Abstract
Tree peony is an important horticultural plant worldwide of great ornamental and medicinal value. Long terminal repeat retrotransposons (LTR-retrotransposons) are the major components of most plant genomes and can substantially impact the genome in many ways. It is therefore crucial to understand their sequence characteristics, genetic distribution and transcriptional activity; however, no information about them is available in tree peony. Ty1-copia-like reverse transcriptase sequences were amplified from tree peony genomic DNA by polymerase chain reaction (PCR) with degenerate oligonucleotide primers corresponding to highly conserved domains of the Ty1-copia-like retrotransposons in this study. PCR fragments of roughly 270 bp were isolated and cloned, and 33 sequences were obtained. According to alignment and phylogenetic analysis, all sequences were divided into six families. The observed difference in the degree of nucleotide sequence similarity is an indication for high level of sequence heterogeneity among these clones. Most of these sequences have a frame shift, a stop codon, or both. Dot-blot analysis revealed distribution of these sequences in all the studied tree peony species. However, different hybridization signals were detected among them, which is in agreement with previous systematics studies. Reverse transcriptase PCR (RT-PCR) indicated that Ty1-copia retrotransposons in tree peony were transcriptionally inactive. The results provide basic genetic and evolutionary information of tree peony genome, and will provide valuable information for the further utilization of retrotransposons in tree peony.
Introduction
Retrotransposons are the most abundant class of mobile genetic elements in plants. They transpose via reverse transcribed RNA intermediates and integrate into new locations within the host genome by means of a `‘copy-and-paste’ mechanism.[Citation1] This mechanism can cause the copy number of the retrotransposons to increase and create stable insertion mutations in the host genome.[Citation2] Retrotransposons are divided into two principal groups: long terminal repeat (LTR) retrotransposons and non-LTR retrotransposons based on the presence or absence of an LTR sequence.[Citation3] The LTR retrotransposons can be further sub-divided into two groups, the Ty1-Copia group and the Ty3-Gypsy group, on the basis of their degree of sequence similarity as well as the order of their genes.[Citation3]
Retrotransposons can not only greatly increase the plant genome size because of the replicative mode of transposition, but can also generate mutations by inserting new copies within or near genes.[Citation4] However, because of the presence of stop codons, frame shifts, and deletions, most retrotransposons appear to be non-functional.[Citation3] Therefore, there is the possibility to study the transposition history of the retrotransposons for understanding their evolution and mutations in the host genome. Ty1-copia retrotransposons are present throughout the plant kingdom, ranging from algae to bryophytes, gymnosperms and angiosperms [Citation5] and are usually present in plant genomes in a high copy number and have high degrees of heterogeneity and insertional polymorphism. These characteristics, shared by many LTR retrotransposons, make the excellent bases for marker systems.[Citation6,7,Citation8] To date, Ty1-copia retrotransposons have been utilized as molecular markers for genetic research in diverse plants due to their high copy numbers and heterogeneity in plant genomes.[Citation8]
Tree peony (Paeonia suffruticosa Andrews.) belongs to the Moutan subfamily of the genus Paeonia, Paeoniaceae. Tree peonies are known as ‘the king of flowers’ due to their rich plenty of horticultural varieties, great ornamental and medicinal value in China. It has been grown for approximately 1400 years in China.[Citation9] Approximately 2000 cultivars of tree peonies are grown throughout the world, and more than 1000 cultivars are found in China.[Citation10]
However, with the rapid increase in the numbers of species or infra-specific taxa in Paeonia sect. Moutan, new technology or new information is needed for tree peony taxonomic assessment.[Citation11] It is proved that retrotransposons have contributed to increasing the genome size in the plant kingdom, and play an important role in the evolution of the genome.[Citation3,Citation12] At the same time, tree peonies have abundant genetic diversity; for example, there are many flower colours in tree peony cultivars (white, red, purple, yellow, secondary and compound colours) and diverse flower shapes (single, lotus, chrysanthemum, rose, crown, globular and others).[Citation13] There are also four cultivar groups based on geographic locations in China: Zhongyuan, Xibei, Xinan and Jiangnan.[Citation14] However, it is unclear how has this genetic diversity formed. Researches in other plants have shown that retrotransposons have played a major role in the formation of colour or other traits.[Citation4,Citation15] That is why, based on the characteristics and function of plant retrotransposons and considering the issues mentioned above in tree peony, the study of retrotransposons may be helpful for the elucidation of tree peony species and cultivars evolution, the formation mechanism of genetic diversity and important agronomic traits.
