fermented banana were weighed in two sterile petri dishes and kept at two opposite sides of the clean transparent plastic box. Approximately, 400–500 wild-type strain of Drosophila melanogaster (+;+;+) flies were anesthetized using ether and were collected in a vial from a bottle. This vial of flies was gently emptied at the center of the box and the lid was closed tightly. The box was kept at room temperature and was observed initially for an hour, making sure that all flies are conscious again and then after 0, 12, and 24 h, the feeding behaviors and preferences were assessed.
To analyze whether the behavior of flies is based on visual or olfactory, an experiment was performed in a three chambered box, wherein, the three chambers were created using two cardboards with a hole so as to allow the passage for flies. Approximately, 250 anesthetized flies were kept in the middle chamber, while gleba and laboratory prepared feed for Drosophila (the laboratory prepared feed contains yeast and some volatile compounds e.g., orthophosphoric acid, 10% methyl benzoate, and propionic acid in concentrated form) were placed inside chambers having hole passage. Schematic of the setting is shown in . When the flies become conscious, the visual block prevents the preference of food based on vision and allows flies to decide the direction of food based on smell only.
Figure 1. Diagrammatic representation of behavioral experiment set ups. (A) initial trial test for attraction toward Gleba as food substrate; (B) to check the preference of food as Gleba and Banana; (C) experiment showing card board bifurcations with a passage hole separating the two food substrate (Gleba and laboratory prepared feed) with anesthetized flies in the center.
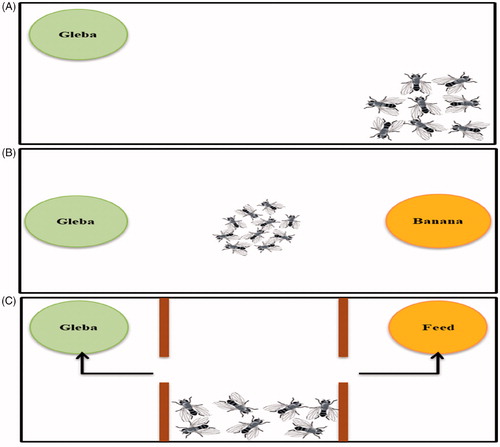
As Drosophila is a human model organism, generally is cultured in a vial with feed following circadian rhythm. All the above experiments were performed under same conditions i.e., following the day and the night cycle.
2.4. GC-MS analysis
Sample extraction: To study the odorant chemicals which attract the Drosophila toward the fruitbody, the fresh detached gleba and pseudostipe were extracted separately by using Hexane solvent. The extracts were filtered through Nylon syringe filter 0.45 µm size. The filtered solution was subjected to GC-MS analysis. GC-MS system consisted of an Agilent 6890N gas chromatograph and having detector (Agilent®5973 Network MSD, Santa Clara, CA). The column consisted of 30 m DB-5 column (J & W Scientific, Folsom, CA). Injection of samples was completed via injector (Split 1:40 at 250 °C, 2.0 µL; carrier gas: helium 1.1 mL/min (60 kPa) at 110 °C; pressure rise: 6 kPa/min). Full scan mass spectra were obtained from 35 to 350 m/z at a rate of 4.5 scans/s and with a 5.00 min solvent delay. Chromatographic peaks and mass spectra were then identified by using the National Institute of Standards and Technology (NIST) reference libraries. The percentage abundance of compounds was calculated by percentage of peak area of each compound by peak area of total compounds. The GC-MS analysis was conducted in duplicate for gleba and pseudostipe.
2.4. Statistical analysis
Data of Gleba feed after 12 and 24 h of feeding were analyzed by T-test, results were considered significantly different at p < 0.05. Data of Gleba versus Banana and Gleba versus laboratory feed after 12 and 24 h were analyzed by a one-way ANOVA with the Tukey post-test to evaluate the differences between treatments. The post-hoc test of the least significant difference (LSD) was used to assess the differences between treatments. Results were considered significantly different at p < 0.01.
