Abstract
Paramyrothecium eichhorniae sp. nov. was observed and collected from Chiang Mai and Phetchaburi Provinces, Thailand. This new species is introduced based on morphological and molecular evidence. This fungus is characterized by its production of sporodochium conidiomata with a white setose fringe surrounding an olivaceous green to dark green slimy mass of conidia, penicillately branched conidiophores, and aseptate and cylindrical to ellipsoid conidia. Phylogenetic analyses of combined LSU rDNA, ITS rDNA, tef1, rpb2, tub2 and cmdA sequence data using maximum parsimony, maximum likelihood and Bayesian approaches placed the fungus in a strongly supported clade with other Paramyrothecium species in Stachybotryaceae (Hypocreales, Sordariomycetes). The descriptions of the species are accompanied by illustrations of morphological features, and a discussion of the related taxa is presented.
1. Introduction
Leaf blight disease of water hyacinth (Eichhornia crassipes (Mart.) Solms) is distributed in different geographical areas of Thailand. Several fungal species, such as Alternaria alternata, A. geophila, A. eichhorniae, Ascochyta chartarum, Bipolaris zeicola (syn. Cochliobolus carbonum), Cercospora rodmanii, Curvularia lunata, Epicoccum nigrum, Fusarium chlamydosporum, F. equiseti, F. pallidoroseum, Globisporangium ultimum (syn. Pythium ultimum), Paramyrothecium roridum (formerly known as Myrothecium roridum) and Stemphylium vesicarium have been reported to be pathogens of water hyacinth [Citation1–3]. Leaf blight disease of water hyacinth has been observed in Thailand, and the fungal pathogen causing the disease was identified as P. roridum (=Myrothecium roridum) using morphological characteristics and ITS rDNA sequence analysis [Citation4–5], as same as the previous report by Okunowo et al. [Citation6] in Nigeria. Moreover, there are many reports that P. roridum has the potential to be a mycoherbicide against water hyacinth and other water weeds [Citation2,Citation6,Citation7]. The host range of P. roridum strain TBRC 10637 (=KKFC448) was evaluated on 77 plant species (40 families), including water hyacinth. This fungus could not infect 74 economically important plants, while symptoms were observed on water hyacinth plants and severe and slight symptoms were observed on duckweed and water lettuce plants [Citation8].
Lombard et al. [Citation9] revised the genus Myrothecium which resulted in the recognition of 13 new genera based on the polyphyletic origin of its species, and more than 15 species have been reported within two renamed genera, Paramyrothecium and Albifimbria. The genus Paramyrothecium was introduced with P. roridum (Tode) L. Lombard & Crous as the type species. Species of Paramyrothecium are reported as saprobe and weakly pathogenic fungi with a worldwide distribution [Citation9]. Paramyrothecium is characterized as follows: sporodochial conidiomata, with or without a white setose fringe surrounding the slimy mass of conidia. Straight to flexuous setae, 1–3(–4)-septate, hyaline conidiophores penicillately branched; conidiogenous cells phialidic or percurrent. Conidia aseptate to 1-septate, cylindrical to ellipsoidal to obovoid, hyaline to pale green, smooth; a sexual morph has not been reported. This genus is similar to Neomyrothecium except that the pulvinate sporodochia with a white setose fringe [Citation9]. Phylogenetic analysis using the cmdA, ITS, rpb2, and tub2 genes showed that members of Paramyrothecium formed a highly supported clade distant from the Myrothecium s. str. clade [Citation9]. However, Krisai-Greilhuber et al. [Citation10] noted that most of the species of Paramyrothecium could not be discriminated morphologically; thus, it was necessary to combine a phylogenetic analysis for accurate taxonomic assignment.
In this study, we introduce a new species in the genus Paramyrothecium, which belongs to Stachybotryaceae (Hypocreales, Sordariomycetes), based on morphological and molecular evidence.
2. Materials and methods
2.1. Fungal specimen
Water hyacinth leaves showing blight symptoms were observed and collected from natural water resources in Chiang Mai and Phetchaburi provinces, Thailand.
2.2. Isolation and morphological studies
The fungal pathogen was isolated using the tissue transplanting method on the potato dextrose agar plates (PDA; Difco, Becton, Dickinson and Company, Bangkok, Thailand). The cultures were deposited in the Kasetsart Kamphaengsaen Fungal Collection (KKFC) and Thailand Bioresource Research Center (TBRC), Thailand. The morphological characteristics of the fungi were examined under a light microscope Olympus BX51 (Olympus, Bangkok, Thailand). The sporodochia were collected directly from the substrate using fine forceps or a needle and then placed in a drop of sterilized water on a microscope slide, and a coverslip was added. The specimens were dried by a dehydration machine at 45 °C for 24–36 h and deposited in the BIOTEC Bangkok Herbarium (BBH).