It has been found that a pair of degenerate oligonucleotide primers based on two highly conserved domains of reverse transcriptase (RT) can successfully be used for polymerase chain reaction (PCR) amplification of copia-like sequence.[Citation1,Citation5,Citation6] To date, Ty1-copia retrotransposons have been studied and characterized widely in plants such as barley, tobacco, tomato, potato, rice, maize, strawberry, mungbean, stramonium, maguey, jute, etc.[Citation8,Citation12,Citation16,17] Despite the large number of retrotransposons that have been isolated, there have been no reports of LTR retrotransposons and their phylogenetic classification in tree peony (P. suffruticosa Andrews.). Until recently, the scarcity of LTR retrotransposon sequences limited the use of retrotransposon-based molecular marker systems in this species.
In order to characterize the heterogeneous population of the copia group of retrotransposons in tree peony, we isolated genomic RT sequences by PCR using primers designed from the conserved domains of the RT region of Ty1-copia retrotransposons. The aim of this investigation was to test for the presence of Ty1-copia-like sequences in tree peony and related species using a PCR-based approach and investigate their sequence heterogeneity, phylogenetic relationships, genetic distribution and transcriptional activity.
Materials and methods
Plant materials and isolation of nucleic acids
The tree peony (P. suffruticosa) cultivar ‘Luoyanghong’, and two related species, P. qiui Y. L. Pei et D. Y. Hong and P. ostii T. Hong et J. X. Zhang., were used for RT sequence isolation. A total of 20 varieties were used for the following dot-blot analysis: five varieties of P. rockii from different regions, two varieties of P. delavayi, another seven related species of tree peony and six varieties from different cultivar groups of P. suffruticosa. shows the information about the materials. Genomic DNA was isolated from young leaves by a modified hexadecyltrimethylammonium bromide (CTAB) method as described by Guo et al.[Citation18] Total RNA was isolated as performed by Gai et al.[Citation19]
Table 1. List of varieties used in this study.
PCR amplification and cloning
The internal domain of the RT gene of Ty1-copia retrotransposons was amplified by PCR using the flanking primers corresponding to the peptide sequences TAFLHG (5′ ACNGCNTTYYTNCAY GG 3′) and YVDDML (5′-ARCATRTCRTCNACRTA-3) following the methods of Kumar et al.[Citation1] PCR products from the RT region of tree peony were cloned into the PMD-18T vector, using the original TA Cloning Kit (TaKaRa, Dalian, China). The ligation products were transformed into DH5α competent cells and positive clones were sequenced by Sun-Biotech Co. (Beijing, China).
Sequence analysis
The nature of cloned sequences was confirmed by performing similarity searches with known retrotransposon sequences from other plants in the National Center for Biotechnological Information (NCBI) database using BLASTN, BLASTX and TBLASTX algorithms. The deduced amino acid sequences of tree peony RT sequences were also compared with the RT sequences of other plants for phylogenetic analysis. Genomic DNA sequences were deposited in the GenBank database and the details of these sequences are given in . Multiple DNA sequence alignments were carried out using Lasergene 8.0. The Bootstrap neighbour-joining (NJ) tree with the tests for 10,000 replications was generated using MEGA 5.0.[Citation20]
Table 2. Accessions of RT sequences isolated from tree peony deposited in GenBank.
