Abstract
In this study, twenty-five yeast strains were isolated from soil samples collected in the gold mining ore in Gia Lai, Vietnam. Among them, one isolate named GL1T could highly tolerate Cu2+ up to 10 mM, and the isolates could also grow in a wide range of pH (3–7), and temperature (10–40 °C). Dried biomass of GL1 was able to remove Cu2+ effectively up to 90.49% with a maximal biosorption capacity of 18.1 mg/g at pH 6, temperature 30 °C, and incubation time 60 min. Sequence analysis of rDNA indicated this strain was closely related to Rhodotorula mucilaginosa but with 1.53 and 3.46% nucleotide differences in the D1/D2 domain of the 28S rRNA gene and the ITS1-5.8S rRNA gene-ITS2 region sequence, respectively. Based on phylogenetic tree analysis and the biochemical characteristics, the strain appears to be a novel Rhodotorula species, and the name Rhodotorula aurum sp. nov. is proposed. This study provides us with more information about heavy metal-tolerant yeasts and it may produce a new tool for environmental control and metal recovery operations.
1. Introduction
The rapid development of industry leads to huge amounts of waste, including heavy metals, being disposed of in the environment [Citation1]. Heavy metals are considered among the most toxic and persistent environmental contamination agents. Copper is a critical metal and is extensively used in industries, leading to a hazardous environment of copper contaminant [Citation1]. Copper is an essential trace element that is involved in the function of many cellular enzymes as a cofactor [Citation2]. However, when copper concentration is higher than the tolerance contents of cells, it can be toxic and a threat to the organisms since copper catalyzes the production of reactive oxygen species, causing serious damage to cytoplasmic components through the oxidation of proteins, split DNA and RNA, and lipid peroxidation [Citation3]. Moreover, under anaerobic conditions, copper is also deleterious for microorganisms including yeast [Citation4]. Also, excessive exposure to copper has been linked to pathologies, such as Alzheimer’s disease [Citation5] and Wilson’s disease [Citation6] in humans. To overcome the problem, copper ions must be removed from contaminated wastewater.
It is known that heavy metal-tolerant microorganisms such as those that have been isolated from areas contaminated by heavy metals are generally excellent candidates for studying and applying bioremediation, as a sustainable technology for the environmental treatment of heavy metal pollution.
Consequently, several heavy metal tolerant yeasts have been isolated and used for removing heavy metals including copper from industrial wastewater and/or contaminated water, such as Cryptococcus sp. [Citation7], Pichia guilliermondii [Citation8], Rhizopus stolonifera [Citation9], Rhodotorula mucilaginosa [Citation10], Loddermyces elongisporus [Citation11].
Whilst identifying microorganisms for copper removal, this study, isolated novel copper-tolerant yeast from gold mining ore in the Gia Lai province of Vietnam. Which belongs to the Rhodotorula genus, but 9, and 22 nucleotides differ in the D1/D2 region of the ribosomal large subunit, and the ITS (internal transcribed spacer) region, respectively. This paper describes it as a new species, Rhodotorula aurum sp. nov., and describes its properties in the removal of copper.
2. Materials and methods
2.1. Sample collection and yeast isolation
Five samples were collected from the gold mine area located in Gia Lai province of Vietnam, with approximately 20 g of soil obtained in sterile plastic bags. Samples were then suspended in enrichment media containing yeast extract 0.5%, glucose 0.5%, NaCl 0.05%, and 0.01% of each (NH4)2SO4, KH2PO4, MgSO4, and CaCl2. After shaking incubation for 72 h at 30 °C, the suspension was plated on YM agar (1% malt extract, 0.5% yeast extract, 0.3% peptone, 2% glucose, and 1.5% agar) supplemented with 50 mg/L copper, plates were then incubated for 3 days at 30 °C.
The morphological, biochemical, and physiological characterizations of isolated yeasts were described according to standard methods reported previously [Citation12]. Images of colony morphology were taken using a Canon camera (model EOS 5D Mark IV; Canon, Tokyo, Japan). Cell shape and morphology were photographed by light microscopy (CX33, Olympus, Tokyo, Japan). The ability of yeast cells to assimilate carbon and nitrogen sources was evaluated according to a previous study [Citation13].
