Abstract
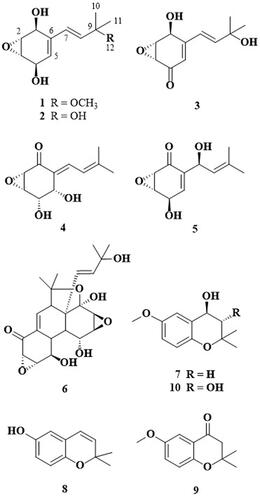
In our ongoing search for new secondary metabolites from fungal strains, one novel compound (1) and nine known compounds (2-10) were isolated from the EtOAc-soluble layer of the culture broth of Panus rudis. The culture broth of P. rudis was extracted in acetone and fractionated by solvent partition; column chromatography using silica gel, Sephadex LH-20, and Sephadex G-10; MPLC; and HPLC. The structures of isolated compounds were elucidated by one- and two-dimensional NMR and LC-ESI-mass measurements. One new compound, panepoxydiol (1), and nine known compounds, (E)-3-(3-hydroxy-3-methylbut-1-en-1-yl)-7-oxabicyclo[4.1.0]hept-3-ene-2,5-diol (2), isopanepoxydone (3), neopanepoxydone (4), panepoxydone (5), panepophenanthrin (6), 4-hydroxy-2,2-dimethyl-6-methoxychromane (7), 6-hydroxy-2,2-dimethyl-3-chromen (8), 2,2-dimethyl-6-methoxychroman-4-one (9), 3,4-dihydroxy-2,2-dimethyl-6-methoxychromane (10), were isolated from the culture broth of P. rudis. This is the first report of isolation of a new compound panepoxydiol (1) and nine other chemical constituents (2-5, 7-10) from the culture broth of P. rudis.
Mushrooms belonging to the genus Panus (Polyporaceae) are characterized by a thin-fleshed but durable basidiomycete. They live on a wide range of broadleaf trees and are distributed in tropical regions around the world [Citation1]. P. rudis was reported to produce hexacyclinol (a new antiproliferative metabolite), panepophenanthrin (a novel inhibitor of ubiquitin activation), and laccase [Citation2–4]. In an investigation of chemical constituents from the culture broth of P. rudis, we isolated a new compound (1) and nine known compounds (2-9, ). In this paper, we describe the isolation, structure determination, and antioxidant activity of these compounds.
A fungal strain of P. rudis was cultured on potato dextrose agar (PDA; BD Difco, Seoul, Korea) for 1 week at 27 °C. Small plugs of agar were punched out and inoculated into 23 1-liter flasks each containing 400 mL of potato dextrose broth (PDB; MB Cell, Seoul, Korea) and cultured for 3 weeks at 27 °C on a rotary shaker incubator at 120 rpm. The whole culture broth (9 L) was extracted in acetone for 24 h at room temperature and then filtered to remove mycelial debris. The filtrate was concentrated to remove acetone and partitioned with ethyl acetate. The ethyl acetate-soluble layer (14.1 g) was separated by silica gel column chromatography eluted with CHCl3:MeOH (100:1, 50:1, 30:1, 20:1, 10:1 0:100, v/v, stepwise). Two fractions, CHCl3:MeOH = 100:1 (2.66 g) and CHCl3:MeOH = 30:1 (3.38 g), displayed potent antioxidant activity. The CHCl3:MeOH = 100:1 (2.66 g) fraction was concentrated and subjected to reversed-phase medium-pressure liquid chromatography (MPLC) eluted with a gradient of increasing methanol in water (10→100% aq. MeOH) to yield an antioxidant fraction M1 and compounds 7 (1.1 g) and 9 (60.0 mg). Compound 8 (13.4 mg) was obtained from fraction M1 by preparative reversed-phase HPLC eluted with 33% aq. MeOH. The CHCl3:MeOH = 30:1 (3.38 g) fraction was concentrated and subjected to reversed-phase MPLC eluted with a gradient of increasing methanol in water (10→100% aq. MeOH) to afford a fraction M2 and compound 10 (12.7 mg). The M2 fraction was subjected to chromatography on a Sephadex LH-20 column eluted with MeOH to yield fractions L1 and L2. Compounds 1 (45.4 mg), 2 (2.6 mg), and 3 (45.1 mg) were obtained from fraction L1 by preparative reversed-phase HPLC eluted with 15% aq. MeOH. Fraction L2 was separated by Sephadex G-10 column chromatography eluted with distilled water to yield fraction G1. Compounds 4 (4.0 mg), 5 (14.3 mg), and 6 (47.5 mg) were purified from fraction G1 by preparative reversed-phase HPLC eluted with 22% aq. MeOH.