Dot-blot analysis
Genomic DNA and the heterogeneous PCR products of the RT domains of Ty1-copia were used for dot-blot analysis. The 270 bp PCR product was labelled using digoxigenin–deoxyuridine triphosphate (DIG–dUTP) by random primed labelling according to the manufacturer's instructions for the DIG DNA Labeling and Detection Kit (Roche, Germany). Hybridization was carried out at 65 °C for 18 h, with washing conditions as recommended by the manufacturer.
Reverse transcriptase PCR (RT-PCR)
First-strand cDNA was synthesized from total RNA using RT XL (AMV) (TaKaRa, Daliang, China). PCR was carried out using the same degenerate primers and cycling conditions as above, except that 1.0 μL of first-strand cDNA was used as the template. Meanwhile, genomic DNA (gDNA) was used as a positive control.
Results and discussion
Isolation of Tyl-copia RT sequences from tree peony genome
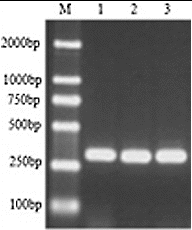
Different degenerate primers corresponding to the conserved RT domains were identified by Flavell et al.,[Citation6] Hirochika and Hirochika,[Citation7] Kumar et al. [Citation1] and Voytas et al. [Citation5] in plants. The highly conserved amino acid sequences of enzyme domains have proven to be a useful basis for the design of PCR primers for the purpose of transposon surveys in a number of plant species.[Citation12,Citation21,Citation22] These primers were also used in the preliminary study. However, only the degenerate oligonucleotide primers corresponding to the TAFLHG and YVDDML regions designed by Kumar et al. [Citation1] were successfully used to amplify the conserved RT domains of the tree peony. An amplicon of ∼270 bp was obtained from the tree peony genome of Luoyanghong, P. qiui and P. ostii, using PCR (). This is in agreement with the results of others [Citation17,Citation23] and indicates that Ty1-copia retrotransposons were distributed in all the studied tree peony genomes and have a similar length of RT sequences.
Sequence characterization of tree peony Ty1-copia RT clones
Nineteen clones containing 270 bp PCR products from Luoyanghong, twelve clones from P. qiui and two clones from P. ostii, were randomly selected for sequence analysis. The results showed that 33 clones with RT sequences of Ty1-copia group retrotransposons were identified. Their DNA sequences were deposited in the NCBI nucleotide sequence database (). Homology-based searching (BLASTx and BLASTn) revealed that these putative RT clones have nucleotide sequence similarities to the RT domains of other known plant Ty1-copia group retrotransposons (data not shown). Six additional RT sequences previously obtained from other plants () which were highly similar to the tree peony putative RT sequences were added for further analysis.
The putative Tyl-copia RT sequences were translated into their amino acids and analysed for the presence of stop codons and frame shifts in their coding regions. Translation of these PCR amplified sequences implied that among the 33 Ty1-copia RT sequences, 19 (approximately 58%) sequences contained in-frame stop codon(s) which inferred that these sequences do not have potential function of a RT fragment. The stop-codon-containing sequences are PS1, PS2, PS4, PS6, PS8, PS9, PS10, PS12, PS14, PS16, PS17, PQ10, PQ12, PQ22, PQ25, PQ26, PQ28, PO302 and PO303, in which one to four stop codons were present within coding regions of the retrotransposons (). The remaining 14 amplified sequences (approximately 42%) were considered to be partial Ty1-copia RT sequences of tree peony with potentially functional RT domain, as they lack any in-frame stop codon. Several of the RT sequences had frame shifts: the 13th amino acid in PS1, the 14th amino acid in PS2, the 18th amino acid in PS4, the 12th amino acid in PS12, the 8th amino acid in PS16, the 14th amino acid in PQ21, the 17th amino acid in PQ26, the 13th amino acid in PO303, the 1st amino acid in PO302, and so on. The age of an element is directly proportional to the number of termination codons incorporated in it since the termination codons are not accumulated in an active element due to its continuous retrotransposition.[Citation24] For example, a study of Ty1-copia retrotransposons in strawberry has identified that 5 of 19 RT gene fragments are characterized with stop codons and/or frameshifts,[Citation25] whereas in persimmon 51% of the Ty1-copia RT sequences have been shown to have stop codons and/or frameshifts.[Citation26] In this study, more than 58% of Ty1-copia RT sequences showed stop codons and/or frameshifts when translated. The percentage is higher than that in other plants, which suggests that there have possibly been frequent activations of tree peony Ty1-copia retrotransposons in the history of this species and maybe this could be one of the reasons that have led to the abundant genetic diversity existing in tree peony.
