?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Fungi of the genus Tuber are ectomycorrhizal fungi that form a symbiotic relationship mainly with oak and hazel trees. Tuber spp. exhibit a highly selective host plant preference; thus, for cultivation purposes it is important to select an appropriate host plant for successful mycorrhization. In addition, as mycorrhizal characteristics differ according to Tuber spp., it is necessary to understand the differences in mycorrhizae according to the fungal species. Tuber huidongense and Tuber himalayense were recently discovered in Korea; therefore, we used spore suspensions from these two species to inoculate two species of oak trees, Quercus acutissima and Quercus dentata, to compare colonization rates and morphologies of the mycorrhizae. The colonization rates demonstrated that the different Tuber spp. favored different host plant species. In addition, unique morphological and anatomical characteristics were observed for T. huidongense and T. himalayense depending on the host species. These findings can lead to new economically important agricultural activities related to truffle cultivation in Korea.
1. Introduction
Fungi in the genus Tuber (Ascomycota, Pezizales) emit a strong, diverse, and long-lasting aroma and the high value of truffles is largely attributed to their aroma. Tuber spp. form ectomycorrhizal relationships with diverse plants, especially oak trees [Citation1]. Different Tuber spp. exhibit a highly selective preference for specific host plants [Citation2]. For example, Tuber gibbosum and Tuber oregonense form a unique symbiotic relationship with Douglas fir (Pseudotsuga menziesii) [Citation3], whereas truffles native to Greece form associations with Quercus coccifera, Quercus ilex, and Quercus pubescents [Citation4].
For Tuber melanosporum, the efficiency and strength of mycorrhization differ depending on the host plant species, showing the highest colonization rate with Q. ilex [Citation4]. Depending on the Tuber spp., there are differences in the characteristics of mycorrhizae that form in the same host plant species [Citation5,Citation6]. When comparing the ectomycorrhiza (ECM) of white truffles formed on the same host plant, the anatomical structure and size of the cystidia, the cell wall thickness of the mantle, and type of transition morphology between the epidermoid and angular types differ for each species [Citation5]. In addition, Tuber spp. exhibit different mycorrhizal morphologies depending on the host plant species [Citation7].
Although there have been few studies on truffles in Korea, our study was facilitated by the recent discovery of two species of truffles, Tuber huidongense and Tuber himalayense [Citation8,Citation9]. The purpose of this study was to provide information on the morphology and anatomical characteristics of ECM and identify suitable host plants by comparing colonization rates following inoculation of T. huidongense and T. himalayense into the host trees Quercus acutissima and Quercus dentata. Quercus acutissima is a common species throughout Korea, whereas Q. dentata is a known host species for both T. huidongense and T. himalayense in Korea.
2. Materials and methods
2.1. Preparation of seedlings
Quercus acutissima acorns were collected from a natural site in Danyang, Korea, and Q. dentata acorns were collected from a market in Youngju, Korea. The acorn shells were removed and the seeds were surface-sterilized with 10% sodium hypochlorite (NaClO) for 30 min. The seeds were then placed in a plastic pot (280 mL) with an autoclaved 1:1 mixture of vermiculite and perlite, and cultured in a greenhouse for 6 months (8 h photoperiod, 55 ± 5% relative humidity, and 24 ± 1 °C).
2.2. Inoculation of Quercus seedlings and mycorrhizal synthesis
The fruiting bodies of T. huidongense and T. himalayense were collected from the rhizospheres of Q. dentata in Pohang [Citation8] and Danyang [Citation9], respectively. The fruiting bodies were surface-sterilized with 70% ethanol and ground with distilled water using a blender. Six-month-old seedlings of Q. acutissima and Q. dentata were inoculated near the roots with a 1 mL spore suspension containing 1.5 × 106 spores of T. huidongense or T. himalayense (determined using a hemocytometer; Paul Marienfeld GmbH & Co. KG, Lauda-Königshofen, Germany), and then roots were wrapped with a non-woven fabric. Tuber huidongense was inoculated into five seedlings of the two host species (10 seedlings total), whereas T. himalayense was inoculated into 10 seedlings of the two host species (20 seedlings total). The inoculated seedlings were transplanted into a plastic pot (280 mL) with an autoclaved 1:1 mixture of vermiculite and perlite; slaked lime was added to maintain the pH at 8. The pots were maintained in a greenhouse for 8 months and watered weekly.
