Abstract
The new genus and species, Jejulea byssolomoides, is described from Jeju Island, Korea. This lichen is characterized by saxicolous, crustose, pale greenish-gray, partly finely filamentous, matt, smooth thallus, prominent convex brown to dark brown ascomata with a concolorous margin constricted at the dark brown base, 300–800 μm diameter, 200–250 μm high, without a distinct proper margin, adhering to the substratum ending in a minute byssoid white external part of cylindrical cells, fusiform 3–5 septate ascospores (17–23 × 4–5 μm). Phylogenetic analyses using ITS and mtSSU sequences place Jejulea in the Pilocarpaceae (Lecanorales). The new taxon is closely related to Byssoloma, a cosmopolitan group of foliicolous lichens, which is most diverse in the tropics. Like Byssoloma, Jejulea also forms a byssoid apothecial margin.
1. Introduction
Although Jeju Island is small in size (1826 km2), it is known for its high number of endemic lichens [Citation1–4] and lichenicolous fungi [Citation5]. Foliicolous lichens in the Gotjawal forest area on Jeju Island have also been described [Citation6]. So far, lichen research has focused mainly on epiphytic, saxicolous [Citation1,Citation2,Citation7], and foliicolous lichens [Citation8,Citation9]. The river valley formed by a solid basalt base can be surprisingly interesting. The discovery of the saxicolous “Byssoloma” is another example of a remarkable endemic species occurring on these islands of the East China Sea.
Pilocarpaceae is a crustose, cosmopolitan family comprising 29 genera, with 424 species [Citation10]. Species are characterized by biatorine or lecideine apothecia and pycnidia or campylidia type of conidiomata. The family is distributed in the tropical zone, where most species grow on leaves. Research of that group has been very intensive recently and descriptions of the new species are not rare [Citation11]. The closest relatives of the new genus Jejulea are the genera Byssoloma, Sporopodium, Tapellaria, Fellhanera, Lasioloma, and Calopadia. The genus Byssoloma Trevis. [Citation12] recently comprises 93 cosmopolitan foliicolous lichen species [Citation13] with the main distribution in tropical and subtropical zones. A few species extend to temperate zones [Citation11]. The genus is characterized by cortical crustose thalli, a byssoid apothecial margin, loosely intricate hyphae of proper exciple, asci with I + darker, tubular structure, and colorless, 1–3 septate spores [Citation14]. Sporopodium Mont. [Citation15] is a genus that also occurs in tropical zones, the campylidia of these 11 species usually feature a distinct socle producing the conidia [Citation14]. Some of the species contain secondary metabolites [Citation16]. The genus Tapellaria [Citation17] forms black, lecideine apothecia, anastomosing paraphyses, and campylidia producing filiform conidia. It is a predominantly foliicolous genus (25 species) with only a small number of the species growing on bark [Citation18]. Most of the species are known from Neotropics, but Tapellaria parvimuriformis was newly described from East Asia [Citation11]. Fellhanera Vězda [Citation19] is a large polyphyletic genus among foliicolous lichens [Citation11] which contains about 114 species [Citation13]. Like the previous genera, it forms small apothecia with a thin margin, paraplectenchymatous excipulum, anastomosing paraphyses, Byssoloma type asci, ellipsoid ascospores, and pycnidial conidiomata [Citation14]. There are other genera very close to Fellhanera: Brasilicia differs in its filiform ascospores, Bapalmuia Sérus [Citation20] has the very same shape as ascospores but differs in its secondary chemistry [Citation14]. Lasioloma [Citation21] differs from Tapellaria and Calopadia in its wooly prothallus, the pilose apothecial margins, and the centrally branched conidia, whereas Tapellaria differs in its black apothecia with purple hypothecium and anastomosing paraphyses. 10 species are corticolous with Neotropical distribution and the rest are foliicolous species with a predominantly Paleotropical distribution [Citation22].