3. Results
3.1. Taxonomical analysis of Itajahya rosea
3.1.1. Taxonomy
Itajahya rosea (Delile) E. Fisch., Ber. dt. bot. Ges. 47(5): 294. 1929 ()
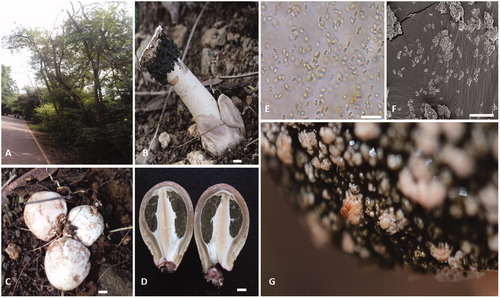
Figure 2. Habitat and features of Itajahya rosea. (A) Leucaena leucocephala plant at Savitribai Phule Pune University Campus in India. (B) Basidiomata of I. rosea growing in soil, scale bar = 1 cm. (C) Immature balls of I. rosea scale bar = 1 cm. (D) Internal structure of immature balls of I. rosea, scale bar = 1 cm. (E) Basidiospores of I. rosea under microscope scale bar = 20 μm. (F) Basidiospore of I. rosea under SEM, scale bar = 30 µm. (G) Drosophila attracted on the gleba and feeding on gleba mass.
Synonym: Phallus roseus Delile 1813
Description: Egg are subglobose or pyriform, 5–6 cm high by 3–4 cm wide, white to yellowish brown, with developed rhizomorph Exoperdium ocher to dun, presence of three layers outer most layer thick 2–2.5 mm, white to beige, middle layer 5–6 mm, mucilagenous, inner layer less than 1 mm, whitish covering over green gleba. Basidioma without indusium, 4–5 cm tall and width 4.2 cm. Receptacle cylindrical with granulose to rugulose surface, receptacle surface is non-merulliod, receptacle surface wig-like, Pseudostipe pink, spongy, hollow, cylindrical, 3–4.5 cm tall and 1.5–2 cm wide, formed by pseudoparenchymatous cells, tappering toward the base, receptaculum conic with reticulate surface. Gleba mucilagenous, deliquiscent, dark green, gleba odor fetid. Calyptra at the apex white-beige discoid, 1.5–2 cm in diameter forming sterile platform above the receptacle. Basidiospores 3.5 × 1.8 µm average, cylindrical hyaline, slightly green, thin walled, smooth ()).
Edibility: Not known.
Habitat: This fungus appears in sandy soils July–October, and is found in cluster associated with the roots of Leucaena leucocephala (Fabaceae) in India.
Distribution: Africa, Southern Yemen, North America, Southern France, Israel, India.
Material examined: India. Savitribai Phule Pune University Campus, MH, India. August 2018, M. Borde, Y. Kshirsagar, R. Jadhav & A. Baghela.
3.1.2. Phylogenetic analysis of Itajahya rosea
The ITS and LSU sequences of I. rosea were deposited in the NCBI GenBank with the accession numbers ITS sequence: MN135577, and LSU sequence: MN134400. The phylogenetic analysis of ITS sequences by the neighbor-joining method confirmed the identity of our fungus, I. rosea formed a sister clade with I. galericulata with bootstrap support (100%) (). Phylogenetic analysis of LSU sequences by the neighbor-joining method confirmed the identity of our fungus, I. rosea formed sister clade with I. galericulata species with bootstrap support (99%) ().
Figure 3. Evolutionary relationships of taxa based on ITS rDNA. The evolutionary history was inferred using the neighbor-joining method. The optimal tree with the sum of branch length = 1.40883632 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Kimura 2-parameter method and are in the units of the number of base substitutions per site. The analysis involved 24 nucleotide sequences. All positions with less than 90% site coverage were eliminated. There was a total of 400 positions in the final dataset. Evolutionary analyses were conducted in MEGA7.