2.3. Pathogenicity test
The healthy water hyacinth plants with 25–50 cm2 in size of leaves were prepared for inoculation. The fungal strain TBRC 10637 was subcultured on PDA and incubated at 28 °C. The photoperiods (12 h) were provided by white fluorescent lamps. Inoculation was done by spraying the leaves of water hyacinth plant with 1 × 108 spores per mL; the control treatment was sprayed with 10 mL of sterile distilled water. This experiment was conducted by using a completely randomized design (CRD), with 10 replications of each treatment. The plants were placed in a growth chamber with 100% relative humidity (RH) for 24 h and then moved to greenhouse conditions. The temperatures in the greenhouse ranged from 26 to 32 °C, with 65–90% RH. The disease symptom was observed at 7 days after inoculation and compared with the leaf blight symptom observed in the nature. Fungal re-isolation was conducted by using the tissue transplanting method. The infected leaves were cut into a 0.5 cm × 0.5 cm size. The samples were surface-disinfected with a 10% sodium hypochlorite solution for 5 min and then washed two times with sterilized distilled water before being plated on the PDA. The cultures were incubated at 28 °C under white fluorescent lamps with a 12 h day per night cycle.
2.4. DNA extraction and PCR amplification
Genomic DNA was extracted from the mycelia on the PDA using a CTAB method [Citation11]. Six nuclear loci, LSU rDNA, ITS rDNA, tef1, rpb2, tub2 and cmdA, were amplified. The primers used to amplify these regions were LROR/LR5 [Citation12], ITS5/ITS4 [Citation13], EF1-728F/EF2, 5F2/7cR [Citation14], T1/T22 [Citation15]) and CAL-228F/CAL2Rd [Citation16–17]. The amplification conditions for the LSU and ITS regions followed the protocol described in Sakayaroj [Citation12], while the amplification conditions for the tef1, rpb2, tub2 and cmdA genes followed the protocol described in Liang et al. [Citation18]. PCR products were sequenced by Macrogen Inc. (Seoul, South Korea) for Sanger dideoxy sequencing by using the same primers as for amplification.
2.5. Sequence alignment and phylogenetic analyses
Thirty-two sequences () were checked for ambiguous bases and assembled using BioEdit v.7.0.5.3 [Citation19]. All the sequences were aligned with MUSCLE [Citation20] and manually edited using BioEdit v.7.0.5.3 [Citation19]. The phylogenetic analyses were performed using maximum parsimony (MP), maximum likelihood (ML) and Bayesian inference (BI).
Table 1. Taxa used in the phylogenetic analyses and the new taxa are deposited sequences shown in bold.
The maximum parsimony analysis was performed by PAUP v.4.0b10 [Citation21] with 10 replicates of stepwise additions, the heuristic search option, the addition of 1,000 random taxa and the tree bisection reconnection (TBR) branch swapping algorithm. All the characters were given equal weight, and the gaps were treated as missing data. Maxtrees was unlimited, branches of zero length were collapsed, and all the multiple, equally parsimonious trees were saved. The robustness of the most parsimonious tree was estimated based on 1,000 bootstrap replications.
The maximum likelihood analysis was performed on the CIPRES supercomputer using the RAxML-HPC2 v.8.2.12 program on XSEDE [Citation22]. One thousand nonparametric bootstrap iterations were run with the GTR model and a discrete gamma distribution.
Bayesian analyses (BA) were conducted in MrBayes v.3.0b4 [Citation23] with a uniform [GTR + I + G] model, Isetnst = 6 rates = invgamma, and prsetstatefreqpr = dirichlet (1,1,1,1). The evolutionary best-fit models of Bayesian analysis (BA) were conducted in MrBayes 3.2.6 [Citation24]. The evolutionary best-fit model was evaluated by means of MrModelTest 2.3 [Citation25] before analysis. Posterior probabilities (PPs) were calculated by the Markov chain Monte Carlo algorithm [Citation26]. Four Markov chains were run for 5,000,000 generations, and trees were sampled every 100 generations. The first 5,000 trees, which represented the burn-in phase of the analysis, were discarded, with 50,000 trees used for calculating the posterior probabilities (BIPP) in the consensus tree.