2.2. Phylogenetic analysis
For yeast identification, one colony of each isolate was picked and inoculated overnight in a YD medium (1% yeast extract, 1% peptone, and 2% glucose). Yeast cells were collected by centrifugation and used for genomic DNA extraction using the Favorgen DNA extraction kit (Favorgen, Ping Tung, Taiwan) according to the manufacturer’s instructions. Subsequently, the internal transcribed spacer (ITS) 1 and 2 regions of the ribosomal DNA, and D1/D2 domains of the large subunit RNA gene were amplified using primers as previously described [Citation14]. PCR was carried out in an MJ mini thermal cycler (Biorad, Hercules, California) using OneTaq® 2X master mix with standard Buffer (New England Biolabs, Ipswich, Massachusetts) in a final volume of 25 μL containing 1 µL of each primer (10 pM), 1 μL of genomic DNA, and 9.5 μl of nuclease-free water. The PCR program was an initial denaturation for 5 min at 95 °C; 35 cycles of denaturation at 95 °C for 30 s, annealing at 54 °C for 30 s, and 72 °C for 30 s, and final extension at 72 °C for 5 min. The PCR products were further verified by being subjected to 1.5% agarose gel electrophoresis and stained with SYBR™ Green I (Invitrogen, Waltham, Massachusetts) for visualization under the MUPID® One LED Illuminator (Nippon genetics Europe, Duren, Germany). Amplicons were then purified using the AccuPrep® PCR/Gel Purification (Bioneer, Daejeon, Korea) and sequenced by Macrogen Inc. (Seoul, South Korea). Sequences were then aligned and compared with known sequences in the Genbank using the Basic Logarithmic Alignment Search Tool (BLAST) algorithm in the National Center for Biotechnology Information (NCBI) database. Multiple sequence alignment was carried out using the MUSCLE algorithm. Phylogenetic tree based on the D1/D2 domains of the large subunit RNA gene using the maximum likelihood (ML) method was constructed by MEGA11 [Citation15] with bootstrap values based on 1000 random resampling to determine the exact taxonomic positions of isolated yeast. Our sequence and the references of sequences used for phylogenetic analysis in this study are shown in Table S1.
2.3. Determination of copper tolerance by yeast isolates
The copper tolerance of yeasts was examined by allowing yeast growth in flasks containing 100 mL a YNB glucose medium (YNB 0.7%, glucose 0.5%) supplemented with different concentrations of Cu2+ (CuSO4·5H2O). Overnight cultured yeast cells were added to flasks (to a final concentration of 105 cells/mL) and incubated at 30 °C for 72 h in a shaking incubator at 150 rpm. The kinetic growth of yeast was checked by measuring optical density at 600 nm on a spectrophotometer (Optizen POP UV-VIS Spectrophotometer, K LAB, Daejeon, Korea). OD600 was also used to check the kinetic growth of yeast in a YNB glucose medium with different pH ranges of 3–8 at 30 °C, and in a range of different temperatures (10–40 °C). All experiments were carried out in triplicate.
2.4. Biosorption assay
The effects of initial pH (2, 3, 4, 5, 6, 7) and temperature (20, 30, 40, 50 °C) on the Cu2+ removal efficiency were investigated according to a previous study [Citation16]. In brief, yeast isolate was cultured in a YM medium for 3 days at 30 °C, the cells were then collected by centrifugation and washed thoroughly with deionized water before being dried at 80 °C for 48 h. Dried biomass was subsequently ground and used as adsorbents. Biosorption was performed under the following conditions: 1 g of biomass was exposed to 200 mL of solution containing 100 mg/L of CuSO4·5H2O in flasks at optimal pH and temperature, and shaken at 150 rpm. After different interval times (5, 10, 20, 40, 60, 90, and 120 min) of incubation, 0.5 mL of the mixture was collected and centrifuged at 10,000 rpm for 10 min. The residual Cu2+ content was analyzed by atomic adsorption spectroscopy (ContrAA 800D, Analytik Jena, Germany). The Cu2+ binding capacity to biomass was calculated as follows: q = (Co− Ct)*V/m, where Co is the initial Cu2+ concentration (100 mg/L); Ct is the final metal ion concentration (mg/L); V is the volume of the reaction solution (in mL); m is yeast biomass dry weight (g); and q is mg of Cu2+ adsorbed per g of biomass.
