Abstract
Improvement of production of an extracellular β-glucosidase with high activity by Brettanomyces anomalus PSY-001 was performed by using recursive protoplast fusion in a genome-shuffling format. The initial population was generated by ultraviolet irradiation, ultrasonic mutagenesis and, then, subjected to recursive protoplast fusion. Mutant strains exhibiting significantly higher β-glucosidase activities in liquid media were isolated. The best mutant strain showed increased cell growth in a flask culture, as well as increased β-glucosidase production. A recombinant strain, F3-25, was obtained after three rounds of genome shuffling and its production of β-glucosidase activity reached 4790 U L−1, which was a nearly eightfold increase compared to the original strain B. anomalus PSY-001. The subculture experiments indicated that F3-25 was genetically stable.
Introduction
Brettanomyces spp. are described as the non-spore forming counterparts of Dekkera spp. and the names of the two genera are often used interchangeably.[Citation1] As Brettanomyces is the more common name, it will be used in this paper. The genus Brettanomyces/Dekkera includes five species: Brettanomyces custersianus, B. naardenensis, B. nanus, Dekkera anomala and D. bruxellensis.[Citation2] Brettanomyces spp. are found during the fermentation and refermentation of special Belgian beers and ales and are involved in the bioflavouring of a particular Belgian trappist beer.[Citation3,4] The release of flavour-active compounds from hop glycosides opens perspectives for the bioflavouring and product diversification of beverages like beer. Higher activities of exo-β-glucanase can be found in Brettanomyces spp. with β-glucosidase activity.[Citation3]
Beta-glucosidases (EC 3.2.1.21) are a group of wide-spread hydrolyases found in microorganisms, plant and animal tissues. Since most of the β-glucosidases from microbial sources are subjected to glucose inhibition, glucose-tolerant β-glucosidases are in high demand.[Citation5] The major applications of β-glucosidases include production of glucose and other soluble sugars from cellulosic wastes in the process of biomass conversion,[Citation6] enhancement of aroma in wine and must by hydrolysis of glycoconjugated precursors [Citation7] and for the enrichment of bioactive isoflavones in soymilk fermentation.[Citation8]
Protoplast fusion is a traditional method, which – although established for over 30 years now – continues to be recognized as a powerful tool for the improvement of many microorganisms. Protoplast fusion shows very broad applicability among microorganisms, both in its intraspecies and interspecies variant, and also between microbes from different kingdoms.[Citation9,10] The technique of protoplast-based genome shuffling has proved to be effective in the rapid improvement of some industrially important microbial strains.[Citation11] Genome shuffling does not require genome sequencing; it is often a preferred method of choice when simultaneous changes have to be introduced at different positions throughout the entire genome. This makes genome shuffling a practical method for the rapid manipulation of complex phenotypes from whole cells and organisms.[Citation12,13]
The aim of this work was to generate high-yield β-glucosidase-producing strains from B. anomalus PSY-001 through the combination of protoplast mutation, fusion and genome-shuffling techniques. The mutant strains were subjected to ultraviolet (UV) irradiation, ultrasonic mutagenesis and then, used for recursive protoplast fusion.
Materials and methods
Yeast strains
The yeast strain used as the parent strain in this study was B. anomalus PSY-001. The identified strain B. anomalus PSY-001 was deposited at the China Center for Type Culture Collection (CCTCC) and was given the CCTCC accession number M209106. The strain was stored in Yeast Extract–Peptone–Dextrose (YPD) medium with 20% (v/v) glycerol at –80 °C.
Medium and cultivation conditions
Yeast strains were grown on 2% YPD medium (1% yeast extract, 2% polypeptone, 2% glucose, and, if necessary, 2% agar) at 30 °C. For the fermentation test of strains, 15% YPD medium (1% yeast extract, 2% polypeptone and 15% glucose) was used. The auxotrophic phenotypes of mutants obtained were determined using minimal medium (MM; 0.17% yeast nitrogen base without amino acids, 2% glucose and 2% agar). If necessary, uracil, L-leucine, L-histidine or L-tryptophan was added to the MM medium to a final concentration of 20, 30, 20 or 20 μg mL−1, respectively.
Strain mutagenesis and mutant screening
Two approaches were employed for mutagenizing the parent strain B. anomalus PSY-001. The cells of B. anomalus PSY-001 were grown in a shake tube containing a 10 mL of YPD medium at 30 °C for 24 h. First, UV irradiation was performed as follows: 0.2 mL of culture broth was spread onto solid YPD plates and exposed directly to a UV lamp with a wavelength of 254 nm and power of 15 W at a distance of 20 cm for 1–60 s. Second, for ultrasonic mutagenesis, 4 mL of the single-cell suspension was treated with 200 W for 0–55 min. The cells of 0.2 mL culture broth were spread onto solid YPD at the centre of the plate; the lawn around the inhibition zone was scrapped after 48 h incubation, and cultivated in liquid YPD for 12 h. The colonies with larger transparent circles were selected to carry out shake-flask analysis. The strains with higher β-glucosidase activities were obtained and taken as the starter for genome shuffling.
