Abstract
To exploit insect-derived fungi, insects were collected from seven different regions in Korea, including Gyeongbuk, Goryeong, and several fungi were isolated from them. A fungal strain designated 21-64-D was isolated from riparian tiger beetle (Cicindela transbaicalica) and morphologically identified as a species belonging to the genus Oidiodendron. Phylogenetic analysis using the nucleotide sequences of internal transcribed spacer (ITS) regions and the partial sequence of the large subunit of the nuclear ribosomal RNA (LSU) gene revealed the distinct phylogenetic position of the isolate among recognized Oidiodendron species including its closest neighbors O. chlamydosporicum, O. citrinum, O. maius, and O. pilicola. The hyphal and conidial morphology of the strain, particularly club-shaped hyphae, clearly differentiated it from its close relatives. Results indicated that 21-64-D is a novel species in the genus Oidiodendron, for which the name Oidiodendron clavatum sp. nov. is proposed.
The genus Oidiodendron belonging to the family Myxotrichaceae was first described by Robak in 1932, with three species isolated from wood pulp, Oidiodendron fuscum, Oidiodendron nigrum, and Oidiodendron rhodogenum [Citation1]. The taxonomic history of the reclassification of the members of the genus and the classification of its novel species have been widely reviewed and reported [Citation2–5]. More than 30 species are currently listed in the Mycobank (https://www.mycobank.org) and Index Fungorum (http://www.indexfungorum.org) databases. The genus Oidiodendron has been isolated from a wide range of habitats, including soil, marine sediments, lichens, air, and various cellulosic substrates, such as litter, wood pulp, bark, moss, and paper [Citation2,Citation4]. Some Oidiodendron species were reported as ericoid mycorrhizal fungi [Citation3,Citation6]. The key morphological features of Oidiodendron are erect and well-differentiated dematiaceous conidiophores, profusely branched above, forming fertile hyphae that fragment basipetally to form arthroconidia. The primary characteristics for identification are the shape, size, and ornamentation of conidia, conidiophore length, and cultural morphology [Citation7].
In Korea, fungi are mainly isolated from soil, plants, and mountainous areas. Many new and unreported species have been reported, and similar experiments are still being conducted. This study attempted to isolate fungi from insects that deviate slightly from the common sources. Fungi and insects reciprocally interact establishing a wide range of symbiotic relationships, from interactions in which fungi act against insects to those in which fungi form mutualistic associations with insects [Citation8]. In mutualistic interactions, one or both participants receive a net benefit such as dispersal, protection, or nutrition. Insect–fungus mutualisms evolved in at least 14 families of insects in six orders (Coleoptera, Blattodea, Lepidoptera, Hymenoptera, Diptera, and Hemiptera) and at least 15 orders of fungi in the Ascomycota and Basidiomycota [Citation9]. In contrast, parasitism is the non-mutualistic form of symbiosis, taking place when only one of the organisms benefits at the expense of the other. Insect species can be infected by obligate or facultative entomopathogenic fungi which exploit them as the only or prevalent nutrient source. These fungi include over 700 species from various genera that can infect and kill several insect and mite species and they are considered as potential biocontrol agents against pest insects [Citation10,Citation11]. Our attempts focused on unexplored sources that were not yet tried and expected to find unreported and novel fungal species. Riparian tiger beetle (Cicindela transbaicalica), widely distributed in Korea and whose adults are seen from May to October [Citation10], was considered a promising source of such fungi.
In this study, a specimen of C. transbaicalica was collected from Gyeongbuk, Goryeong-gun, in the riverside of Korea (35°39′34.4″N, 128°20′07.4″E) in April 2021, transferred to the laboratory, and stored at 4 °C until use. The insects were ground and mixed with double-distilled water, and the suspension was serially diluted. Then, 100 µL of each dilution was spread on potato dextrose agar (PDA; Difco, Detroit, MI, USA) plates and incubated at 25 °C for 2–3 days. Single colonies were transferred to fresh PDA plates and incubated at 25 °C for 4–5 days. The colony, designated 21-64-D, was selected for further molecular analysis based on its cultural characteristics. The strain was maintained in 20% glycerol at −80 °C for further study.