2. Materials and methods
2.1. Morphological studies
Observations and measurements of photobiont cells, thallus and apothecium anatomy, asci and ascospores were made of hand-cut sections mounted in water and diluted KOH (K) solution. Asci were also observed in Lugol’s Iodine (I), with and without pretreatment in K. Mean value (x) and standard deviation (SD) were calculated, and the results are given as (minimum value observed) x ± SD (maximum value observed). x, SD, and n (the total number of ascospores measured) are given within parentheses. Thin-layer chromatography (TLC; Merck, Darmstadt, Germany) was carried out according to [Citation23]. Macro images were captured with a Canon 5DSR digital SLR camera (Canon, Tokyo, Japan) and an Olympus Zuiko 20 mm macro lens (Olympus, Tokyo, Japan). Microscopic images were captured with a Canon 5DSR digital SLR camera (Canon, Tokyo, Japan) mounted on an Olympus BX41 DIC microscope (Olympus, Tokyo, Japan). Illustrations were prepared using Adobe Photoshop. Measurements of the hymenium, hypothecium, cortex, and spore size (30–50 spores per specimen) were made in water mounts. The voucher specimen was deposited in the Korean Lichen Research Institute, Sunchon National University, Suncheon, South Korea.
2.2. DNA extraction, amplification, and sequencing
Genomic DNA was extracted from the six fresh lichen specimens using the CTAB protocol [Citation24]. The nuclear ribosomal internal transcribed spacer (nrITS) and mitochondrial small subunit ribosomal RNA (mtSSU) regions were amplified using AccuPower® PCR PreMix (Bioneer, Daejeon, South Korea). The primers used were ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) for ITS [Citation25], mrSSU1 (5′-AGCAGTGAGGAATATTGGTC-3′) and mrSSU3R (5′-ATGTGGCACGTCTATAGCCC-3′) for mtSSU [Citation26]. PCR amplification was done using a T100™ Thermal Cycler machine (Bio-Rad, Hercules, CA, USA) performed under the following conditions: an initial cycle of 5 min at 94 °C, followed by 30 cycles of 30 s at 94 °C, 30 s at 55 °C, 10 min at 72 °C, and then finally 10 min at 72 °C for nrITS, and an initial cycle of 3 min at 94 °C, followed by 35 cycles of 1 min at 94 °C, 1 min at 52 °C, 1 min at 72 °C, and then finally 7 min at 72 °C for mtSSU.
2.3. Multiple sequence alignment
Six new sequences (nrITS and mtSSU) were obtained from this study, and their closest relatives (i.e., Byssoloma species) based on BLAST searches were retrieved from GenBank. Members representing all genera were currently accepted (). For phylogenetic analyses, sequences were assembled by ATGC version 1.03 (GENETYX Co., Tokyo, Japan) and multiple sequence alignment (MSA) was performed using MAFFT v. 7 with G-INS-1 algorithm for nrITS and L-INS-i algorithm for mtSSU [Citation27]. Unclearly aligned position sequences were manually modified using MEGA v. 7 [Citation28]. nrITS and mtSSU based on a combined phylogenetic tree were estimated based on Maximum-Likelihood (ML) and Bayesian Posterior Probabilities (PP). ML and PP best-fit model of nucleotide substitution and parameters were estimated by IQ-TREE 2.2.0 [Citation29] based on Bayesian information criterion (BIC), and SYM + I + G4 model for nrITS and TVM + F + I + G4 for mtSSU were chosen. ML analysis was performed using IQ-TREE 2. 2. 0 [Citation29] and 1000 bootstrap replications. Bayesian analysis was conducted based on the Markov chain Monte Carlo method (MCMC), 10 million generations with every 100th sampling using MrBayes v. 3.2.7 [Citation30]. The first 25% sample of trees was discarded and visualized in Figtree v. 1.4.4. Micarea micrococca and Micarea byssacea located in Pilocarpaceae were chosen as outgroups.
Table 1. List of species and DNA sequence information employed for phylogenetic analysis.