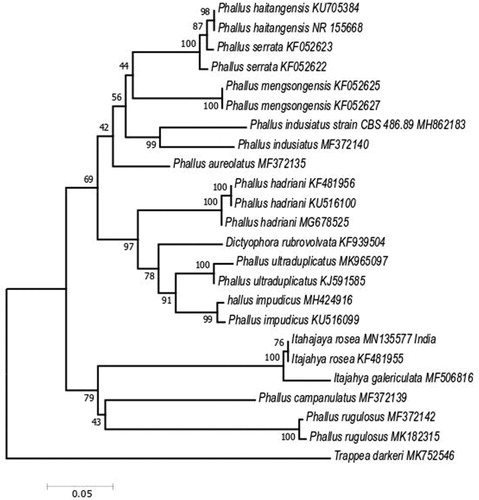
Figure 4. Evolutionary relationships of taxa based on LSU rDNA. The evolutionary history was inferred using the neighbor-joining method. The optimal tree with the sum of branch length = 0.36008874 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Kimura 2-parameter method and are in the units of the number of base substitutions per site. The analysis involved 26 nucleotide sequences. All positions with less than 95% site coverage were eliminated. There was a total of 459 positions in the final dataset. Evolutionary analyses were conducted in MEGA7.
3.2. Behavioral studies on Drosophila
The Drosophila were found to be attracted toward gleba portion of I. rosea fruiting body (). Under laboratory conditions, the Drosophila were found to get significantly attracted toward the gleba of I. rosea (). Initially for few hours it was not clear whether the flies would choose to feed on gleba or not because a random distribution pattern of flies around the box was observed as flies take time to acclimatize to the new environment though few were feeding on gleba; however, within few hours, it became clear that most of the flies were getting attracted to the gleba preferring over the banana. It was observed that significant number of flies were in fungal substrate (gleba) zone and feeding on the gleba after 24 h of the inclusion in the box ().
Figure 5. Bar graph showing fly count (A) flies attracted to Gleba as the only food substrate at 0–24 h. Values represent the mean of three biological replicates ± SD. A *p < 0.05 indicates no statistical significance based on T-test with two independent treatments; (B) comparison of flies attracted toward Gleba and Banana at 0–24 h. Values represent the mean of three biological replicates ± SD. A ** p < 0.01 indicates no statistical significance based on one-way ANOVA, different letters above each bar indicate significant differences in flies attraction to Gleba and Banana after 12 and 24 h (Tukey test, ** p < 0.01) and (C) comparison of flies attracted toward Gleba and Laboratory prepared feed at 0–24 h. Values represent the mean of three biological replicates ± SD. A ** p < 0.01 indicates no statistical significance based on one-way ANOVA, different letters above each bar indicate significant differences in flies attraction to Gleba and Banana after 12 and 24 h (Tukey test, ** p < 0.01).
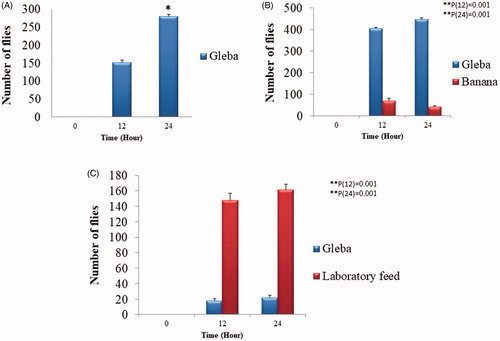
The three chamber experiment showed that flies were significantly attracted more toward laboratory prepared feed as compared to gleba after 12 and 24 h, as it contains yeast and some volatile compounds (e.g., orthophosphoric acid, 10% methyl benzoate, and propionic acid) in concentrated form which exhibits anti-microbial properties and helps in attracting flies. During this experiment, flies could not see the type of feed kept in two chambers, while they could smell them and flied to their favorite food chamber (). Therefore, it confirms that flies response was an olfactory response rather than a visual response.