Genomic DNA was extracted from mycelia using the HiGene Genomic DNA prep kit (Biopact, Daejeon, South Korea). For molecular identification, internal transcribed spacer (ITS) regions were amplified using primers ITS1F and ITS4 [Citation12], and a large subunit of the nuclear ribosomal RNA (LSU) gene was amplified using primers LROR and LR5 [Citation13]. The amplified polymerase chain reaction products were purified using the EXOSAP-IT kit (Thermo Fisher Scientific, Waltham, MA). The purified DNA was sequenced by Macrogen Co., Ltd. (Daejeon, South Korea). The sequences of the species related to the genus Oidiodendron were retrieved from the National Center for Biotechnology Information (). The sequences were initially aligned using Clustal X 2.0 [Citation14], and phylogenetic analyses were performed using MEGA 7 [Citation15]. The construction of phylogenetic trees was carried out using neighbor joining (NJ) [Citation16], maximum likelihood (ML) [Citation17], and maximum parsimony (MP) [Citation18] algorithms.
Table 1. List of species used in phylogenetic analyses along with their GenBank accession numbers.
A BLAST search of the NCBI database revealed that the LSU sequence of strain 21-64-D shared 97.9%, 97.7%, and 99.4% identity with closely related Oidiodendron pilicola CBS 158.76, Oidiodendron chlamydosporicum CBS 132.72, and Oidiodendron truncatum CBS 629.70, respectively. However, based on the ITS region sequence similarity, the closest neighbors of the isolate were Oidiodendron maius ISO.29, Oidiodendron eucalypti CPC32659, and Oidiodendron tenuissimum CBS 126945, with relatively low similarity values of 92.6%, 92.3%, and 92.1%, respectively. These results clearly indicated that the comparative analysis based on the sequence of only one gene did not allow precise identification of the closest relatives. Therefore, multilocus sequence analysis was performed using concatenated sequences of the ITS regions and the LSU gene of strain 21-64-D.
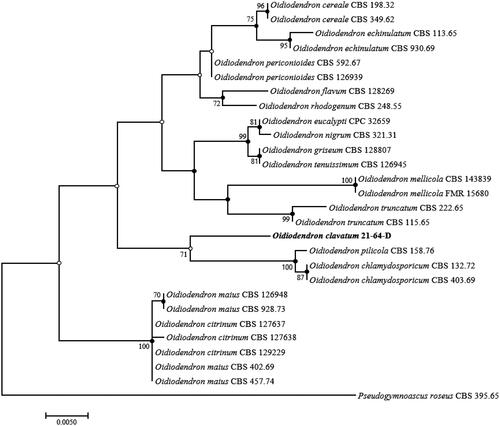
The constructed ML phylogenetic tree () clearly demonstrated that the isolate belongs to the genus Oidiodendron but is distinct from other Oidiodendron species. Based on the tree topology, its phylogenetically closest neighbors are O. chlamydosporicum, O. maius, and Oidiodendron citrinum (an additional strain not mentioned above). Unfortunately, the ITS sequence was unavailable for O. pilicola in the GenBank database. Therefore, a second ML phylogenetic tree was constructed using the sequences of LSU gene to clarify the phylogenetic relationship level between strain 21-64-D and O. pilicola. As shown in , 21-64-D formed a separate cluster, indicating the novelty of the isolate at the species level from O. pilicola and the other above-mentioned Oidiodendron species. Moreover, both ML trees shared corresponding nodes with NJ and MP trees, as indicated by filled circles in and . Accordingly, the novel strain is considered a single, novel, phylogenetically distinct Oidiodendron species. Based on the topology of the phylogenetic trees, four species, O. chlamydosporicum, O. citrinum, O. maius, and O. pilicola, were selected to compare their morphological characteristics to that of 21-64-D to confirm the results of the phylogenetic analysis. The morphological features of the isolate were observed under a light microscope (BX-50, Olympus, Tokyo, Japan). Strain 21-64-D was cultured on PDA, malt extract agar (MEA; Difco), oatmeal agar (OA; Difco), and cornmeal agar (CMA; Difco), and the size, shape, and color of its colonies were recorded after 28 days of cultivation at 25 °C.
Figure 1. ML phylogenetic tree based on the concatenated sequences of ITS regions and the LSU gene showing the phylogenetic position of strain 21-64-D among Oidiodendron species. Bootstrap values greater than 50% (based on 1000 replications) are shown at branching points. Filled circles indicate that the corresponding nodes were also recovered in trees generated using NJ and MP algorithms. Open circles indicate that the corresponding nodes were also recovered in the tree generated using the NJ or MP algorithm. The isolated strain is shown in bold. Pseudogymnoascus roseus CBS 395.65 was used as an outgroup. Bar, 0.01 substitutions per nucleotide position.