3. Results
3.1. Phylogenetic analyses
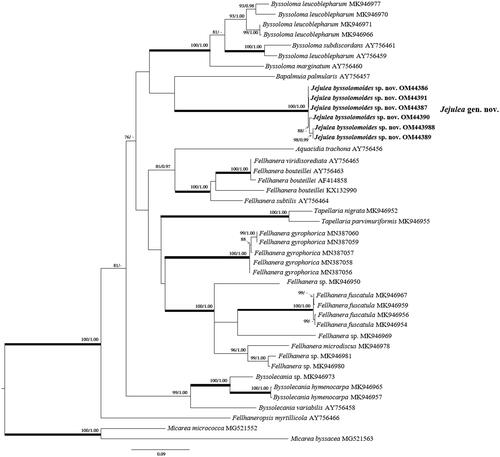
The data set of nrITS and mtSSU consisted of 37 nrITS and 81 mtSSU sequences of Pilocarpaceae obtained from NCBI with six sequences of two-locus newly obtained from Jejulea byssolomoides (). Maximum-likelihood bootstrap value (ML) ≥70% and Bayesian Posterior Probabilities (PP) ≥95% were added above the branches. In the mtSSU phylogenetic tree, J. byssolomoides is closely located in a clade composed of four genera, Bapalmuia, Byssolecania, Byssoloma, Tapellaria, and formed an independent clade (). In the nrITS phylogenetic tree, J. byssolomoides was clustered in the Byssoloma clade with Bapalmuia palmularis (). The branch length indicates the difference between the three genera. However, statistical support of the external node dividing the clustered three genera Bapalmuia, Byssoloma, and Jejulea in nrITS was not significant. Thus, the approximate taxonomic position of J. byssolomoides was confirmed in nrITS. Two genera Byssolecania and Tapellaria formed independent clades strongly supported by ML and PP.
Figure 1. The phylogenetic tree was based on mtSSU sequences. Maximum-likelihood bootstrap value (ML) ≥70% and Bayesian Posterior Probabilities (PP) ≥95% were marked above branches. Thickened branches indicate ML/PP = 100/1.00. The newly obtained sequence of Jejulea byssolomoides used in this study is shown in bold.
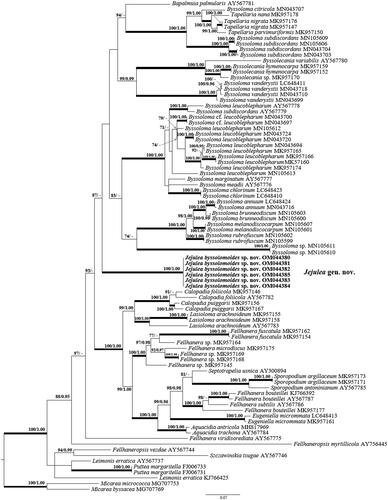
Figure 2. The phylogenetic tree was based on nrITS sequences. Maximum-likelihood bootstrap value (ML) ≥70% and Bayesian Posterior Probabilities (PP) ≥95% were marked above branches. Thickened branches indicate ML/PP = 100/1.00. The newly obtained sequence of Jejulea byssolomoides used in this study is shown in bold.
3.2. Taxonomy
Jejulea byssolomoides J.P. Halda, J.-J. Woo & J.-S. Hur gen. et sp. nov. ().
Figure 3. Jejulea byssolomoides (KoLRI 36855, holotype). (A, B) Thallus with ascomata (arows); (C) Cross section of ascoma; (D, E) Detail of mature ascospores in ascus (mounted in water). Scales: (A) – 10 mm, (B) – 1000 μm, (C) – 50 μm, (D, E) – 10 μm.

MycoBank No.: MB#842471 (genus) and MB#842472 (species).
Jejulea byssolomoides belongs to the Pilocarpaceae and is phylogenetically related to Byssoloma leucoblepharum, Byssoloma subdiscordans, Byssoloma marginatum, and B. palmularis but differs by the combination of the following characters: fusiform 3–5 septate ascospores (17–23 × 4–5 μm), an indistinct proper margin with a white byssoid external part of cylindrical cells (20–25 μm), dark brown hypothecium and absence of pycnidia.
3.3. Type
South Korea, Yeongcheon-dong, Seogwipo, Jeju-si, Jeju-do, 33°18′0.79′′N 126°34′34.54′′E, alt. 307 m, on the vertical face of a sheltered basalt rock along a stream, 18 August 2015, J. P. Halda, S.-O. Oh & D. Liu 152633 (KoLRI 036855 – Holotype, KoLRI 036876, 036882, 036883, 036886, 036887, 036888, 036889, 036891, 036893, 057219, 057220, 057221, 057222, 057223, 057224 – Isotypes).