3.3. GC-MS analysis
GC-MS analysis from pseudostipe of I. rosea was identified for the first time showed that, total twenty two compounds were detected from both the gleba and pseudostipe. Nineteen compounds from gleba and nine compounds from pseudostipe and their molecular formula, retention time and percent Abundance were mention in (). Nine compounds with total abundance (78.11%) were found in pseudostipe of I. rosea namely, (1) pentadecane, (2) hexadecane,2,6,10-trimethyl-, (3) docosane, (4) nonadecane, (5) eicosane, (6) heneicosane, (7) 1,2-benzenedicarboxylic acid bis(2-methylpropyl) ester, (8) 4-(5′-nitro-2′-thienyl)-3-iodo-2-butanone, (9) phenol,2,4-bis(1,1-dimethylethyl)- (). The most abundant compounds were 1,2-benzenedicarboxylic acid bis(2-methylpropyl) ester, Heneicosane, Nonadecane, Eicosane, and Pentadecane. Nineteen compounds identified from Gleba with total abundance (84.76%) included (1) hexadecane, (2) pentadecane, (3) hexadecane, 2,6,10-trimethyl-, (4) hexadecane,2,6,10,14-tetramethyl, (5) (+)-(5S,9S)-5,9-dimethylpentadecane, (6) (cis)-1-butyl-2-undecylcyclopropane, (7) nonadecane, (8) eicosane, (9) benzene1,2-dimethoxy-, (10) 9,12-octadecadienoic acid (Z,Z)-, (11) benzoic acid ethyl ester (), (12) hexadecanoic acid, (13) 1,2-benzenedicarboxylic acid, bis(2-methylpropyl) ester, (14) anthraergostatetraenol hexahydrobenzoate, (15) N,N'-dicarbazoylhydrazine, (16) (S)-(−)-4-benzoyloxy-5-oxopentyl pivalat, (17) ethyl 5-methyl-4-(2-phenylimidazol-1-yl)butyl-1,2,4-triazine-6-carboxylate, (18) phenol,2,4-bis(1,1-dimethylethyl)-, (19) 2-(7-(4-methoxyphenyl)-5,6-diphenyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylidene)-malononitrile (). Three fatty acids were reported most abundant, 9,12-octadecadienoic acid (Z,Z)-, benzoic acid ethyl ester, and hexadecanoic acid followed by anthraergostatetraenol hexahydrobenzoate and mixture of volatiles such as Eicosane, Nonadecane, Pentadecane. Seven compounds were found to be common in gleba and pseudostipe.
Figure 6. Chemical structure of compounds reported from pseudostipe of Itajahya rosea by GC-MS analysis.
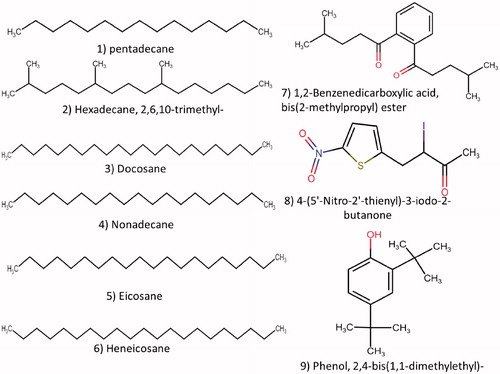
Figure 7. Chemical structure of compound numbers 1–11 (a) and 12–19 (b) reported from gleba of Itajahya rosea by using GC-MS analysis.
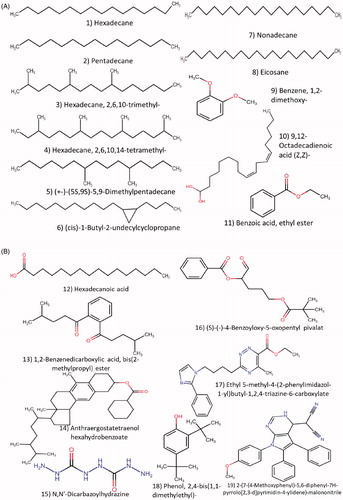
Table 2. List of compounds identified in Itajahya rosea from pseudostipe and gleba by using GC-MS analysis.