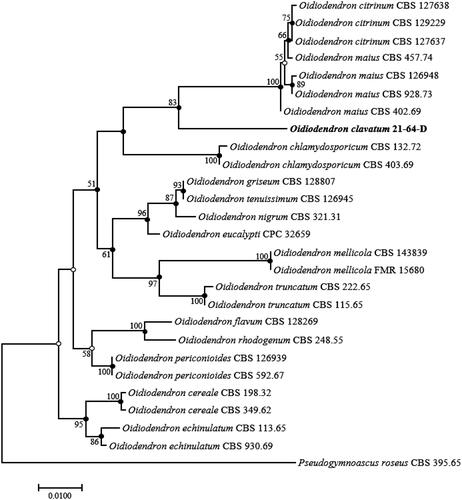
Figure 2. ML phylogenetic tree based on the sequences of the LSU gene showing the distinct position of Oidiodendron clavatum from O. pilicola and other members of the genus. Bootstrap values greater than 50% (percentage of 1000 replications) are shown at branching points. Filled circles indicate that the corresponding nodes were also recovered in trees generated using NJ and MP algorithms. Open circles indicate that the corresponding nodes were also recovered in the tree generated using the NJ or MP algorithm. The isolated strain is shown in bold. Pseudogymnoascus roseus CBS 395.65 was used as an outgroup. Bar, 0.005 substitutions per nucleotide position.
Taxonomy
Oidiodendron clavatum S.Y. Lee, L.N. Ten & H.Y. Jung sp. nov. ().
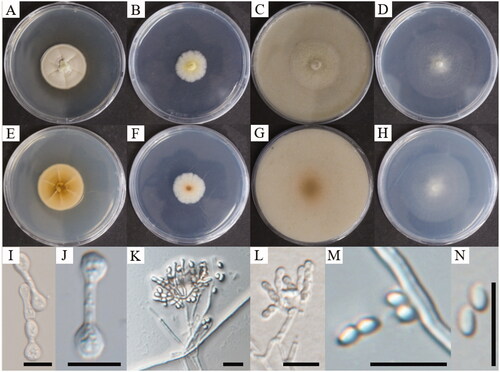
Figure 3. Cultural and morphological characteristics of Oidiodendron clavatum. Colonies on PDA (A, E), MEA (B, F), OA (C, G), and CMA (D, H) after 28 days of cultivation at 25 °C. Hyphae expand into club-shaped (I), club-shaped hypha (J), conidiophores (K, L), and conidia (M, N). Scale bar, 10 μm (I–N).
MycoBank no.: MB843126.
Etymology: The specific epithet is derived from the Latin word clavatum (“club”) and refers to the shape of hyphae.
Typus: The culture was isolated from the insects (Cicindela transbaicalica) in Gyeongbuk, Goryeong, Korea (35°39′34.4″N, 128°20′07.4″E). The stock culture (NIBRFGC000509256) was deposited in the National Institute of Biological Resources (NIBR) as a metabolically inactive culture.
Habitat: The fungus is associated with the riparian tiger beetle (C. transbaicalica).
Description: Colony growth was rapid at 25 °C on PDA, MEA, OA, and CMA. After 28 days of incubation on PDA, the colonies were light yellow, reverse pale yellow to brown in the center, and reached 34.0–34.6 mm in diameter. On MEA, the colonies were 24.7–26.4 mm in diameter and bright yellow to white and reverse white. On OA, the colonies were pale yellow aerial hypha and brown reverse, reaching 36.7–38.0 mm in diameter. On CMA, the colonies were 39.0–39.8 mm in diameter and white to dusky yellow with flat mycelium, center black graininess, and transparent white on the reverse side of the Petri dish. Additionally, lots of conidia were found with well-developed conidiophores at the centers of colonies. Conidiophores were opaque and abundant, short, smooth with about four to five conidia chains formed on top. Hyphae were hyaline, branched, smooth-walled, and club-shaped. Conidia was pale brownish, elliptical, and thin-walled, and the size was 2.3 − (3.1) − 3.9 × 1.6 − (2.0) − 2.6 μm ().