The matrix and the resulting tree have been deposited at TreeBASE under submission number 29197 (http://purl.org/phylo/treebase/phylows/study/TB2:S29197).
3. Results
3.1. Phylogenetic analyses
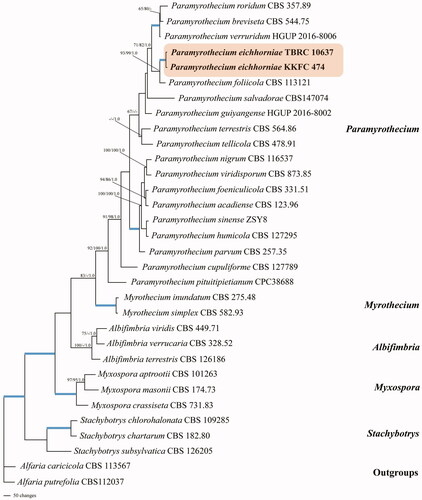
The assembled sequences comprised 32 taxa (). Alfaria caricicola (CBS 113567) and and Alfaria putrefolia (CBS 112037) were used as outgroups. After alignment, the best tree was subjected to maximum parsimony, which combined LSU rDNA, ITS rDNA, tef1, rpb2, tub2 and cmdA. The dataset consists of 4043 characters, of which 2434 were constant, 366 were variable parsimony-uninformative and 1253 were parsimony informative with a length of 4532 steps (CI = 0.570, RI = 0.687, RC = 0.392 and HI = 0.430). The best scoring RAxML tree had a final optimization likelihood value of −25813.607984. The bootstrap support values for the maximum parsimony (BSMP, left) and maximum likelihood (BSML, middle) analyses were greater than 50%. The branches with Bayesian posterior probabilities (BPP, right) greater than 0.95 are indicated at the nodes.
The phylogenetic analyses showed that all the collected strains were clustered in the family Stachybotryaceae. The two strains of P. eichhorniae sp. nov. (TBRC 10637 and KKFC 474), which were recovered as distinct species, were grouped with P. foliicola with bootstrap and posterior probability support (97% BSMP, 99% BSML and 1.00 BPP) in the tree ().
Figure 1. Phylogenetic relationships of Paramyrothecium spp. from combined ITS, LSU, tef1, rpb2, tub2 and cmdA analyses. Bootstrap values (1,000 replicates) over 50% for MP and RAxML and over 0.95 for Bayesian posterior probabilities are added to the left of the nodes (MP/ML/PP), multiplied by 100; the blue lines in the tree represent bootstrap (BSMP and BSML) support of 100% and a posterior probability (BPP) of 1.00.
3.2. Morphological analysis
The genus Paramyrothecium was introduced by Lombard et al. [Citation9]. Its original diagnosis was of sporodochial conidiomata, with or without a white setose fringe surrounding the slimy mass of conidia, hyaline conidiophores with penicillately branched, aseptate to 1-septate ellipsoidal to obovoid conidia. It was considered that the species identification using morphology is imprecise because their morphological features cannot clearly differentiate species. We summarized the morphological characters of species of Paramyrothecium and provided the details of the host and distribution in . For the single gene tree of each loci see Supplementary Figures S1–6.
Table 2. Known Paramyrothecium species with host, location, and synopsis of morphological characteristics.
4. Taxonomy
Paramyrothecium eichhorniae J. Unartngam, A. Unartngam & U. Pinruan, sp. nov. .
Figure 2. Paramyrothecium eichhorniae sp. nov. (BBH 48295, holotype). (a) Leaf blight disease symptom on water hyacinth. (b) Sporodochial conidiomata on substrate. (c) Sporodochial conidiomata on PDA. (d–f) Colonies on PDA, CMA, and OA after 15 days (left, from above; right, from below). (g–h) Setae. (i–j) Conidiogenous cells. (k–n) Conidia. Scale bars: a = 2 cm, b = 100 μm, c = 0.3 mm, d–f = 1 cm, g–h = 10 μm, and i–n = 5 μm.

Index Fungorum number: IF556554
Etymology: Name refers to Eichhornia, the plant genus from which this fungus was collected.
Sexual morph: Unknown.