2.3. Molecular identification of ECM
To determine the successful formation of ECM in each inoculated seedling, genomic DNA was extracted from a mycorrhizal root tip using the DNeasy Plant Mini kit (QIAGEN GmbH, Hilden, Germany). The ribosomal DNA internal transcribed spacer (ITS) region was then amplified using the primer pair ITS1F/ITS4 [Citation10]. The nucleotide sequences were analyzed (SolGent Co. Ltd., Daejeon, Korea) and identified using BLAST (https://www.ncbi.nlm.nih.gov/).
2.4. Morphological and anatomical characteristics of ECM
After 8 months of growth, seedlings were harvested and the morphological and anatomical characteristics of the ECM of T. huidongense and T. himalayense were observed. Morphological characteristics were observed using a dissection microscope (Olympus SZX7, Tokyo, Japan). After cross-sectioning the roots using a cryostat (CM1850, Leica, Heidelberg, Germany), the anatomical characteristics of the ECM were observed and recorded using a light microscope (Axio Imager A1, Carl ZEISS, Oberkochen, Germany).
2.5. Mycorrhizal root colonization rates
After 8 months of growth, seedlings were carefully removed from their pot and the roots were washed with distilled water to remove as much soil as possible. Thereafter, three locations along the root were randomly selected at 2 cm intervals. Subsequently, 100 root tips for each location of the seeding roots were examined, and the number of mycorrhizal root tips (T) and non-mycorrhizal root tips (N) were counted under a dissection microscope (Olympus SZX7). The percent root colonization rate (CR) was calculated as follows [Citation11]:
3. Results
3.1. Inoculation of seedlings and mycorrhizal synthesis
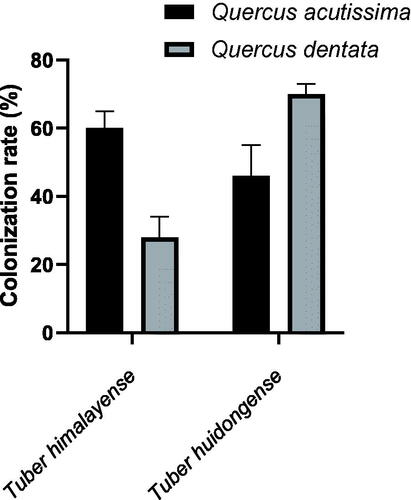
ECM formation in both host plants was observed approximately 2.5 months after inoculation with spore suspensions of T. huidongense and T. himalayense. Tuber huidongense colonized all five seedlings of Q. acutissima and all five seedlings of Q. dentata. Tuber himalayense similarly colonized all 10 seedlings of Q. acutissima; however, only eight of the ten Q. dentata seedlings were colonized.
3.2. Molecular analysis of ECM
All of the ITS sequences amplified using mycorrhizal root tip DNA were similar to those of T. huidongense and T. himalayense ITS sequences deposited in GenBank. Analysis of the constructed phylogenetic trees confirmed that the base sequence of ECM belonged to the same phylogenetic group as that from the DNA of the fruiting bodies ( and ).
Figure 1. Neighbor-joining phylogenetic tree of Tuber huidongense GB20001 ascoma based on concatenated alignment of internal transcribed spacer (ITS) DNA sequences. Tuber formosanum was considered as an outgroup. The T. huidongense GB20001 sequence was isolated from the fruiting body. A total of 1–5 DNA sequences were derived from the ectomycorrhizae of Quercus acutissima and 6–10 DNA sequences were derived from the ectomycorrhizae of Q. dentata. Numbers in the figure represent bootstrap values (1000 replicates).