Figure 2. Sequence alignment of the deduced amino acid sequences corresponding to the reverse transcriptase domain (RT) of the Ty1-copia group retrotransposons in tree peony. Gaps and stop codons are indicated as (--) and (.), respectively. Numerals on the right are the number of amino acid residues in the sequences. The three shaded boxes indicate the conserved residue of the sequences. The details of RT sequences are given in .
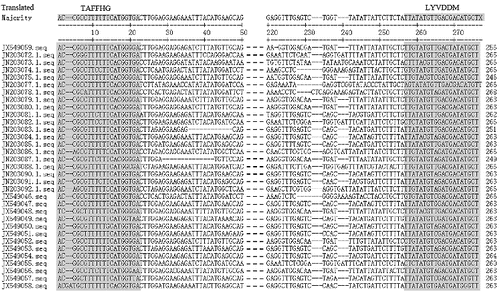
The alignment and the in silico analysis of the deduced amino acid sequences of all 33 sequences revealed the presence of blocks of residues of highly conserved regions among them (). They contained characteristic amino acid motifs for the RT gene (5′-TAFF(L)HG; central region, YGLKQ and 3′YVDDM). Most of these sequences showed strong homology to the RT conserved domains of the Ty1-copia-like retrotransposons of various plant species.[Citation1] Although the amino acid motifs of few sequences of Ty1-copia retrotransposons of tree peony did not show any similarity (), the homology-based searches (BLASTn and BLASTx) confirmed these as sequences of Ty1-copia-like retrotransposons. These results suggest that the above-mentioned clones represent a portion of the RT domain of the Ty1-copia group retrotransposons in tree peony.
Li [Citation14] summarized the previous researches and presented the viewpoint that P. jishanensis, P. rockii, P. qiui and P. ostii are the wild ancestor of P. suffruticosa based on the morphology and cultivation history, which was further supported by Zhang et al.[Citation11] Hou et al. [Citation27] reported that P. ostii had the closest relationships with the Zhongyuan cultivar group of P. suffruticosa by eight amplified-fragment-length-polymorphism (AFLP) primer analysis. However, the alignments in our study showed that the sequence similarity between Luoyanghong and P. qiui was higher than the one between Luoyanghong and P. ostii. The P. ostii sequences are more divergent than that of Luoyanghong (P. suffruticosa). In fact, eight sequences were obtained from P. ostii, but only two were identified as putative Tyl-copia RT sequences after Blast search and alignments. This reflects the extreme sequence heterogeneity existing in P. ostii. It indicated that the genetic relationship between P. suffruticosa and P. qiui is closer than that between P. suffruticosa and P. ostii. This disagreement with Hou et al. [Citation27] may be due to the limited primers used in their study.
Phylogenetic analysis of tree peony Ty1-copia RT sequences
Due to high variability in the nucleotide sequences, multiple sequence alignments were performed on amino acid sequences, using Lasergene. Within plants, sequence analyses of the RT genes revealed extremely high heterogeneity even in the same species.[Citation28] High level of heterogeneity was found among the 33 peptide sequences, all showing between 10.7% (PQ29 and PO302) and 96.6% (PS13 and PQ23) identity to one another.