3.4. Etymology
The name of the genus refers to Jeju Island, the type locality. The epithet “byssolomoides” points to the closest relative genus, Byssoloma.
3.5. Morphology
Thallus thin, crustose, superficial, spreading, 1–8 cm diameter, 20–40 μm thick, spreading, pale greenish-gray, partly finely filamentous, matt, smooth; hypothallus indistinct. Algal cells chlorococcoid, spherical 6–15(–17) μm diameter interstitial hyphae short-celled, 2–3 μm thick. Apothecia biatorine, applanate, orbicular, 300–800 μm diameter, 200–250 μm high, often grows in small groups of 2–4 ascomata. Excipulum well-developed, margin indistinct, becoming thinner outwards and adhering to the substratum ending in a minute white external part of cylindrical cells (20–25 μm); disk 250–750 μm diameter, almost flat, not elevated at the margin, brown to dark brown, blackish from the center, epruinose. Proper exciple obviously thinner or tapering outwards as a minute white hyphal rim, colorless, subgelatinous; hyphae branched and anastomosing, uninflated (ca. 2 μm wide lumina), embedded in a gelatinous matrix, generally oriented toward the tapering edge. Hypothecium ca. 100 μm thick at the center, dark brown to partly black, subparaplectenchymatous, composed of more or less vertically arranged cells, darker than subhymenium. Subhymenium 15–20 μm thick, brown. Hymenium 40–75 μm thick, pale brown, dark brown below; paraphyses sparsely branched and anastomosing, with 1–2 μm diameter lumina. Asci clavate, with a blurred IKI + layer and a tholus with an IKI + blue inner tube (Byssoloma-type), ca. 35 × 12 μm. Ascospores 8 per ascus, fusiform, 3–5 septate sometimes with perispore 1–2 μm thick, (14–)17–23(–26) × (3–)4–5(–6) μm [x = 19.6 × 4.4 μm; SD 3.3; 0.6 μm; n = 48; l/w ratio = 4.5], colorless, sometimes slightly constricted at the septa. Pycnidia not observed.
3.6. Chemistry
No lichen product was detected by TLC.
3.7. Ecology and distribution
Known from the type locality in Seogwipo, Jeju Island, South Korea. The species was found growing on shaded volcanic rocks (basalt) along a stream protected by the forest’s margin together with Coenogonium lueckingii, Flakea papillata, Porina curnowii, P. eminentior, P. leptalea, P. mastoidea, Strigula nipponica, Verrucaria aethiobola, and Willeya iwatsukii. Jeju Island is a shield volcano that is composed of basaltic lava flows and minor pyroclastic rocks. The climate is characterized by hot humid summers and cool winters as a result of the influence of the East Asian monsoon.
3.8. Remarks
Jejulea byssolomoides superficially resembles Byssoloma (see above) with its white byssoid margin of apothecia and septate ascospores but differs in the different ascus type and in having a true exciple of palisade plectenchyma. No species of the genus Byssoloma have been confirmed from Korea. Among saxicolous East-Asian species Gyalideopsis lunata [Citation31] differs in its colorless hymenium and hypothecium, hyaline proper margin, and shorter submuriform to muriform ascospores (12–17 × 7–10 μm vs. 3–5 septate, 17–23 × 4–5 μm in J. byssolomoides).
The closest related species B. leucoblepharum and B. subdiscordans differ in ecology: they grow especially on leaves of evergreen shrubs and trees, and also in morphology: thinner thallus, smaller pale to black apothecia 300–600 μm diameter, disk plane, with a persistent densely byssoid white margin. Excipulum made of colorless loosely woven hyphae, 50–150 μm broad. Hypothecium 20–50 μm high, light to dark brown. Apothecial base aeruginous. Hymenium 45–60 μm high, colorless. Asci 35–55 × 9–12 μm. Ascospores oblong, 3-septate, without constrictions at the septa, 10–18 × 2.5–3.5 μm (3–5 μm in B. subdiscordans), colorless. Pycnidia subglobose to cup-shaped, 0.1–0.15 mm diameter, brownish-gray with a black center. Conidia pyriform, non-septate, 4–5 × 1.2–1.8 μm, colorless. Byssoloma marginatum forms a compact apothecial margin because the excipulum is composed of hyphae embedded in the gelatinous matrix. The apothecial disk is colored dark grayish brown and ascospores (10–16 × 3–4 μm), 3-septate, colorless.