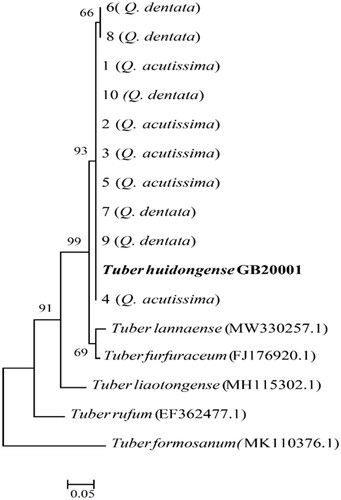
Figure 2. Neighbor-joining phylogenetic tree of Tuber himalayense CB20001 ascoma based on concatenated alignment of internal transcribed spacer (ITS) DNA sequences. Tuber pseudohimalayense was considered as an outgroup. The T. himalayense CB20001 sequence was isolated from the fruiting body. A total of 1–8 DNA sequences were derived from the ectomycorrhiza of Quercus acutissima and 9–18 DNA sequences were derived from the ectomycorrhizae of Q. dentata. Numbers in the figure represent bootstrap values (1000 replicates).
3.3. Morphological and anatomical characterization of ECM
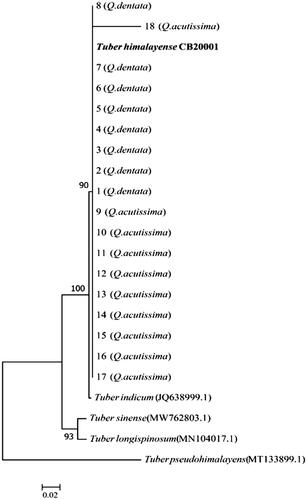
After 8 months of inoculation, the T. huidongense and T. himalayense ECM that formed in the two host plants was characterized. Common to both host plants, the ECM mantle of T. huidongense was irregularly interlocked and the mycelium was simple, curved, tortuous, septate, and exhibited some right-angle ramification (). However, the diameter of the unramified ends and the length and diameter of the cystidia in the ECM were significantly different between the two host plants (independent t-test, p < 0.05; ). The ECM mantle of T. himalayense in both host plants was generally brown, with localized dark areas, and an irregular interlocking pattern (). The mycelium was simple, tortuous, and septate. There were significant differences in the length of the ECM, length, and diameter of the unbranched end, thickness of the mantle, and length of the hyphae in the excised progenitor systems between the two host plants (independent t-test, p < 0.05; ).
Figure 3. Macro-morphological and anatomical characteristics of Tuber huidongense mycorrhizae with Quercus acutissima (A–D) and Q. dentata (E–H). (A, E) Shape of mycorrhizal root tips. (B, F) Cross-section of mycorrhizal root tips. (C, G) Outer mantle surface structure. (D, H) Separate hyphae emanating from the outer mantle layer. (Scale bars: A, E = 1 mm; B, F = 20 μm; C, D, G, H = 50 μm).
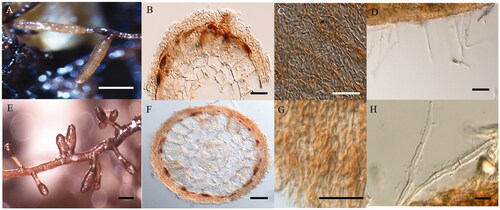
Figure 4. Macro-morphological and anatomical characteristics of Tuber himalayense mycorrhizae with Quercus acutissima (A–D) and Q. dentata (E–H). (A, E) Shape of mycorrhizal root tips. (B, F) Cross-section of mycorrhizal root tips. (C, G) Outer mantle surface structure. (D, H) Separate hyphae emanating from the outer mantle layer. (Scale bars: A, E = 1 mm; B, F = 30 μm; C, D, G, H = 25 μm).