3. Results and discussion
3.1. Yeast isolation
After 4 days of enrichment culture, 25 yeasts were isolated from 5 samples. One isolate named GL1T was selected according to its ability to tolerate high Cu2+ concentration. Sequence analysis of the D1/D2 domain of the 28S rRNA gene showed that the GL1T strain was closely related to Rhodotorula mucilaginosa but with 1.53% nucleotide differences (8 substitutions and 1 gap out of 586 nucleotides). In the same way, the ITS1-5.8S rRNA gene-ITS2 region sequence of the strain GL1T differed from R. mucilaginosa by 22 nucleotides (19 substitutions and 3 gaps out of 636 nucleotides). These conserved sequences were submitted to Genbank with accession numbers OP214778, and OQ073533, respectively.
Kurtzman et al. (1998) [Citation14] indicated that yeast strains exhibiting nucleotide substitutions >1% of the D1/D2 domain usually are considered different species. Results from the phylogenetic tree on the basis of the D1/D2 sequence indicated that GL1 belongs to the Rhodotorula species and that is a new sister species of R. mucilaginosa (). In addition, it can assimilate several hydrocarbon sources including glucose, fructose, maltose, trehalose, glycerol and soluble starch. But in contrast to R. mucilaginosa, Rhodotorula GL1 was able to assimilate ethanol and inositol, but it could not assimilate citrate, arabinose, and xylose ().
Figure 1. Phylogenetic tree showing the position of Rhodotorula sp. GL1 (Rhodotorula aurum sp. nov.) and their related species constructed by the maximum-likelihood method based on the D1/D2 nucleotide sequences. The numbers given at the branching points indicate levels of bootstrap support (%) with which a given branch appeared in 1000 bootstrap replications. Only values >50 are shown. Sporidiobolus pararoseus CBS 491 (NG 067256) was used as an outgroup. Bar, 0.02 nucleotide substitution rate. The GenBank accession numbers are reported in brackets.
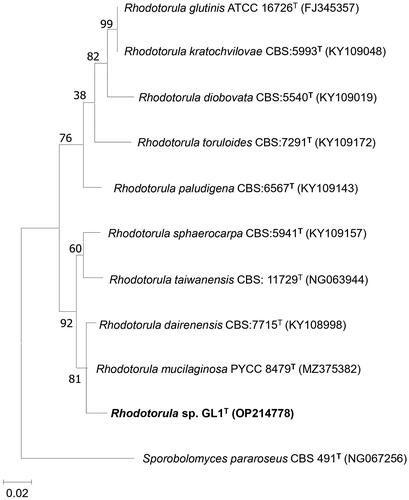
Table 1. Biochemical characteristics of Rhodotorula sp. GL1 compared to other Rhodotorula species.
According to the findings presented here, we propose that Rhodotorula sp. GL1T is a novel species in the Rhodotorula genus and is named Rhodotorula aurum sp. nov. (aurum in Latin meaning gold).
3.2. Phenotypic and physiological characteristics
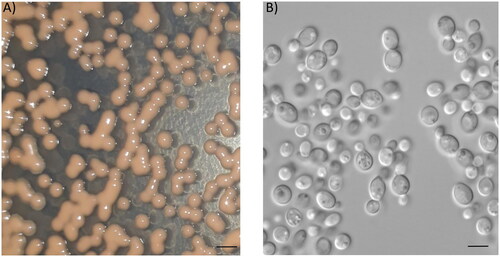
Yeast colonies Rhodotorula aurum sp. nov. strain were glistening, butyrous to mucoid, smooth with entire margins, and red-pigmented white at 3 days old on PDA agar. Yeast cells are ovoid to elliptical in shape with size in the range of 4–7 µm, and budding cell is commonly unipolar (). Moreover, yeast cell didn’t produce spores, hyphae, and pseudohyphae after 21 days at 25 °C in yeast sporulation medium (Formedium, Norfolk, UK) or in corn meal agar (Himeidia, Mumbai, India). Rhodotorula aurum sp. nov. could grow at temperatures ranging from 10 to 40 °C (Figure S1A). The optimal temperature was observed at 30 °C since growth performance reached the maximum, whereas at 45 °C no growth was observed (data not show), and poor growth was found at 10 and 40 °C. Similar results were also observed in the study of Mundra et al. (2011) [Citation18], who reported that Rhodotorula sp. PS4 could grow at temperatures in the range of 5–40 °C. While the ability to grow at different temperatures in R. calyptogenae and R. minuta was varied, some strains could grow at temperatures above 35 °C, others were not able to grow at this temperature [Citation19].pH is a critical factor for yeast growth, most of yeasts grow well at a pH of between 4.5 and 6.5, but some species can grow in a more acidic or alkaline medium [Citation20]. In agreement with this, Rhodotorula aurum sp. nov. grew well in the pH range of 3–7, and the optimal pH for yeast growth was determined at pH 5-6 (Figure S1B). The strain was able to grow at pH 8, but the growth kinetic was significantly decreased compared to that in the pH range of 3–7. Similar results were also observed in previous studies, in which pH 5.5 was determined as the optimal pH for Rhodotorula sp. RY1801 [Citation21], R. acheniorum [Citation22], and R. glutinis [Citation23]. A higher pH (7) for Rhodotorula sp. PS4 growing and dissolving phosphate was determined [Citation18].