4. Discussion
Four species of Itajahya have been discovered till date, however, only I. galericulata has been reported from India. The present study represents the first record of I. rosea from India. The most relevant distinguishing characteristic features in between I. rosea and I. galericulata have been the presence of a calyptra at the apex of the receptacle and the pinkish pseudostipe. The species is morphologically very close to I. galericulata. The peridium of the immature basidiome is an important feature separating the species: presence of three-layered in I. rosea and presence of four-layered peridium in I. galericulata. Itajahya galericulata has been documented in dry sandy environments of New Mexico and Arizona in Brazil either in clay banks of forest streams or among roots of dead trees [Citation6,Citation22]. In contrast, in South Africa, it was found to be associated with litter of J. mimosifolia [Citation23]. Itajahya rosea as a rare species characteristic of desert and semiarid regions, as reported from Yemen and Northeastern Brazil [Citation8,Citation24,Citation25]. Similarly, the Australian I. hornseyi is also reported from a sandy soil habitat [Citation26]. The Leucaena leucocephala is originated in Southern Mexico and Northern Central America where the climate is temperate and tropical zone. The Leucaena leucocephala plant is invasive species may show association with I. rosea from its origin and there is possibility of dissemination of this association from Mexico to India via Africa and could be through seed-borne. The present molecular study showed that two gene regions namely, ITS and LSU have shown clearly that I. rosea was separated from other species. Our results confirm that I. rosea from India is phylogenetically closed to the I. galericulata, however, still branches out as a separate species. It also resulted that the inclusion of Itajahya genus in Phallales by phylogenetic studies.
As in the case of many herbivores orientation toward host odors can be assumed to be of importance to fungivorous insects colonizing new patches [Citation27]. Fungal odors have been shown to attract insects living on fungi or on substrates decayed by fungi [Citation28–34]. For example, stinkhorn mushrooms attract flies and some other insects by emitting a foul-smelling odor and these insects disperse their spores [Citation11,Citation12,Citation15].
In our study, we have shown for the first time that Drosophila species gets attracted to the gleba of I. rosea. Further, we have also demonstrated that the laboratory reared Drosophila could also get attracted to the isolated crude gleba material under the laboratory conditions. The laboratory-based experiments have also shown that the laboratory reared Drosophila preferred the gleba over banana as a substrate for feeding. This behavioral study confirmed that there were some volatile compounds being produced by I. rosea, therefore, for the first time, we identified those compounds by GC-MS analysis.
The GC-MS analysis of gleba and pseudostipe from I. rosea were studied, total 19 compounds were reported from gleba and some of their potential role and functions are discussed here. Blends of odorant compounds that include docosane, heneicosane, hexadecane, pentadecane, nonadecane, and 1,2-dimethoxybenzene produced by I. rosea play a role in attraction of Drosophila. Some of these alkanes are previously reported in P. impudicus [Citation35]. Heneicosane is reported to be an attractant of mosquitoes in the genus Aedes [Citation36]. A single odorant in gleba may be less effective to attract but blend of odorants is effective for attraction toward food source [Citation37,Citation38]. Other odorant such as 1,2-dimethoxybenzene is reported to key compound produced by flower for pollinator attraction [Citation39,Citation40]. Thus, the Blends of odorant compounds produced by I. rosea showed an adaptive mechanisms for attraction of Drosophilla.
Fatty acids, such as 12-octadecadienoic acid (Z,Z)- unsaturated fatty acid also known as Linoleic acid, are abundantly present in gleba, Benzoic acid ethylester is an ester formed by condensation of benzoic acid and ethanol and third one hexadecenoic acid which activates olfactory receptors of Drosophila and also has fruit odor quality [Citation41,Citation42]. These fatty acids serve as an appetitive signal for Drosophilla and they are detected through sweet-sensing neurons by Phospolipase C signaling in gustatory system, which promote the feeding [Citation43]. It has also been hypothesized that the Drosophila feed on gleba containing spores, which if not digested might come out through feces [Citation44–46]. Thereby helps in dispersal; however, there is need to perform more experiments to claim the spore dispersal through feces.