Compound 1 was obtained as a brown oil with a specific rotation value of −64.8° ([α]D; 25 °C, c = 0.1, MeOH). Its molecular formula was established as C12H18O4 through a high-resolution ESI-mass measurement (m/z 249.1105 [M + Na]+, Δ + 0.2 mmu) (Supplementary Figure S1). The UV spectrum of 1 showed a maximum absorption band (log ε) at 233.0 (4.2) nm. The 1H NMR spectrum of 1 showed signals due to three olefinic methines at δ 6.12 (d, J = 16.4 Hz), 6.07 (d, J = 16.4 Hz), and 5.71; four oxygenated methines at δ 4.61, 4.36, 3.50, and 3.38; a methoxy at δ 3.16; and two methyl groups at δ 1.29 and 1.28 (Supplementary Figure S2). The 13C NMR spectrum in combination with HMQC spectrum displayed a sp2 carbon at δ 136.5; three sp2 methine carbons at δ 138.1, 130.1, and 126.8; an oxygenated quaternary carbon at δ 76.8; four oxygenated methine carbons at δ 65.4, 64.0, 57.3, and 56.2; a methoxy carbon at δ 50.9; and two methyl carbons at δ 26.5 and 26.0 (; Supplementary Figures S3 and S4). The 1H-1H COSY spectrum established two partial structures, as shown in (Supplementary Figure S5). The HMBC correlations from H-1, H-2, and H-4 to C-6; from H-5 to C-7; from H-7 to C-1, C-5, and C-9; from H-8 to C-9, C-10, and C-11; and from H-10 and H-11 to C-8 and C-9 indicated the presence of the (E)-3-(3-hydroxy-3-methylbut-1-en-1-yl)-7-oxabicyclo[4.1.0]hept-3-ene-2,5-diol moiety. An additional HMBC correlation from the methoxy protons at δ 3.16 to C-9 suggested its attachment to C-9 (Supplementary Figure S6). The relative stereochemistry of 1 was suggested as shown in by comparison of NMR chemical shift values of known compounds 2 and 3 [5, 6]. The absolute stereochemistry still remains to be determined. Based on the aforementioned evidence, the chemical structure of 1 was determined to be a new compound and was named panepoxydiol.
Figure 2. Two-dimensional NMR correlations of compound 1, isolated from the culture broth of Panus rudis.
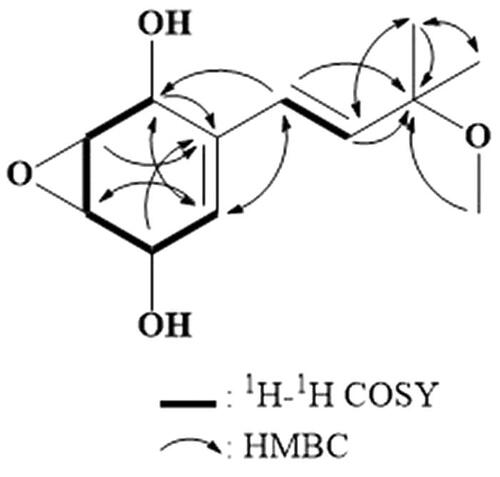
Table 1. 1H and 13C NMR spectral data for compound 1 in CD3OD.
Other isolated compounds 2-10 were identified as (E)-3-(3-hydroxy-3-methylbut-1-en-1-yl)-7-oxabicyclo[4.1.0]hept-3-ene-2,5-diol (2), isopanepoxydone (3), neopanepoxydone (4), panepoxydone (5), panepophenanthrin (6), 4-hydroxy-2,2-dimethyl-6-methoxychromane (7), 6-hydroxy-2,2-dimethyl-3-chromen (8), 2,2-dimethyl-6-methoxychroman-4-one (9), and 3,4-dihydroxy-2,2-dimethyl-6-methoxychromane (10) by comparison of NMR spectroscopic data with published literature values [Citation3,Citation5–9].