Figure 2. Morphology of Rhodotorula aurum sp. nov. (A) Colonies grown on YM plate for 2 days. Bar 1 mM. (B) Cells under microscopy (magnification of 100×). Bar 10 µM.
The results of biochemical tests indicate the similarity of Rhodotorula aurum sp. nov. and other strains, in that Rhodotorula aurum sp. nov. can assimilate many different sugars, but could not utilize xylose, arabinose, and citrate ().
3.3. Effect of copper on kinetic growth of Rhodotorula aurum sp. nov.
The growth of Rhodotorula aurum sp. nov. in YNB glucose containing different copper concentrations varied, at low concentrations (0.5–2 mM) Cu2+ Rhodotorula aurum sp. nov. could grow well as a control (without copper). However, the growth ability of yeast cells was severely affected when copper was above these concentrations by extending the lag phase and reducing the maximal OD600 it could reach (). These results are in agreement with previous studies, which suggested that high concentrations of heavy metals cause stress for yeast cells, prolonging lag phage extension or decreasing specific growth rate [Citation24,Citation25]. As indicates Rhodotorula aurum sp. nov. could grow in a medium containing 10 mM copper. This finding is consistent with that of Villegas et al. (2005) [Citation26], who found R. mucilaginosa RCL-11 could grow in a medium supplemented with 10 mM copper. In contrast, 8 mM of copper concentration is the maximum level of copper tolerated by R. mucilaginosa LM9 [Citation27].
Figure 3. Effect of Cu2+ on the growth (A) and cell morphology (B) of Rhodotorula aurum sp. nov. Bar 10 µM.
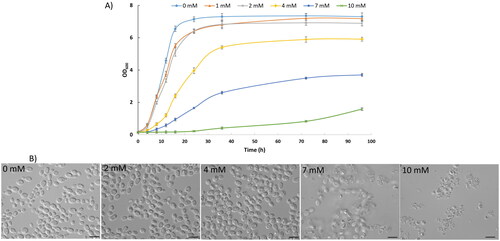
The effect of copper on cell morphology was also investigated as yeast cells were cultured in a medium without Cu2+ and in medium containing different concentrations of Cu2+. As shown in , cells exposed to copper exhibit altered morphology with some strangely rough dots inside, but cells were severely affected and damaged the cell wall when Cu2+ reached 7 mM or more (10 mM) which could explain the dynamic growth being decreased. A detailed understanding of the mechanism by which copper alters yeast cell morphology may need further study, but the dots (dark bodies) of yeast cells in medium containing Cu2+ (≤4 mM) may represent resistance mechanisms of yeasts through sequestration and extrusion of cytoplasmic copper ions rather than avoidance or compartmentalization [Citation28].