For comparative purposes, we included in the phylogenetic analysis Ty1-copia group RT sequences from other species deposited in the GenBank database (). The phylogenetic relationship among the RT sequences of Tyl-copia group retrotransposons was represented as a NJ-tree based on P-distance and supported with 10,000 replicates of bootstrapping (). The phylogenetic analysis showed that the sequences could be grouped into six distinct families (F1–F6) and that most Tyl-copia RT sequences in tree peony were closely related to the representative elements of other plant species. The first family in included five sequences all from Luoyanghong and seemed to be associated with GQ852889.1 isolated from Epimedium coactum, DQ376441 from Orobanche ramosa, GU573476.1 from Lycium ruthenicum, as well as GU197836.1 from Fragaria x ananassa. The second family comprised of three sequences from Luoyanghong and three ones from P. qiui and did not include any from other plant sequences. The third family was found to be largest, consisting of five sequences from Luoyanghong and six sequences from P. qiui. ADF45720.1 from Eleocharis quinqueflora was associated with family 3 and its members were very similar to each other, indicating that they were recently duplicated. Family 4 containing one single sequence (PO302) was separated as an independent group because of its long distance to the remaining sequences. Family 5 is the most complicated; it included two sequences from Luoyanghong, two sequences from P. qiui and one from P. ostii and appeared to be related to DQ494250.1 from Prunus mume ABF57057. The RT sequences showed the high diversity of retrotransposons within tree peony. The sequences isolated from different species of tree peony are mixed together and could not be distinguished from each other, indicating that most probably a vertical transmission had happened during the evolution of these retrotransposons in tree peony. Furthermore, when the identified families span species boundaries, it could be suggested that they existed early in plant evolution prior to modern plant species divergence.[Citation29] The divergent families probably represent parallel evolution of groups of sequences from a common ancestor and the result of the phylogenetic analysis showed that the RT sequences from tree peony had high homology with those from other species, such as Lycium ruthenicum and Prunus mume (), which supports a horizontal mechanism of transmission.
Figure 3. Phylogenetic analysis of the deduced amino acid sequences representing fragments of the RT domain of Ty1-copia retrotransposons in tree peony and other plants, using the neighbour-joining method. The numbers in the branches represent bootstrap support for 10000 replicates. The details of the sequence names are given in .
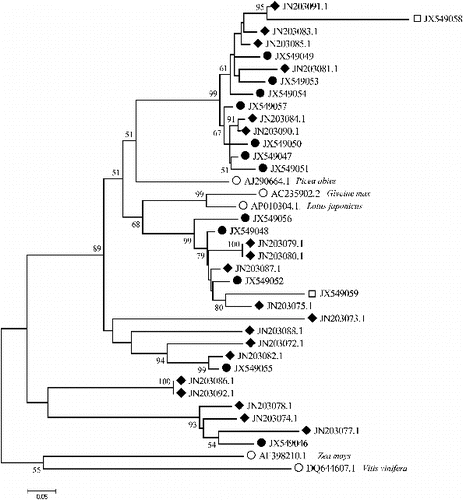
The phylogenetic analysis revealed that tree peony Ty1-copia group retrotransposon families 4, 5 and 6 possess long branches. On the other hand, the remaining families (1–3) are characterized with short branches. Branch lengths are known to be proportional to sequence divergence and may be considered a result of faster sequence evolution brought about at least partly by the error-prone nature of RT.[Citation29,30] The sequence of PO302 from P. ostii and F4 in comprises a unique sequence in a phylogenetic context, with the longest branch, which indicated that P. ostii are more primitive than P. suffruticosa and P. qiui. Clones of Luoyanghong form a large clade composed of closely related sequences, however, sequences of P. qiui, especially of P. ostii are more variable. These data, combined with other comprehensive analyses,[Citation14] suggest that retroelements could have played a role in differential genome evolution, similar to that in other plant groups.[Citation21]
Dot-blot analysis