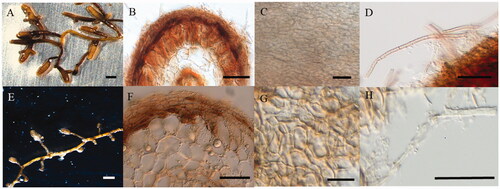
Table 1. Dimensional measurements of Tuber huidongense ectomycorrhiza (ECM) in two Quercus plants.
Table 2. Dimensional measurements of Tuber himalayense ectomycorrhiza (ECM) in two Quercus species.
3.4. ECM root colonization rates
The mean CR of T. huidongense in Q. dentata was significantly higher than that in Q. acutissima (p < 0.05), whereas the opposite was observed in the case of T. himalayense (). The mean CR of T. himalayense in Q. acutissima was significantly higher than that in Q. dentata (p < 0.01).
4. Discussion
In this study, mycorrhization was confirmed in both Q. acutissima and Q. dentata after 2.5 months of inoculation with spores of T. huidongense or T. himalayense. In previous studies, T. huidongense formed ECM with Castanea mollissima and Pinus armandii after approximately 5 months of inoculation [Citation12], whereas T. himalayense formed ECM with Quercus spp. and Pinus densiflora species after 4 months of inoculation [Citation13]. Thus, the time required for colonization may vary depending on the host plant species. Here, Q. dentata and Q. acutissima were shown to be the preferable host species for the cultivation of T. huidongense and T. himalayense, respectively.
Tuber spp. show a preference for specific host plant species [Citation14]; therefore, selection of the appropriate host plant is an important factor for effective mycorrhization of Tuber spp. [Citation2]. In this study, T. huidongense had a significantly higher CR in Q. dentata than Q. acutissima, and T. himalayense had a significantly higher CR in Q. acutissima than in Q. dentata. Thus, it was demonstrated that T. huidongense and T. himalayense exhibit a preference at the level of the host plant species. Interestingly, fruiting bodies of T. himalayense have been generally reported in the rhizosphere of Q. dentata [Citation9,Citation15]; however, T. himalayense showed a higher CR in Q. acutissima than Q. dentata in the current study. Initial CR of T. himalayense could be higher in Q. dentata seedling than in Q. acutissima [Citation13]; however, further studies are needed to determine whether initial CR affects the formation of the fruiting body.
The morphological and anatomical characteristics of T. huidongense and T. himalayense ECM were described in this study, representing the first characterization of T. huidongense ECM formed on oak trees. Morphological differences in T. huidongense ECM between the host plants Castanea mollissima and Pinus armandii were previously reported [Citation12], further supporting the conclusion that ECM can show distinct morphological differences according to the host plants, even when inoculating with the same Tuber species. Most of the T. huidongense ECM that formed in C. mollissima and P. armandii exhibited only bright colors, whereas the ECM that formed in oak trees was dark in color [Citation12]. However, it is difficult to conclude that this result is due to the host plant species; the color of ECM changes with age and it can be affected by the specific conditions applied to the growing seedlings [Citation16].
The current study also characterized the properties of T. himalayense ECM formed on Q. dentata for the first time. There have been previous reports of the properties of T. himalayense ECM formed on Q. acutissima, Quercus serrata, Quercus phillyraeoides, and P. densiflora [Citation13]. Most of the ECM characteristics reported for T. himalayense in these host plants were similar to those in Q. dentata; however, we showed that the ECM mantle in Q. dentata differed from the polygonal or angular-shaped mantle cells in P. densiflora [Citation13]. In this study, slight differences in ECM morphology were observed depending on each Tuber spp., even if the ECM was formed in the same host plant. For example, when the ECM characteristics of T. huidongense and T. himalayense were compared 8 months after inoculation, brown spots were observed in some ECM in T. huidongense, and some of the mycelia had right-angle ramifications. In addition, the mantel color of T. himalayense ECM was darker than that of T. huidongense.