3.4. Biosorption assays
3.4.1. Effect of initial pH on the removal efficiency of Cu2+
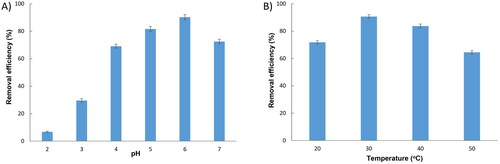
It is well known that pH has significant effects on the biosorption capacity of heavy metals because it is actively engaged in the competition between protons and heavy metal ions for the surface biosorption sites [Citation29]. shows that, in a strong acidic medium (pH 2), the adsorption of Cu2+ on the biosorbent surfaces was slow as only 6.75% of Cu2+ was removed. This is because at very low pH a high concentration of H+ ions competes with Cu2+ to fill the active sites on the surface of the biosorbent. The removal efficiency was dramatically increased from 29.6 to 81.67% as pH increased from 3 to 5, and reached a maximum of 90.28% at pH 6 since increasing pH leads to a decrease in H+ ions in the solution which brings about an increase in the number of active sites for Cu2+ adsorption. However, when pH reached 7, OH- competed with functional groups of biosorbent surfaces, and also reacted with Cu2+, then a precipitate of Cu(OH)2 may appear that interferes with the adsorption process, causing a reduction in the adsorption efficiency to 72.52%. These results are consistent with those of Say et al. (2001) [Citation30] who used Phanerochaete chrysosporium for biosorption of Cu2+, and with those of Chen et al. (2021) who also found that 6 is the optimal pH for biosorption of Cu2+ by yeast Pichia pastoris [Citation31]. Meanwhile, pH 5 was determined as the optimal pH value for Cu2+ adsorption by Saccharomyces cerevisiae [Citation32], by Papiliotrema huenov [Citation16]. pH in the range of 3.5–4 was the most favorable for dried yeast S. cerevisiae in adsorption of Cu2+ [Citation33], a higher pH (7) was optimum for copper ion adsorption by Spirulina platensis [Citation34]. Thus, the optimal pH for copper ion adsorption may be dependent on the biological material used. The pH 6 was therefore chosen as the optimal pH for subsequent experiments.
3.4.2. Effect of temperature on biosorption of Cu2+
Temperature is among the most important factors that influence the kinetics of biosorption [Citation35]. The Cu2+ adsorbed by the Rhodotorula aurum sp. nov. was evaluated under four different temperatures: 20, 30, 40, and 50 °C. As the results shown in , at 20 °C the removal efficiency of copper was 71.84%, and it was significantly increased to 90.66% when the temperature increased to 30 °C. However, the removal efficiency gradually decreased to 83.75% at 40 °C, and then dramatically reduced to 64.52% when the temperature reached 50 °C. This reduction in adsorption when the temperature increased was possibly due to the weakening of adsorptive forces between Cu2+ and the active sites of surface biosorbents [Citation36] or to a change in the functional groups on the surface of yeast [Citation37]. Our results demonstrate that biosorption of Cu2+ by Rhodotorula aurum sp. nov. is endothermic, an increase in temperature led to expedited ion diffusion. This finding broadly supports the work of other studies about the effect of temperature on biosorption [Citation16,Citation38,Citation39].
3.4.3. Biosorption of Cu2+ by Rhodotorula aurum sp. nov. biomass under optimal conditions
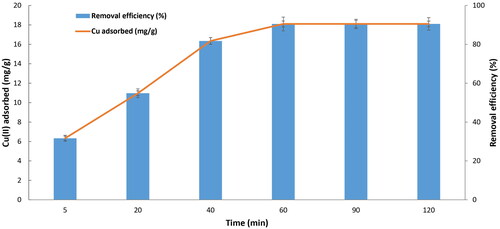
The results from indicate that the adsorption of Cu2+ to yeast biomass reached equilibrium within 60 min. The biosorption capacity was increased from 6.32 to 18.1 mg/g when the contact time increased from 5 to 60 min, and the Cu2+ removal efficiency in the solution increased from 31.66 to 90.49%, respectively. Thus, at pH 6, temperature 30 °C, and incubation time 60 min the maximum Cu2+ adsorption to the yeast biomass was around 18.1 mg/g, which is significantly higher compared to those of previous studies in which the maximal Cu2+ binding capacity ranged from 2.59 to 15.85 mg/g using different sorbents such as Saccharomyces cerevisiae [Citation32,Citation33], Candida spp. [Citation26] and Pichia stipitis [Citation39]. However, the findings of the current study are far below those observed by Salvadori et al. (2014) [Citation36] who found the biosorption capacity of copper was 26.2 mg/g when using the dead biomass of R. mucilaginosa isolated from a wastewater mine in Brazil. It has been reported that the pretreatment of yeast biomass by phosphate [Citation40], by NaOH, or by Na2CO3 [Citation41] dramatically improves biosorption capacity. Thus to improve the Cu2+ biosorption capacity using biomass Rhodotorula aurum sp. nov. as a biosorbent, it is necessary to develop an effective pretreatment method, which is presently underway and the results will be reported upon completion.