The (+)-(5S,9S)-5,9-dimethylpentadecane is probably one of the sex pheromone produced in gleba of I. rosea, the same pheromone was also reported from female coffee leaf minor, Leucoptera coffeella one of the pest of coffee trees in Brazil [Citation47]. The presence of such pheromones support the breeding of Drosophila after visit on I. rosea. One steroidal terpenoid compound abundantly produced from gleba named, anthraergostatetraenol hexahydrobenzoate is also responsible for feeding, similar compound reported from other fungi [Citation48,Citation49]. Similarly, various woodland Drosophila species were reported as a food generalist, facultative fungal breeders and also a number of mycophagous organisms are attracted to P. impudicus for the program of oviposition and breeding [Citation50]. There is diverse variety of various insects like Coleoptera, Diptera, and more were reported to be obligatory or opportunistic breeders on different mushroom fruit bodies [Citation51–55]. Therefore, the attractiveness of gleba food odor blends containing few key odorants compounds which are responsible for behavioral response of Drosophila and blends of fatty acids and pheromones are responsible for feeding and breeding mechanism.
5. Conclusions
To summarize, the I. rosea was first time reported in association with Leucaena leucocephala from India and molecular phylogenetic analysis revealed that it formed sister clade with I. galericulata. During its life cycle, I. rosea produced blends of volatile compounds and three abundant fatty acids and pheromone. The mixture of volatiles was responsible for attraction of Drosophila species toward the gleba. Three important abundant fatty acids, which activated the olfactory response for searching of food. Thus, flies were feeds on gleba. The pheromones produced by gleba is responsible for breeding program of Drosophila. Since the fruiting bodies are short lived, we will in future undertake a prospective study to decipher the volatile compounds of different stages of fruiting and how it affects Drosophila interaction and possible role of this interaction in fungal spore dispersal.
Acknowledgments
The authors are thankful to Director, Agharkar Research Institute, Pune, for providing the necessary facilities and support for the research. Authors are thankful to Bhupendra V. Shravage for a fruitful discussion on behavioral studies on Drosophila.
Disclosure statement
The authors declare that there are no conflicts of interest. All the experiments undertaken in this study comply with the current laws of the country where they were performed.
References
- Hosaka K, Bates ST, Beever RE, et al. Molecular phylogenetics of the gomphoid-phalloid fungi with an establishment of the new subclass Phallomycetidae and two new orders. Mycologia. 2006;98(6):949–959.
- Fries RE. Cber einige Gasteromyceten aus Bolivia und Argen-tinien. Arkives for Botanik. 1909;8(11):3–7.
- Spegazzini C. Fungi Argent. novi vel criti. Anales Del Museo Nacional de Buenos Aires. 1899;6:183.
- Malençon G. Phallus roseus A. Delile 1813, alias Itajahya rosea(Delile) Ed. Fischer 1929. Bull Soc mycolog France. 1984;100:15–33.
- Kreisel H. A preliminary survey of the genus Phallus sensu lato. Czech Mycol. 1996;48(4):273–281.
- Möller Alfred. BrazilischenPilzblumen, 79–100 et 148, in Schimp. Botanische Mittheilungen aus den Tropen. 1895. 7, p. 79.
- Malençon G. Itajahya rosea (Delile) E. Fischer, très rare Phaloidée decouverte dans l’Ardrar Mauritanien par M. le Pr. Th. Manad. Bulletin de la Société d´Histoire Naturelle de l’Afrique du Nord 1953. 44, p.70–75.
- Ottoni BS, Silva BDB, Fazolino EP, et al. Phallus roseus, first record from the neotropics. Mycotaxon. 2010;112(1):5–8.
- Delile AR. Phallus roseus Delile, Flore d’Egypte. Explic. Pl. (Paris) 1813. 2, p. 300.
- Cabral TS, Marinho P, Goto BT, et al. Abrachium, a new genus in the Clathraceae, and Itajahya reassessed. Mycotaxon. 2012;119(1):419–429.
- Ingold CT. Fungal spores: their liberation and dispersal. Oxford: Clarendon, 1971. p. 302.