To determine the copy numbers of Ty1-copia elements in a plant genome, Southern blot analysis is usually carried out.[Citation21,Citation26,Citation31] At first, we also attempted to conduct Southern blot and designed some primers to obtain probes based on the consensus sequences from alignments. However, the results were not satisfactory, since the signal was too weak. That is why, dot blotting was employed to investigate the existence of Ty1-copia elements in the tree peony genome. Dot blotting has been widely used in the analysis of retrotansposon sequences to determine the copy numbers.[Citation23,Citation25,Citation32,33] The heterogenous populations of 270 bp Ty1-copia whole PCR products were used as probes for dot-blot hybridization in this study to acquire the relative numbers of Copia retrotransposons. However, due to the lack of information about the length of the tree peony genome at present, we could not obtain the specific copy number of Copia elements in tree peony.[Citation23,Citation25,Citation32]
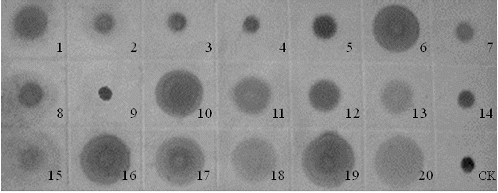
Dot-blot analysis in this study showed the existence of dispersed copies of the Ty1-copia element in tree peony genome because the hybridization signal was detected in all the materials (). The detection of multiple bands under both high- and low-stringency hybridization conditions indicates that a large number of sequences homologous to RT are integrated throughout the tree peony genome. However, the intensity of the hybridization signal in different varieties was different (), which suggests a presence of a different number of Ty1-copia elements in the corresponding genomes. For example, the signals in P. qiui, P. ostii, P. rockii and P. decomposita were inferior to those of other species, which indicated lower numbers in them. This result is in accordance with the evolution analysis of Li.[Citation14] However, even in the same species of P. rockii, different signals were detected in the varieties collected from various localities. Indeed, it was confirmed that different genetic diversity existed in different populations of P. rockii.[Citation9,10] It may be due to the different selection pressures. Another interesting result is that medium signals were detected in P. ludlowii and P. lutea. Both of them are yellow tree peonies. This indicated their special status in the species of tree peony. Li [Citation14] and Zhang et al. [Citation11] all stated that P. ludlowii and P. lutea were not involved in the evolution of P. suffruticosa. The hybridization intensities of varieties of P. suffruticosa, whether it is from Zhongyuan, Xibei, Xinan or Jiangnan, were all stronger than those of P. qiui, P. ostii, P. rockii and P. decomposita. This suggested that the copy numbers in P. suffruticosa are more than those in P. qiui, P. ostii, P. rockii and P. decomposita, which, combined with the results of Li [Citation14] and Zhang et al.,[Citation11] indicates that the retrotransposons of P. suffruticosa may have been multiplied in comparison to that of P. qiui, P. ostii, P. rockii or/and P. decomposita. This result may possibly indicate that this element is an ancient component of the tree peony genome, introduced before the divergence of the species and conserved during evolution. Of course, more proofs are needed to determine the genetic relationships among the species of tree peony.
Transcriptional analysis
Plant retrotransposons are usually transcriptionally inactive during the developmental stages to lessen the detrimental effect on the host.[Citation3] The feature of retrotransposons transcriptional activity in tree peony was studied by an RT-PCR approach using degenerate oligonucleotide primers. Total RNA was extracted from leaves of in vitro grown tree peony plants, and it was reverse transcribed to obtain the corresponding cDNA. However, no product corresponding to the expected size of Ty1-copia retrotransposons was amplified from cDNAs, while the expected product was amplified from the control (gDNA). The results indicated that Ty1-copia retrotransposons were transcriptionally inactive in tree peony.