4. Conclusion
In this study, a highly copper-resistant strain named Rhodotorula aurum sp. nov. was isolated from a gold mining area. It is affiliated with R. mucilaginosa but exhibited some differences in the nucleotide sequence of the SLU gene (>1%) and in biochemical characteristics. The dried biomass of Rhodotorula aurum sp. nov. was able to absorb 18.1 mg Cu2+/g biomass that makes it potentially useful in the bioremediation of heavy metals.
5. Taxonomy
Rhodotorula aurum sp. nov., A, B, & C.
MycoBank: MB 848923
Etymology: Rhodotorula aurum sp. nov. in reference to gold mining located in Chưpah, Gia Lai Province, and identified by KCT Nguyen, PH Truong, TC Ho, CT Le, KD Tran, TL Nguyen, MT Nguyen, and PV Nguyen.
Strain GL1T exhibits red-pigmented white and smooth colonies that are glistening, butyrous to mucoid, with entire margins in 3 days old on PDA agar. Microscopic morphology shows ovoid and elliptical budding yeast-like cells which are unipolar, and have a size of 4–7 µm. Spores, hyphae, and pseudohyphae were not observed after 21 days at 25 °C of cultivation. The maximum growth of Rhodotorula aurum sp. nov. was observed at 30 °C, but the yeast cells exhibit poor growth at 10 and 40 °C. Strain GL1T was able to grow in medium containing 10 mM of Cu2+ and in pH ranged 3–7. Strain GL1T could assimilate ethanol and inositol, but neither use citrate, arabinose or xylose.
The type strain GL1T was isolated from Gia Lai province, Vietnam (GPS: 14°21'47.7"N 108°12'53.9"E), and deposited in the Institute of Biotechnology, Hue University Vietnam with accession number 062022.
Supplemental Material
Download MS Word (14.4 KB)Supplemental Material
Download MS Word (12 KB)Supplemental Material
Download JPEG Image (279.4 KB)Acknowledgments
The authors thank Dr. Derek Wilkinson for the English editing and proofreading of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Izydorczyk G, Mikula K, Skrzypczak D, et al. Potential environmental pollution from copper metallurgy and methods of management. Environ Res. 2021;197:111050. doi: 10.1016/j.envres.2021.111050.
- Burkhead JL, Gogolin Reynolds KA, Abdel‐Ghany SE, et al. Copper homeostasis. New Phytol. 2009;182(4):799–816. doi: 10.1111/j.1469-8137.2009.02846.x.
- Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97(6):1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x.
- Strain J, Culotta VC. Copper ions and the regulation of Saccharomyces cerevisiae metallothionein genes under aerobic and anaerobic conditions. Mol Gen Genet. 1996;251(2):139–145. doi: 10.1007/BF02172911.
- Brewer GJ. The risks of copper toxicity contributing to cognitive decline in the aging population and to Alzheimer’s disease. J Am Coll Nutr. 2009;28(3):238–242. doi: 10.1080/07315724.2009.10719777.
- Llanos RM, Mercer JFB. The molecular basis of copper homeostasis copper-related disorders. DNA Cell Biol. 2002;21(4):259–270. doi: 10.1089/104454902753759681.
- Abe F, Miura T, Nagahama T, et al. Isolation of a highly copper-tolerant yeast, Cryptococcus sp., from the Japan Trench and the induction of superoxide dismutase activity by Cu2+. Biotechnol Lett. 2001;23:2027–2034.
- de Silóniz M-I, Balsalobre L, Alba C, et al. Feasibility of copper uptake by the yeast Pichia guilliermondii isolated from sewage sludge. Res Microbiol. 2002;153(3):173–180. doi: 10.1016/s0923-2508(02)01303-7.
- Eman MF, Fatma FA-M, Soad AE. Biosorption of heavy metals onto different eco-friendly substrates. J Toxicol Environ Heal Sci. 2017;9:35–44.