- Malloch D, Blackwell M. Dispersal of fungal diaspores. In: Carroll GC, Wicklow DT, editors. The fungal community: its organization and role in the ecosystem. 2nd edn. New York (NY): Marcel Dekker; 1992. p. 147–171.
- Driessen G, Hemerik L. Aggregative responses of parasitoids and parasitism in populations of Drosophila breeding in fungi. Oikos. 1991;61(1):96–107.
- Pudil F, Uvira R, Janda V. Volatile compounds in stinkhorn (Phallus impudicus L. ex Pers.) at different stages of growth. Eur Sci J. 2014;10:163–171.
- Tuno N. Spore dispersal of Dictyophora fungi (Phallaceae) by flies. Ecol Res. 1998;13(1):7–15.
- Aamir S, Sutar S, Singh SK, et al. A rapid and efficient method of fungal genomic DNA extraction, suitable for PCR based molecular methods. PPQ. 2015;5(2):74–81.
- Vilgalys R, Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol. 1990;172:4238–4246.
- White TJ, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. New York (NY): Academic Press Inc.; 1990. p. 315–322.
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425.
- Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA. 2004;101(30):11030–11035.
- Kumar S, Stecher G, Tamura K, et al. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874.
- Long WH, Stouffer DJ. Studies in the Gasteromycetes – IX. The genus, Itajahya in North America. Mycologia. 1943;35:620–628.
- Marincowitz S, Coetzee MPA, Wilken PM, et al. Phylogenetic placement of Itajahya: an unusual Jacaranda fungal associate. IMA Fungus. 2015;6(2):257–262.
- Moreno G, Khalid AN, Alvarado P, et al. Phallus hadriani and P. roseus from Pakistan. Mycotaxon. 2013;125(1):45–51.
- Kreisel H, Al-Fatimi M. Further basidiomycetes from Yemen. Feddes. Repertorium. 2008;119(5–6):463–483.
- Hansford CG. Australian fungi. II. New records and revisions. Proc Linnean Soc New South Wales. 1954;79:97–141.
- Bernays EA, Chapman RF. Host–plant selection by phytophagous insects. Chapman & Hall, New York (NY), Boston (MA): Springer; 1994. p. 1–283.
- Pacioni G, Bologna MA, Laurenzi M. Insect attraction by Tuber: a chemical explanation. Mycol Res. 1991;95(12):1359–1363.
- Bengtsson G, Hedlind K, Rundgren S, et al. Selective odor perception in the soil Collembola. J Chem Ecol. 1991;17(11):2113–2125.
- Jonsell M, Nordlander G. Field attraction of Coleoptera to odours of the wood decaying polypores Fomitopsis pinicola and Fomes fomentarius. Ann Zool Fenn. 1995;32:391–402.
- Honda H, Ishiwatari T, Matsumoto Y. Fungal volatiles as oviposition attractants for the yellow peach moth, Conogethes punctiferalis (Guenée) (Lepidoptera; Pyralidae). J Insect Physiol. 1988;34(3):205–211.
- Phelan LP, Lin H. Chemical characterization of fruit and fungal volatiles attractive to dried-fruit beetle Carpophilus hemipterus (L.) (Coleoptera: Nitidulidae). J Chem Ecol. 1991;17(6):1253–1262.
- Pierce AM Jr, Pierce HD, Oehlschlager AC, Borden JH, et al. 1-Octen-3-ol, attractive semiochemical for foreign grain beetle, Ahasverus advena (Waltl) (Coleoptera: Cucujidae). J Chem Ecol. 1991;17(3):567–580.
- Pfeil RM, Mumma RO. Bioassay for evaluating attraction of the phorid fly, Megaselia halterata to compost colonized by the commercial mushroom, Agaricus bisporus and to 1-octen-3-ol and 3-octanone. Entomol Exp Appl. 1993;69(2):137–144.
- Borg–Karlson AK, Englund FO, Unelius CR. Dimethyloligosulphide, major volatiles released from Sauromatum guttatum and Phallus impudicus. Phytochemistry. 1994;35(2):321–323.