Holotype: BBH 48295
Asexual morph: Conidiomata sporodochial, stromatic, superficial, cupulate, scattered or gregarious; outline oval or irregular in outline, 55 − 500 μm in diam, 60–200 μm deep with a white setose fringe surrounding an olivaceous green to dark green slimy mass of conidia. Setae arising from sporodochia, thin-walled, hyaline, 1 − 3-septate, smooth, unbranched, straight to flexuous, 40 − 120 μm long, 2 − 3 μm wide, terminating in an acute rounded apex. Conidiophores growing from the basal stroma, consisting of a stipe and a penicillately branched conidiogenous apparatus, stipes unbranched, hyaline, septate, smooth, 15–40 × 2–3 μm, primary branches aseptate, unbranched, smooth, 10–17 × 2–3 μm; secondary branches aseptate, unbranched, smooth, 10 − 15 × 2 − 3 μm; terminating in a single whorl of 3–5 conidiogenous cells arising apically. Conidiogenous cells phialidic, cylindrical to subcylindrical, hyaline, smooth, straight to slightly curved, (8−)11 − 17(−20) x 2 − 3 μm, conspicuous collarettes and periclinal thickenings. Conidia aseptate, hyaline, smooth, cylindrical to ellipsoidal, 5 − 6.5 × 1.5 − 2.5 μm (n = 30, x̅ = 5.6 × 2.3 μm), rounded at both ends.
Known distribution: Amphoe Saraphi, Chiang Mai Province, Thailand.
Habit and habitat: on leaf of Eichhornia crassipes.
Culture characteristics: Colonies on PDA, Corn meal agar (CMA) and Oat meal agar (OA) approx. 9 cm in diam. after 14 d at 25 °C, circular with entire, white mycelium, hyaline, smooth; reverse on PDA creamy pink, sporulating in culture.
Material examined: THAILAND, Chiang Mai Province, on leaf of Eichhornia crassipes, 20 September 2012, O. Piyaboon and J. Unartngam (holotype BBH 48295); culture ex-holotype TBRC 10637.
Additional material examined: THAILAND, Phetchaburi Province, on the leaf of Eichhornia crassipes, 15 October 2012, O. Piyaboon and J. Unartngam (culture KKFC 474).
Note: Phylogenetically, P. eichhorniae is most closely related to P. foliicola L. Lombard & Crous (). Morphologically, it differs from P. foliicola on the longer conidiophore (up to 40 μm long) while in P. foliicola it is shorter (up to 25 μm long). The conidia of P. eichhorniae (5 − 6.5 × 1.5 − 2.5 μm) are slightly larger than those of P. foliicola (5 − 6 × 1 − 2 μm). The setae of P. eichhorniae (40 − 120 × 2 − 3 μm) are sometimes slightly longer than those of P. foliicola (60 − 100 × 2 − 3 μm). Furthermore, P. foliicola produces a rosy buff exudate that diffuses into the growth medium, which was not seen on P. eichhorniae. However, we found that both species could not be discriminated by morphology, it is greater way that a combined their phylogeny and morphology. Thus, the present strains were identified as the new species P. eichhorniae.
4.1. Pathogenicity test studies
The characteristics of leaf blight disease of water hyacinth in a natural water source included round-to-teardrop-shaped leaf spots and blights with conidial mass (). Pathogenicity test by spraying the spore suspension on water hyacinth leaves showed early leaf blight signs on the water hyacinths leaves and dead tissues appeared. All of the inoculated leaves showed symptoms and the sporodochia appeared on the leaves after 2 weeks of inoculation similar to the symptoms of leaf blight disease of water hyacinth in nature ().
5. Discussion
Taxonomic studies of Paramyrothecium have been based on morphological features and molecular analyses. In this study, the fungus causing leaf blight disease on water hyacinth plants collected in Chiang Mai and Phetchaburi Provinces belongs to the genus Paramyrothecium. P. eichhorniae is introduced as a new species and is well separated from other species of Paramyrothecium in the phylogenetic analyses of combined LSU rDNA, ITS rDNA, tef1, rpb2, tub2 and cmdA sequence data. This new species group with P. foliicola, however, its morphological characters are distinctive, with the conidiophore stipes of P. foliicola being shorter than those of P. eichhorniae. The conidia of P. foliicola are smaller than those of P. eichhorniae, and colony on the growth medium produces a rosy buff exudate, which was not seen on the P. eichhorniae cultures. Moreover, this is the first report of disease caused by Paramyrothecium was on water hyacinth. However, the present isolates on water hyacinth in Chiang Mai had previously been misclassified under P. roridum in 2014 using morphological characteristics and ITS rDNA sequence analysis [Citation4–5]. This study supported the comments of Krisai-Greilhuber et al. [Citation10] that the identification of Paramyrothecium species using morphology is imprecise because the morphological features cannot clearly differentiate species (). Combining morphology and analyses of the gene sequence data are needed.