- Grujić S, Vasić S, Radojević I, et al. Comparison of the Rhodotorula mucilaginosa biofilm and planktonic culture on heavy metal susceptibility and removal potential. Water, Air, Soil Pollut. 2017;228:73.
- Rehman A, Farooq H, Hasnain S. Biosorption of copper by yeast, Loddermyces elongisporus, isolated from industrial effluents: its potential use in wastewater treatment. J Basic Microbiol. 2008;48(3):195–201. doi: 10.1002/jobm.200700324.
- Kurtzman CP, Fell JW, Boekhout T, et al. Methods for isolation, phenotypic characterization and maintenance of yeasts. In: Kurtzman CP, Fell JW, Boekhout T, editors, The yeasts: A Taxonomic Study. Vol 1, 5th ed. Amsterdam (Netherlands): Elsevier. p. 87–110.
- Nguyen KCT, Nguyen PV, Truong HTH. Heavy metal tolerance of novel papiliotrema yeast isolated from Vietnamese mangosteen. Mycobiology. 2020;48(4):296–303. doi: 10.1080/12298093.2020.1767020.
- Kurtzman CP, Robnett CJ. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Van Leeuwenhoek. 1998;73(4):331–371. doi: 10.1023/a:1001761008817.
- Tamura K, Stecher G, Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. 2021;38(7):3022–3027. doi: 10.1093/molbev/msab120.
- Nguyen Van P, Thi Hong Truong H, Pham TA, et al. Removal of manganese and copper from aqueous solution by yeast Papiliotrema huenov. Mycobiology. 2021;49(5):507–520. doi: 10.1080/12298093.2021.1968624.
- Ruas FAD, Amorim SS, Leão VA, et al. Rhodotorula mucilaginosa isolated from the manganese mine water in Minas Gerais., Brazil: potential employment for bioremediation of contaminated water. Water Air Soil Pollut. 2020;231:1–14.
- Mundra S, Arora R, Stobdan T. Solubilization of insoluble inorganic phosphates by a novel temperature-, pH-, and salt-tolerant yeast, Rhodotorula sp. PS4, isolated from seabuckthorn rhizosphere, growing in cold desert of Ladakh, India. World J Microbiol Biotechnol. 2011;27(10):2387–2396. doi: 10.1007/s11274-011-0708-4.
- Shivaji S, Bhadra B, Rao RS, et al. Rhodotorula himalayensis sp. nov., a novel psychrophilic yeast isolated from roopkund lake of the Himalayan Mountain ranges, India. Extremophiles. 2008;12(3):375–381. doi: 10.1007/s00792-008-0144-z.
- Yalcin SK, Ozbas ZY. Effects of pH and temperature on growth and glycerol production kinetics of two indigenous wine strains of Saccharomyces cerevisiae from Turkey. Braz. J. Microbiol. 2008;39(2):325–332. doi: 10.1590/S1517-83822008000200024.
- Zhao Y, Guo L, Xia Y, et al. Isolation, identification of carotenoid-producing Rhodotorula sp. from marine environment and optimization for carotenoid production. Mar Drugs. 2019;17(3):161. doi: 10.3390/md17030161.
- Nasrabadi MRN, Razavi SH. Optimization of β-carotene production by a mutant of the lactose-positive yeast Rhodotorula acheniorum from whey ultrafiltrate. Food Sci Biotechnol. 2011;20(2):445–454. doi: 10.1007/s10068-011-0062-1.
- Latha BV, Jeevaratnam K, Murali HS, et al. Influence of growth factors on carotenoid pigmentation of Rhodotorula glutinis DFR-PDY from natural source. Indian J Biotechnol. 2005;4:353–357.
- Bankar A, Zinjarde S, Shinde M, et al. Heavy metal tolerance in marine strains of Yarrowia lipolytica. Extremophiles. 2018;22(4):617–628. doi: 10.1007/s00792-018-1022-y.
- Liu B, Wang C, Liu D, et al. Hg tolerance and biouptake of an isolated pigmentation yeast Rhodotorula mucilaginosa. PLOS One. 2017;12(3):e0172984. doi: 10.1371/journal.pone.0172984.