These results could be considered as a first step towards understanding the performance of Ty1-copia retrotransposons in tree peony and more work is needed in future in order to expand our knowledge about the activity of Ty1-copia retrotransposons and to utilize them in genetic analysis of tree peony. The isolation of full-length Ty1-copia retrotransposons and the development of molecular markers based on the retrotransposons of tree peony are ongoing.
Additional information
Funding
References
- Kumar A, Pearce SR, McLean K, Harrison G, Heslop-Harrison J, Waugh R, Flavell AJ. The Ty1-copia group of retrotransposons in plants: genomic organisation, evolution, and use as molecular markers. Genetica. 1997;100:205–217.
- Flavell AJ, Pearce SR, Heslop-Harrison P, Kumar A. The evolution of Ty1-copia group retrotransposons in eukaryote genomes. Genetica. 1997;100:185–195.
- Kumar A, Bennetzen JL. Plant retrotransposons. Annu Rev Genet. 1999;33:479–532.
- Kobayashi S, Goto-Yamamoto N, Hirochika H. Retrotransposon-induced mutations in grape skin color. Science. 2004;304:982.
- Voytas DF, Cummings MP, Koniczny A, Ausubel FM, Rodermel SR. Copia-like retrotransposons are ubiquitous among plants. Proc Natl Acad Sci USA. 1992;89: 7124–7128.
- Flavell AJ, Dunbar E, Anderson R, Pearce SR, Hartley R, Kumar A. Ty1–copia group retrotransposons are ubiquitous and heterogeneous in higher plants. Nucleic Acids Res. 1992;20:3639–3644.
- Hirochika H, Hirochika R. Ty1-copia group retrotransposons as ubiquitous components of plant genomes. Jpn J Genet. 1993;68:35–46.
- Kalendar R, Flavell AJ, Ellis TH, Sjakste T, Moisy C, Schulman AH. Analysis of plant diversity with retrotransposon-based molecular markers. Heredity. 2011;106:520–530.
- Yuan JH, Cheng FY, Zhou SL. Genetic structure of the tree peony (Paeonia rockii) and the Qinling Mountains as a geographic barrier driving the fragmentation of a large population. PLoS One. 2012;7:e34955.
- Yuan Jh, Cheng FY, Zhou SL. The phylogeographic structure and conservation genetics of the endangered tree peony, Paeonia rockii (Paeoniaceae), inferred from chloroplast gene sequences. Conservation Genet. 2011;12:1539–1549.
- Zhang JM, Wang JX, Xia T, Zhou SL. DNA barcoding: species delimitation in tree peonies. Sci China C Life Sci. 2009;52:568–578.
- Khaliq I, Khan MA, Pearce S. Ty1-copia retrotransposons are heterogeneous, extremely high copy number and are major players in the genome organization and evolution of Agave tequilana. Genet Resour Crop Evol. 2012;59:575–587.
- Shu Q, Wang L, Wu J, Du H, Liu Z, Ren H, Zhang J. Analysis of the formation of flower shapes in wild species and cultivars of tree peony using the MADS-box subfamily gene. Gene. 2012;493:113–123.
- Li JJ. The origin, evolution and classification of cultivars. In: Li JJ, editor. Chinese tree peony and herbaceous peony. Beijing: Chinese Forestry Press; 1999. p. 59–62.
- Sato M, Kawabe T, Hosokawa M, Tatsuzawa F, Doi M. Tissue culture-induced flower-color changes in Saintpaulia caused by excision of the transposon inserted in the flavonoid 3′, 5′ hydroxylase (F3′5′H) promoter. Plant Cell Rep. 2011;30:929–939.
- Schulman AH, Flavell AJ, Paux E, Ellis TH. The application of LTR retrotransposons as molecular markers in plants. Methods Mol Biol. 2012;859:115–153.
- Ma Y, He P, Sun HY, Zhao GL, Dai HY, Zhang ZH. Isolation and characterization of transcriptionally active Ty1-copia retrotransposons in Fragaria×ananassa. Agric Sci China. 2010;9:337–345.