4. Discussion
Its geographical isolation and the special climatic conditions on Jeju Island are the main factors determining the emergence of new species of lichenized fungi, of which several dozen have been described herein in recent years (e.g., Caloplaca chejuensis, Fellhanera chejuensis, Fauriea jejuensis, Graphis jejuensis, Orientophila chejuensis, and Protoparmeliopsis chejuensis).
Jejulea byssolomoides is a distinct and mainly lichen-inhabiting lineage in the Pilocarpaceae characterized by its saxicolous thallus having a larger and wide 3–5 septate, 17–23 × 4–5 μm ascospores so it cannot be confused with any other species of Pilocarpaceae. Other lichen genera with a byssaceous ascomata growth form and a chlorococcoid photobiont such as Byssoloma Trevis., Bapalmuia Sérus., Sporopodium Mont., Tapellaria Müll. Arg., Fellhanera Vězda, Lasioloma Santesson, and Calopadia Vězda are phylogenetically unrelated, with different types of fruiting bodies and sizes of ascospores. The new species also resembles the saxicolous Septotrapelia usnica known from Indonesia, Singapore, and Sri Lanka [Citation32], but the species contains usnic acid and zeorin and forms a granulose thallus, slightly bent, 3-septate ascospores not constricted at the septa (21–27 × 5–6 μm) [Citation33].
Ascomata with hairy margins are not common among lichens. They are known in some species in the family Porinaceae which produce hairy perithecial ascomata (Trichothelium) and in Pilocarpaceae (Lasioloma, Byssoloma) [Citation14]. The saxicolous Gyalidea species, also known from coastal Korea, differ in the absence of white cylindrical cells growing from the margin of the excipulum, a different type of ascus (lightly thickened apical tholus, KI-, a small ocular chamber) [Citation34] and in the different shape and size of the ascospores (1-septate to muriform) [Citation2,Citation35].
Acknowledgment
The authors thank Simon O’Flynn for proofreading the text.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Kondratyuk SY, Lőkös L, Halda JP, et al. New and noteworthy lichen-forming and lichenicolous fungi 7. Acta Bot Hungarica. 2018;60(1–2):115–184.
- Kondratyuk SY, Halda JP, Lőkös L, et al. New and noteworthy lichen-forming and lichenicolous fungi 8. Acta Bot Hungarica. 2019;61(1–2):101–135.
- Moon KH, Nakanishi M, Kashiwandani H. New or noteworthy species of Graphidaceae (Ostropales, Ascomycota) in Korea. Jap J Bot. 2012;87(5):320–325.
- Wang XY, Liu D, Lőkös L, et al. New species and new records of Buellia (lichenized ascomycetes) from Jeju province, South Korea. Mycobiology. 2016;44(1):14–20.
- Joshi Y, Kondratyuk S, Lokos L, et al. New species and new records of lichenicolous fungi from South Korea. Mycosphere. 2015;6(2):195–200.
- Woo J-J, Lucking R, Oh S-Y, et al. Two new foliicolous species of Strigula (Strigulaceae, Strigulales) in Korea offer an insight into phorophyte-dependent variation of thallus morphology. Phytotaxa. 2020;443(1):1–12.
- Aptroot A, Moon KH. 114 New reports of microlichens from Korea, including a description of five new species, show that the microlichen flora is predominantly Eurasian. Herzogia. 2014;27(2):347–365.
- Jayalal U, Oh SO, Lücking R, et al. Contributions to the foliicolous lichens flora of South Korea. Mycobiology. 2013;41(4):202–209.
- Oh S-Y, Woo J-J, Hur J-S. Distribution of foliicolous lichen Strigula and genetic structure of S. multiformis on Jeju Island, South Korea. Microorganisms. 2019;7(10):430.