- Kumar P, Lomash V, Jatav PC, et al. Prenatal developmental toxicity study of n-heneicosane in Wistar rats. Toxicol Ind Health. 2016;32(1):118–125.
- Dweck HKM, Ebrahim SAM, Khallaf MA, et al. Olfactory channels associated with the Drosophila maxillary palp mediate short- and long-range attraction. eLife. 2016;5:14925.
- Riffell JA, Lei H, Hildebrand JG, et al. Neural correlates of behavior in the moth Manduca sexta in response to complex odors. Proc Natl Acad Sci USA. 2009;106:19219–19226.
- Jürgens A, Witt T, Gottsberger G. Flower scent composition in night-flowering *Silene species (Caryophyllaceae). Biochem Syst Ecol. 2002;30(5):383–397.
- Dötterl S, Füssel U, Jürgens A, et al. 1,4-Dimethoxybenzene, a floral scent compound in willows that attracts an oligolectic bee. J Chem Ecol. 2005;31:2993–2998.
- Anholt RRH. Chemosensation and evolution of Drosophila host plant selection. iScience. 2020;23:100799.
- Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160.
- Masek P, Keene AC. Drosophila fatty acid taste signals through the PLC pathway in sugar-sensing neurons. PLoS Genet. 2013;9(9):e1003710.
- Lilleskov EA, Bruns TD. Spore dispersal of a resupinate ectomycorrhizal fungus, Tomentella sublilacina, via soil food webs. Mycologia. 2005;97:762–769.
- Kües U, Navarro-Gonzaléz M. Communication of fungi on individual, species, kingdom and above kingdom levels. In: Anke T, Weber S, Editors. Physiology and genetics. Selected basic and applied aspects. The mycota. Berlin: Springer; 2009. p. XV:79–106.
- Kobayashi M, Kitabayashi K, Tuno N. Spore dissemination by mycophagus adult drosophilids. Ecol Res. 2017;32(4):621–626.
- Zarbin PHG, Princival JL, Lima ER, et al. Unsymmetrical double Wittig olefination on the syntheses of insect pheromones. Part 1: synthesis of 5,9-dimethylpentadecane, the sexual pheromone of Leucoptera coffeella. Tetrahedron Lett. 2004;45(2):239–241.
- Afieroho OE, Ugoeze KC. Gas Chromatography-Mass Spectroscopic (GC-MS) Analysis of n-hexane extract of Lentinus tuber-regium (Fr) Fr (Polyporaceae) Syn Pleurotus tuber regium Fr sclerotia. Trop J Pharm Res. 2014;13(11):1911–1915.
- Aneesh S, Thoppil JE. Study on cytotoxicity and chemical profiling of Hexagonia tenuis (Hook.) Fr., a potential bracket fungus (Polyporaceae). Int J Pharm Biol Sci. 2019;9(3):822–831.
- Driessen G, Van Alphen JJM, Hemerik L. Drosophila species, breeding in the stinkhorn (Phallus impudicus Pers.) and their larval parasitoids. Neth J Zool. 1989;40(3):409–427.
- Hosaka K, Uno K. A preliminary survey on larval diversity in mushroom fruit bodies. Bull Natl Museum Nat Sci Ser B (Botany). 2012;38:77–85.
- Jonsell M, González Alonso C, Forshage M, et al. Structure of insect community in the fungus Inonotus radiatus in riparian boreal forests. J Nat Hist. 2016;50(25–26):1613–1631.
- Kinzner MC, Tratter M, Bächli G, et al. Oviposition substrate of the mountain fly Drosophila nigrospersa (Diptera: Drosophilidae). PLoS One. 2016;11(10):e0165743.
- Collett TS, Zeil J. Insect learning flights and walks. Curr Biol. 2018;28(17):R984–R988.
- Epps MJ, Penick CA. Facultative mushroom feeding by common woodland ants (Formicidae, Aphaenogaster spp.). Food Web. 2018;14:9–13.