Notes: O. clavatum (strain 21-64-D) has a close molecular phylogenetic relationship with O. maius, O. citrinum, O. chlamydosporicum, and O. pilicola. The morphological characteristics of strain 21-64-D distinguish it from closely related species based on hyphae, conidiophores, and conidia (). The average length of conidia of 21-64-D (3.1 × 2.0 µm; length-to-width ratio (L/W)=1.6) is longer than that of O. citrinum (2.8 × 1.8 µm; L/W = 1.6) and O. chlamydosporicum (2.5 × 1.7 µm; L/W = 1.5). In contrast, it is shorter than O. maius (3.3 × 1.7 µm; L/W = 1.9). The average width of the conidia of strain 21-64-D (3.1 × 2.0 µm; L/W = 1.6) is bigger than that of the three closely related species, O. maius (3.3 × 1.7 µm; L/W = 1.9), O. chlamydosporicum (2.5 × 1.7 µm; L/W = 1.5), and O. citrinum (2.8 × 1.8 µm; L/W = 1.6). Moreover, the conidial L/W values differentiate O. clavatum (1.6) from O. maius (1.9), O. chlamydosporicum (1.5), and O. pilicola (1.8). The conidia of isolate 21-64-D are pale brown, whereas the four close relatives are hyaline. The biggest difference of 21-64-D from O. pilicola is the shape of conidia. O. clavatum has elliptical-shaped conidia, whereas O. pilicola produces barrel-shaped conidia. The shape of conidiophores and hyphae also clearly distinguishes the novel species from its close phylogenetic neighbors. O. clavatum has branched conidiophores, whereas O. maius and O. citrinum produce unbranched conidiophores. Notably, melanized chlamydospores are a characteristic feature of O. chlamydosporicum, while strain 21-64-D does not generate them. Furthermore, club-shaped hyphae are the most distinctive morphological character of O. clavatum, the same shape of hyphae was not observed in the closest Oidiodendron species. The colony of strain 21-64-D on CMA is white to dusky yellow with black graininess in the center, additionally differentiating it from the close phylogenetic relatives. O. maius has off-white to gray, appressed colonies. O. citrinum produces yellow-green, appressed colonies, and O. chlamydosporicum has cream or pale gray to green-gray or brown, darker at margins, appressed colonies. In addition, the average size of the colonies on CMA clearly differentiates O. clavatum (39.4 mm) from O. chlamydosporicum (12.5 mm).
Table 2. Morphological comparison of Oidiodendron clavatum with closely related species.
The above-mentioned morphological characteristics support the phylogenetic analysis results of strain 21-64-D, clearly confirming the novelty of the isolate at the species level among the other recognized Oidiodendron species. The application of DNA sequence analysis and molecular phylogenetic analyses had a major impact on the systematics of Myxotrichaceae. In particular, a relationship between Oidiodendron species and teleomorphic taxa within Myxotrichaceae was strongly confirmed by molecular analysis using ITS sequences [Citation7]. Later, these data were successfully used by Rice and Currah [Citation5] to perform a survey of the named Oidiodendron species and related anamorphs of the genus Myxotrichum. Besides ITS, LSU sequences were also used for the phylogenetic analysis of Oidiodendron, but two loci were applied separately from each other. In particular, phylogenetic analyses using LSU sequences were conducted to establish O. eucalypti [Citation6] and Oidiodendron mellicola [Citation19]. To the authors’ knowledge, there was only one attempt to use combined ITS and LSU sequences, which was carried out for the identification of isolated Oidiodendron strain KNUE20T046, but a limited number of Oidiodendron species were included for phylogenetic analysis [Citation20]. The results, particularly the identification of unexpected O. citrinum as the close relative of strain 21-64-D, clearly indicated that molecular analysis using two concatenated genetic markers allows more accurate differentiation and classification of novel Oidiodendron strains.
Oidiodendron is a cosmopolitan genus whose members, as mentioned above, can be found in various environmental niches. Strain 21-64-D was isolated from riparian tiger beetle. This is the first Oidiodendron species isolated from insects to the authors' knowledge. The widespread distribution of the genus is connected with their excellent adaptive capacity, and now Oidiodendron strains are considered promising sources of secondary metabolites with various biological activities [Citation21]. Seven new compounds, named chetracins B, C, D and oidioperazines A to D, were isolated from O. truncatum. Among them, chetracin B revealed cytotoxic activity in the nanomolar range against a panel of five human cancer lines [Citation22]. This Oidiodendron species is also known as a producer of oidiodendronic acid and oidiodendrolides A, B, and C, which are effective antifungal agents against pathogenic yeasts, Candida albicans, and Cryptococcus neoformans [Citation23]. Two nematicides, 4-hydroxyphenylacetic and oidiolactone D, antimicrobial harzianic acid, and antibacterial dihydrosecofuscin and secofuscin were isolated from Oidiodendron sp. [Citation24], Oidiodendron flavum [Citation21], and Oidiodendron griseum [Citation25], respectively. O. clavatum as a novel species provides additional opportunity to expand research on bioactive compounds, and its further investigation would be worthwhile. In addition, strain 21-64-D is the first insect-associated species of the genus Oidiodendron. These results extend the distribution of the genus and highlight the need for further studies on its ecological and biological roles.