Supplemental Material
Download MS Word (2.3 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Liyange NP, Gunasekera SA. Integration of Myrothecium roridum and 2,4-D in water hyacinth management. J Biol Chem. 1989;193:265–275.
- Tegene S, Hussein T, Tessema T, et al. Exploration of fungal pathogens associated with water hyacinth (Eichhornia crassipes (mart.) Solms-Laubach) in Ethiopia. Afr J Agric Res. 2012;7:11–18.
- Ray P, Sushilkumar , Pandey AK. Efficacy of pathogens of water hyacinth (Eichhornia crassipes) singly and in combination for its biological control. J Biol Control. 2008; 22:173–177.
- Piyaboon O, Unartngam A, Unartngam J. Effectiveness of Myrothecium roridum for controlling water hyacinth and species identification based on molecular data. Afr J Microbiol Res. 2014;8:1444–1452.
- Piyaboon O, Unartngam A, Unartngam J. Genetic relationships of Myrothecium roridum isolated from water hyacinth in Thailand using ISSR markers and ITS sequence analysis. J Agric Sci Technol. 2016;12:249–261.
- Okunowo WO, Osuntoki AA, Adekunle AA, et al. Occurrence and effectiveness of an indigenous strain of Myrothecium roridum tode: fries as a bioherbicide for water hyacinth (Eichhornia crassipes) in Nigeria. Biocontrol Sci Technol. 2013;23(12):1387–1401.
- Lee HB, Kim JC, Hong KS, et al. Evaluation of fungal strain, Myrothecium roridum F0252, as a bioherbicide agent. Plant Pathol J. 2008;24(4):453–460.
- Piyaboon O, Pawongrat R, Unartngam J, et al. Pathogenicity, host range and activities of a secondary metabolite and enzyme from Myrothecium roridum on water hyacinth from Thailand. Weed Biol. Manag. 2016;16(3):132–144.
- Lombard L, Houbraken J, Decock C, et al. Generic hyper-diversity in Stachybotriaceae. Persoonia. 2016;36:150–246.
- Krisai-Greilhuber I, Chen Y, Jabeen S, et al. Fungal systematics and evolution: FUSE 3. Sydowia. 2017;69:229–264.
- O'Donnell K, Cigelnik E, Weber NS, et al. Phylogenetic relationship among ascomycetous truffle and the true and false morels inferred from 18S and 28S ribosomal DNA sequence analysis. Mycologia. 1997;89(1):48–65.
- Sakayaroj J. Phylogenetics relationships of marine Ascomycota. Ph.D. Thesis, Prince of Songkla University, Thailand. 2005.
- White TF, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M.A., Gelfand, D.H., Sninsky, F.S. & White, T.J. (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, California, 1990. pp.315–322.
- O'Donnell K, Sarver BAJ, Brandt M, et al. Phylogenetic diversity and microsphere array-based genotyping of human pathogenic fusaria, including isolates from the multistate contact lens-associated U.S. keratitis outbreaks of 2005 and 2006. J Clin Microbiol. 2007;45(7):2235–2248.
- O'Donnell K, Cigelnik E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol Phylogenet Evol. 1997;7(1):103–116.
- Carbone I, Kohn LM. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91(3):553–556.
- Groenewald JZ, Nakashima C, Nishikawa J, et al. Species concepts in Cercospora: spotting the weeds among the roses. Stud Mycol. 2013;75(1):115–170.
- Liang J, Li G, Zhou S, et al. Myrothecium-like new species from turfgrasses and associated rhizosphere. MycoKeys. 2019;51:29–53.
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98.
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797.
- Swofford DL. PAUP: Phylogenetic analysis using parsimony, version 4.0b10. Sunderland (MA): Sinauer Associates, Inc. Publishers. 2002.
- Miller M, Pfeiffer W, Schwartz T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop 2010 (GCE), New Orleans, Louisiana, November 2010. pp. 1–8.
- Huelsenbeck JP, Ronquist F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17(8):754–755.
- Ronquist F, Huelsenbeck JP. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1572–1574.
- Nylander JAA. MrModelTest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University, Uppsala, Sweden. 2004.
- Larget B, Simon DL. Markov chain Monte Carlo algorithms for the Bayesian analysis of phylogenetic trees. Mol Biol Evol. 1999;16(6):750–759.