- Lücking R, Hodkinson BP, Leavitt SD. Corrections and amendments to the 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota. Bryologist. 2017;120(1):58–69.
- Wang WC, van den Boom P, Sangvichien E, et al. A molecular study of the lichen genus Byssoloma trevisan (Pilocarpaceae) with descriptions of three new species from China. Lichenologist. 2020;52(5):387–396.
- Trevisan V. Spighe e paglie. Padova (Italy): Fasc. primo.; 1853. p. 1–64.
- Robert V, Stegehuis G, Stalpers J. The MycoBank engine and related databases [Internet]; 2005 [cited 2022 Feb 8]. Available from: https://www.mycobank.org/.
- Lücking R. Foliicolous lichenized fungi. Flora Neotropica Monograph. 2008;103:1–866.
- Montagne C. Cryptogamia guyanensis seu plantarum cellularium in Guyana gallica annis 1835–1849 a cl. Leprieur collectarum enumeratio universalis. Ann Des Sci Naturel Trois sér Bot. 1851;16:47–81.
- Lücking R, Lumbsch HT. New species or interesting records of foliicolous lichens. VI. Sporopodium aeruginascens (Lecanorales), with notes on the chemistry of Sporopodium. Mycotaxon. 2001;78:23–27.
- Müller J. Lichenes epiphylli novi. Basel (Switzerland): H. Georg; 1890. p. 1–22.
- Neuwirth G, Stocker-Worgotter E. Tapellaria palaeotropica (Pilocarpaceae), a new foliicolous lichen species from the Seychelles, and a world key to the genus. Lichenologist. 2017;49(3):253–258.
- Vězda A. Neue gattungen der familie Lecideaceae s. lat. (lichenes). Folia Geobot Phytotax. 1986;21(2):199–219.
- Sérusiaux E. New taxa of foliicolous lichens from Western Europe and Macronesia. Nordic J Bot. 1993;13(4):447–461.
- Santesson R. Foliicolous lichens I. A revision of the taxonomy of obligately foliicolous, lichenized fungi. Symbolae Bot Upsal. 1952;12(1):1–590.
- McCarthy PM. A new corticolous species of Lasioloma (lichenized Ascomycota, Pilocarpaceae) from North-Eastern Queensland. Australasian Lichenol. 2020;87:58–61.
- Orange A, James PW, White FJ. Microchemical methods for the identification of lichens. London (UK): British Lichen Society; 2001.
- Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987;19:11–15.
- White TJ, Bruns TD, Lee SB, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols. Part three. Genetics and evolution. Waltham (MA): Academic Press; 1990. p. 315–322.
- Zoller S, Scheidegger C, Sperisen C. PCR primers for the amplification of mitochondrial small subunit ribosomal DNA of lichen-forming ascomycetes. Lichenologist. 1999;31(5):511–516.
- Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–780.
- Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874.
- Minh BQ, Schmidt HA, Chernomor O, et al. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol. 2020;37(5):1530–1534.
- Ronquist F, Teslenko M, van der Mark P, et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61(3):539–542.
- Harada H, Kawakami S. Gyalideopsis lunata sp. nov. (lichenized Ascomycota, Gomphillaceae) with rudimentary hyphophores, from gifuken, Central Japan. Lichenology. 2011;10(1):23–28.
- Kukwa M, Piątek M. First records of the lichen Septotrapelia usnica (Lecanorales, Ascomycota) from West Africa. Polish Bot J. 2014;59(1):105–108.
- Bungartz F, Hillmann G, Kalb K, et al. Leprose and leproid lichens of the Galapagos, with a particular focus on Lepraria (Stereocaulaceae) and Septotrapelia (Pilocarpaceae). Phytotaxa. 2013;150(1):1–28.
- Henssen A, Lücking R. Morphology, anatomy, and ontogeny in the Asterothyriaceae (Ascomycota: Ostropales), a misunderstood group of lichenized fungi. Ann Bot Fennici. 2002;39:273–299.
- Kondratyuk SY, Lőkös L, Halda JP, et al. New and noteworthy lichen-forming and lichenicolous fungi 5*. Acta Bot Hungarica. 2016;58(3–4):319–396.