In conclusion, morphological and phylogenetic analyses showed that strain 21-64-D is distinct from previously identified Oidiodendron species. It should be considered a novel species within the genus with the name O. clavatum sp. nov.
Disclosure statement
The authors declare no potential conflict of interest.
Additional information
Funding
References
- Robak H. Investigations regarding fungi on Norwegian ground woodpulp and fungal infections at wood pulp mills. Saertrykk Av Nyt Mag Naturvidenskaberne. 1932;71:185–330.
- Barron GL. New species and new records of Oidiodendron. Can J Bot. 1962;40(4):589–607.
- Couture M, Fortin JA, Dalpe Y. Oidiodendron griseum Robak: an endophyte of ericoid mycorrhiza in Vaccinum spp. New Phytol. 1983;95(3):375–380.
- Calduch M, Gené J, Cano J, et al. Three new species of Oidiodendron Robak from Spain. Stud Mycol. 2004;50:159–170.
- Rice AV, Currah RS. Oidiodendron: a survey of the named species and related anamorphs of Myxotrichum. Stud Mycol. 2005;53:83–120.
- Crous PW, Wingfield MJ, Burgess TI, et al. Fungal planet description sheets: 716–784. Persoonia. 2018;40:240–393.
- Hambleton S, Egger KN, Currah RS. The genus Oidiodendron: species delimitation and phylogenetic relationships based on nuclear ribosomal DNA analysis. Mycologia. 1998;90(5):854–868.
- Nicoletti R, Becchimanzi A. Ecological and molecular interactions between insects and fungi. Microorganisms. 2022;10(1):96.
- Biedermann PH, Vega FE. Ecology and evolution of insect-fungus mutualisms. Annu Rev Entomol. 2020;65:431–455.
- Islam W, Adnan M, Shabbir A, et al. Insect–fungal-interactions: a detailed review on entomopathogenic fungi pathogenicity to combat insect pests. Microb Pathog. 2021;159:105122.
- Kim TH, Paik JC, Jeong KH. Tiger beetles (Carabidae, Cicindelinae) of Korea. Kor J Soil Zool. 2005;10:1–15.
- White TJ, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. New York: Academic Press, Inc.; 1990. p. 315–322.
- Vilgalys R, Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol. 1990;172(8):4238–4246.
- Larkin MA, Blackshields G, Brown NP, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–2948.
- Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874.
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–425.
- Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 1981;17(6):368–376.
- Fitch WM. Toward defining the course of evolution: minimum change for a specific tree topology. Syst Zool. 1971;20(4):406–416.
- Rodríguez-Andrade E, Stchigel AM, Terrab A, et al. Diversity of xerotolerant and xerophilic fungi in honey. IMA Fungus. 2019;10:20.
- Choi JW, Gwon JH, Park H, et al. Four unreported endophytic fungi isolated from roots of Quercus spp. in Korea. Kor J Mycol. 2021;49:325–335.
- Ouyang X, Hoeksma J, Beenker WA, et al. Harzianic acid has multi-target antimicrobial activity against gram-positive bacteria. bioRxiv. 2021.
- Li L, Li D, Luan Y, et al. Cytotoxic metabolites from the Antarctic psychrophilic fungus Oidiodendron truncatum. J Nat Prod. 2012;75(5):920–927.
- Hosoe T, Nozawa K, Lumley TC, et al. Tetranorditerpene lactones, potent antifungal antibiotics for human pathogenic yeasts, from a unique species of Oidiodendron. Chem Pharm Bull. 1999;47(11):1591–1597.
- Ohtani K, Fujioka S, Kawano T, et al. Nematicidal activities of 4-hydroxyphenylacetic acid and oidiolactone D produced by the fungus Oidiodendron sp. Z Naturforsch C J Biosci. 2011;66(1–2):31–34.
- Navarri M, Jégou C, Bondon A, et al. Bioactive metabolites from the deep subseafloor fungus Oidiodendron griseum UBOCC-A-114129. Mar Drugs. 2017;15(4):111.