By observing the ECM of T. huidongense and T. himalayense, it was possible to understand their characteristics according to Tuber species, and their fluctuations according to host plant species. However, it is difficult to distinguish Tuber spp. through ECM observations alone. Therefore, additional studies on T. huidongense and T. himalayense ECM are required. This study expanded the host plant range of T. huidongense and T. himalayense by demonstrating the formation of ECM in Q. acutissima, in addition to Q. dentata. For these results to lead to new agricultural activities, additional follow-up experiments are needed to determine the selective conditions for ECM synthesis of T. huidongense and T. himalayense.
Acknowledgment
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Fischer C, Oliach D, Bonet Lledos JA, et al. Best practices for cultivation of truffles. Solsona (Spain): Centre Tecnològic Forestal de Catalunya; 2017.
- Bonito GM, Trappe JM, Rawlinson P, et al. Improved resolution of major clades within Tuber and taxonomy of species within the Tuber gibbosum complex. Mycologia. 2010;102(5):1042–1057.
- Bonito GM, Gryganskyi AP, Trappe JM, et al. A global meta-analysis of Tuber ITS rDNA sequences: species diversity, host associations and long-distance dispersal. Mol Ecol. 2010;19(22):4994–5008.
- Bonet JA, Fischer CR, Colinas C. Cultivation of black truffle to promote reforestation and land-use stability. Agron Sustain Dev. 2006;26(1):69–76.
- Kovács GM, Jakucs E. Morphological and molecular comparison of white truffle ectomycorrhizae. Mycorrhiza. 2006;16(8):567–574.
- Guerin-Laguette A, Cummings N, Hesom-Williams N, et al. Mycorrhiza analyses in New Zealand truffières reveal frequent but variable persistence of Tuber melanosporum in co-existence with other truffle species. Mycorrhiza. 2013;23(2):87–98.
- Huang LL, Guerin-Laguette A, Wang R, et al. Characterization of Tuber indicum (Pezizales, Tuberaceae) mycorrhizae synthesized with four host trees exotic to China. Symbiosis. 2020;82(3):215–224.
- Park H, Gwon JH, Lee JC, et al. Report on Tuber huidongense, a truffle species previously unrecorded in Korea. Kor J Mycol. 2020;48:505–510.
- Park H, Gwon JH, Lee JC, et al. Morphological and phylogenetic characteristics of Tuber himalayense collected from rhizosphere of Quercus dentata in Korea. Kor J Mycol. 2021;49:101–108.
- White TJ, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. San Diego (CA): Academic Press; 1990. p. 315–322.
- Boutahir S, Iotti M, Piattoni F. Morphological and molecular characterization of Tuber oligospermum mycorrhizas. Afr J Agric Res. 2013;8:4081–4087.
- Wan SP, Yu FQ, Tang L, et al. Ectomycorrhizae of Tuber huidongense and T. liyuanum with Castanea mollissima and Pinus armandii. Mycorrhiza. 2016;26(3):249–256.
- Kinoshita A, Obase K, Yamanaka T. Ectomycorrhizae formed by three Japanese truffle species (Tuber japonicum, T. longispinosum, and T. himalayense) on indigenous oak and pine species. Mycorrhiza. 2018;28(7):679–690.
- Molina R, Trappe JM. Patterns of ectomycorrhizal host specificity and potential among Pacific Northwest conifers and fungi. For Sci. 1982;28:423–458.
- Nakamura N, Abe JP, Shibata H, et al. Genotypic diversity of the Asiatic black truffle, Tuber himalayense, collected in spontaneous and highly productive truffle grounds. Mycol Prog. 2020;19(12):1511–1523.
- Wang R, Guerin-Laguette A, Butler R, et al. The European delicacy Tuber melanosporum forms mycorrhizae with some indigenous Chinese Quercus species and promotes growth of the oak seedlings. Mycorrhiza. 2019;29(6):649–661.