Antioxidant activity of compounds 1-10 was evaluated using the ABTS radical scavenging assay method [Citation10]. Commercialized antioxidants, butylated hydroxyanisole (BHA), and Trolox were used as positive controls. Compound 8 displayed potent antioxidant activity with an IC50 value of 57.2 ± 7.2 μM in the ABTS radical scavenging assay, which is approximately two-times less active than BHA (IC50, 26.5 ± 1.6) and Trolox (IC50, 29.9 ± 1.3). The other isolated compounds showed no activity up to 200 µM.
P. rudis, a fungal strain of mushroom origin, was selected by its moderate antifungal activity and the induction of plant disease resistance during initial screening to select fungi with biological activity. In this study, we investigated the chemical constituents of the culture broth of P. rudis, a fungus of mushroom origin. Consequently, chemical constituents 1-10 were isolated, and their chemical structures were determined using spectroscopic methods. To the best of our knowledge, compound 1 was a new compound, and (E)-3-(3-hydroxy-3-methylbut-1-en-1-yl)-7-oxabicyclo[4.1.0]hept-3-ene-2,5-diol (2), isopanepoxydone (3), neopanepoxydone (4), panepoxydone (5), 4-hydroxy-2,2-dimethyl-6-methoxychromane (7), 6-hydroxy-2,2-dimethyl-3-chromen (8), 2,2-dimethyl-6-methoxychroman-4-one (9), and 3,4-dihydroxy-2,2-dimethyl-6-methoxychromane (10) were isolated for the first time from the culture broth of P. rudis. Compound 8 was previously reported to display antibiotic activity against Candida albicans [Citation11]. In this study, compound 8 also exhibited potent ABTS radical scavenging activity.
Supplemental Material
Download MS Word (874.8 KB)Acknowledgements
The authors thank Ms. Ji-Young Oh, Center for University-wide Research Facilities (CURF) at Jeonbuk National University, for NMR measurements.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Vargas-Isla R, Capelari M, Menolli NJ, et al. Relationship between Panus lecomtei and P. strigellus inferred from their morphological, molecular and biological characteristics. Mycoscience. 2015;56(6):561–571.
- Schlegel B, Härtl A, Dahse H-M, et al. Hexacyclinol, a new antiproliferative metabolite of Panus rudis HKI 0254. J Antibiot. 2002;55(9):814–817.
- Sekizawa R, Ikeno S, Nakamura H, et al. Panepophenanthrin, from a mushroom strain, a novel inhibitor of the ubiquitin-activating enzyme. J Nat Prod. 2002;65(10):1491–1493.
- Zhang M, Wu F, Wei Z, et al. Characterization and decolorization ability of a laccase from Panus rudis. Enzyme and Microb Technol. 2006;39(1):92–97.
- Shotwell JB, Koh B, Choi HW, et al. Inhibitors of NF-κB signaling: design and synthesis of a biotinylated isopanepoxydone affinity reagent. Bioorg Med Chem Lett. 2002;12(23):3463–3466.
- Barros-Filho BA, de Oliveira MC, Mafezoli J, et al. Secondary metabolite production by the basidiomycete, Lentinus strigellus, under different culture conditions. Nat Prod Commun. 2012;7(6):771–773.
- Lei X, Johnson RP, Porco JA. Total synthesis of the ubiquitin-activating enzyme inhibitor (+)-panepophenanthrin. Angew Chem. 2003;115(33):4043–4047.
- Cotelle N, Moreau S, Bernier JL, et al. Antioxidant properties of natural hydroquinones from the marine colonial tunicate Aplidium californicum. Free Radic Biol Med. 1991;11(1):63–68.
- Cota BB, Rosa LH, Fagundes EMS, et al. A potent trypanocidal component from the fungus Lentinus strigosus inhibits trypanothione reductase and modulates PBMC proliferation. Mem Inst Oswaldo Cruz. 2008;103(3):263–270.
- Lee IK, Jung JY, Kim YS, et al. p-Terphenyls from the fruiting bodies of Paxillus curtisii and their antioxidant properties. Bioorg Med Chem. 2009;17(13):4674–4680.
- Favre-Godal Q, Pinto S, Dorsaz S, et al. Identification of antifungal compounds from the root bark of Cordia anisophylla J.S. Mill. J Braz Chem Soc. 2019;30:472–478.