- Villegas LB, Amoroso MJ, de Figueroa LIC. Copper tolerant yeasts isolated from polluted area of Argentina. J Basic Microbiol. 2005;45(5):381–391. doi: 10.1002/jobm.200510569.
- Rajpert L, Skłodowska A, Matlakowska R. Biotransformation of copper from kupferschiefer black shale (Fore-Sudetic monocline, Poland) by yeast Rhodotorula mucilaginosa LM9. Chemosphere. 2013;91(9):1257–1265. doi: 10.1016/j.chemosphere.2013.02.022.
- Villegas LB, Amoroso MJ, de Figueroa LIC. Responses of Candida fukuyamaensis RCL‐3 and Rhodotorula mucilaginosa RCL‐11 to copper stress. J Basic Microbiol. 2009;49(4):395–403. doi: 10.1002/jobm.200800218.
- Febrianto J, Kosasih AN, Sunarso J, et al. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. J Hazard Mater. 2009;162(2–3):616–645. doi: 10.1016/j.jhazmat.2008.06.042.
- Say R, Denizli A, Arıca MY. Biosorption of cadmium (II), lead (II) and copper (II) with the filamentous fungus Phanerochaete chrysosporium. Bioresour Technol. 2001;76(1):67–70. doi: 10.1016/s0960-8524(00)00071-7.
- Chen X, Tian Z, Cheng H, et al. Adsorption process and mechanism of heavy metal ions by different components of cells, using yeast (Pichia pastoris) and Cu 2+ as biosorption models. RSC Adv. 2021;11(28):17080–17091. doi: 10.1039/d0ra09744f.
- do Nascimento JM, de Oliveira JD, Rizzo ACL, et al. Biosorption Cu (II) by the yeast Saccharomyces cerevisiae. Biotechnol Rep. 2019;21:e00315. doi: 10.1016/j.btre.2019.e00315.
- Cojocaru C, Diaconu M, Cretescu I, et al. Biosorption of copper (II) ions from aqua solutions using dried yeast biomass. Colloids Surfaces A Physicochem Eng Asp. 2009;335(1–3):181–188. doi: 10.1016/j.colsurfa.2008.11.003.
- Al-Homaidan AA, Al-Houri HJ, Al-Hazzani AA, et al. Biosorption of copper ions from aqueous solutions by spirulina platensis biomass. Arab J Chem. 2014;7(1):57–62. doi: 10.1016/j.arabjc.2013.05.022.
- Uslu G, Tanyol M. Equilibrium and thermodynamic parameters of single and binary mixture biosorption of lead (II) and copper (II) ions onto Pseudomonas putida: effect of temperature. J Hazard Mater. 2006;135(1–3):87–93. doi: 10.1016/j.jhazmat.2005.11.029.
- Salvadori MR, Ando RA, Oller do Nascimento CA, et al. Intracellular biosynthesis and removal of copper nanoparticles by dead biomass of yeast isolated from the wastewater of a mine in the Brazilian Amazonia. PLOS One. 2014;9(1):e87968. doi: 10.1371/journal.pone.0087968.
- Luk CHJ, Yip J, Yuen CWM, et al. Biosorption performance of encapsulated Candida krusei for the removal of copper (II). Sci Rep. 2017;7(1):2159. doi: 10.1038/s41598-017-02350-7.
- Fadel M, Hassanein NM, Elshafei MM, et al. Biosorption of manganese from groundwater by biomass of Saccharomyces cerevisiae. Hbrc J. 2017;13(1):106–113. doi: 10.1016/j.hbrcj.2014.12.006.
- Yilmazer P, Saracoglu N. Bioaccumulation and biosorption of copper (II) and chromium (III) from aqueous solutions by Pichia stipitis yeast. J Chemical Tech Biotech. 2009;84(4):604–610. doi: 10.1002/jctb.2088.
- Ojima Y, Kosako S, Kihara M, et al. Recovering metals from aqueous solutions by biosorption onto phosphorylated dry baker’s yeast. Sci Rep. 2019;9(1):225. doi: 10.1038/s41598-018-36306-2.
- Farhan SN, Khadom AA. Biosorption of heavy metals from aqueous solutions by Saccharomyces cerevisiae. Int J Ind Chem. 2015;6(2):119–130. doi: 10.1007/s40090-015-0038-8.