- Guo DL, Hou XG, Zhang J. Sequence-related amplified polymorphism analysis of tree peony (Paeonia suffruticosa Andrews.) cultivars with different flower colours. J Hortic Sci Biotech. 2009;84:131–136.
- Gai S, Zhang Y, Mu P, Liu C, Liu S, Dong L, Zheng G. Transcriptome analysis of tree peony during chilling requirement fulfillment: assembling, annotation and markers discovering. Gene. 2012;497:256–262.
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739.
- Dixit A, Ma KH, Yu JW, Cho EG, Park YJ. Reverse transcriptase domain sequences from Mungbean (Vigna radiata) LTR retrotransposons: sequence characterization and phylogenetic analysis. Plant Cell Rep. 2006;25: 100–111.
- Singh A, Nirala N, Narula A, Das S, Srivastava PS. Isolation and characterization of Ty1-copia group of LTRs in genome of three species of Datura: D. innoxia, D. stramonium and D. metel. Physiol Mol Biol Plants. 2011;17(3):255–261.
- Xiao W, Su Y, Sakamoto W. Isolation and characterization of Ty1/copia-like retrotransposons in mung bean (Vigna radiata). J Plant Res. 2007;120:323–328.
- Gao X, Havecker ER, Baranov PV, Atkins JF, Voytas DF. Translational recoding signals between gag and pol in diverse LTR retrotransposons. RNA. 2003;9:1422–1430.
- Ma Y, Sun H, Zhao G, Dai H, Gao X, Li H, Zhang Z. Isolation and characterization of genomic retrotransposon sequences from octoploid strawberry (Fragaria× ananassa Duch.). Plant Cell Rep. 2008;27:499–507.
- Nakatsuka A, Iwami N, Matsumoto S, Itamura H, Yamagishi M. Ty1-copia group retrotransposons in persimmon (Diospyros kaki Thunb.). Genes Genet Syst. 2002;77: 131–136.
- Hou XG, Yin WL, Li JJ, Wang HF. AFLP analysis of genetic diversity of 30 tree peony (Paeonia suffruticosa Andr.) cultivars. Sci Agric Sin. 2006;39:1709–1715.
- Pearce SR, Li D, Flavell A, Harrison G, Heslop-Harrison J, Kumar A. TheTy1-copia group retrotransposons inVicia species: copy number, sequence heterogeneity and chromosomal localisation. Mol Gen Genet. 1996;250:305–315.
- Hafez EE, Zaki EA. Phylogenetic and molecular evolutionary analyses of Ty1-copia group retrotransposons in cultivated Egyptian cotton, Gossypium barbadense L. Afr J Biotechnol. 2005;4(11):1275–1280.
- Eickbush TH, Furano AV. Fruit flies and humans respond differently to retrotransposons. Curr Opin Genet Dev. 2002;12:669–674.
- Kim H, Yamamoto M, Hosaka F, Terakami S, Nishitani C, Sawamura Y, Yamane H, Wu J, Matsumoto T, Matsuyama T. Molecular characterization of novel Ty1-copia-like retrotransposons in pear (Pyrus pyrifolia). Tree Genet Genom. 2011:1–12.
- Ahmed S, Shafiuddin M, Azam SM, Islam MS, Ghosh A, Khan H. Identification and characterization of jute LTR retrotransposons: their abundance, heterogeneity and transcriptional activity. Mob Genet Elem. 2011;1: 18–28.
- Sun HY, Dai HY, Zhao GL, Ma Y, Ou CQ, Li H, Li LG, Zhang ZH. Genome-wide characterization of long terminal repeat-retrotransposons in apple reveals the differences in heterogeneity and copy number between Ty1-copia and Ty3-gypsy retrotransposons. J Integr Plant Biol. 2008; 50:1130–1139.