Protoplast formation
Cell walls were digested using snailase from Yuanye Bio-Technology Co. Ltd (Shanghai, China). Enzymolysis was performed by incubation with 20 mg mL−1 snailase for 1 h at 30 °C.
Protoplast fusion
Biparental-inactivated protoplasts were employed for the protoplast fusion. Protoplast inactivation by UV irradiation was carried out by transferring 5 mL of protoplast suspension to a sterile plate without cover and exposing it for 40 s under a 15 W UV lamp at a distance of 30 cm. Protoplast inactivation by ultrasonic mutagenesis was carried out by transferring 5 mL of protoplast suspension to a sterile plate without cover and exposing it to 200 W ultrasonic treatment for 40 min. The cells of the initial strains subjected to UV irradiation and ultrasonic mutagenesis were inoculated in 250 mL Erlenmeyer flasks containing 20 mL of YPD medium. Cells were harvested at OD660 of about 1.0 by centrifugation at 3500 × g for 15 min and were then washed twice with citric acid–sodium citrate buffer.
For protoplast fusion, 0.5 mL of the protoplast suspension from the target strain was mixed and centrifuged for 10 min at 3500 × g. The pellet was gently resuspended in 1 mL PE buffer (Yeast Protein Extraction Reagent, Takara) and mixed with 4 mL (40% w/v) polyethylene glycol (PEG) 4000 in PE buffer. After 5 min incubation at 30 °C, the mixture was collected and washed three times with 5 mL of PE buffer (by centrifugation for 5 min at 2000 × g). The collected pellet was resuspended in 5 mL of PE buffer and diluted with the same PE buffer at a 1:3 ratio. This diluted suspension (0.1 mL) was plated on regeneration medium and incubated at 30 °C for 3 days.
Genome shuffling of five high-yield β-glucosidase-producing strains
For genome shuffling, 0.5 mL aliquots of the protoplast suspension of each strain were mixed together. After 10 min centrifugation at 500 × g, the pellet was gently resuspended in 1 mL of PE buffer and then mixed with 4 mL (40% w/v) PEG 4000 in PE buffer and incubated at 30 °C for 10 min. The resulting mixture was washed with PE buffer and diluted as described above. The diluted suspension (0.L mL) was plated on regeneration medium and incubated at 30 °C for 3 days.
Bioassay and shake-flask screening for mutants
A high-throughput cultivation method was employed to rapidly screen large numbers of resulting mutants as described earlier.[Citation14] The fermentation medium in the shake flask was composed of bran (53.15 g L−1), yeast extract powder (3.03 g L−1), KCl (0.204 g L−1) and CaCl2 (0.611 g L−1); the pH was adjusted to 6.8 before sterilization. This culture medium has been optimized to support efficient production of β-glucosidase with B. anomalus in our laboratory and used throughout all the shake-flask experiments.
Enzyme assay
The β-glucosidase activity was determined with p-nitrophenyl-β-D-glucopyranoside (p-NPG, Sigma, USA) as a substrate, hence called pNPGase activity, as follows: 1 mL of 5 mmol L−1 p-NPG (in 50 mmol L−1 citric acid–sodium citrate buffer pH 4.8) was incubated with the enzyme solution at 50 °C for 10 min. The reaction was stopped by adding 1 mL of sodium carbonate (1.0 mol L−1, pH 10); the liberated p-nitrophenol was measured at 405 nm. Then, one unit of enzyme activity was determined as the amount of enzyme required to release 1 μmol of p-nitrophenol in 1 min under the assay conditions.[Citation15]
Results and discussion
Strain mutagenesis and mutant screening
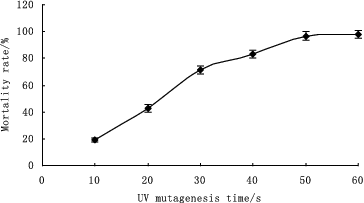
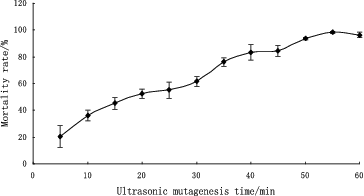
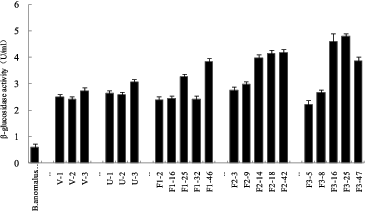
Genome shuffling practically mimics the features of natural evolution through recursive genetic recombination. Thus, it requires a diverse population of mutants with an improvement of the desired phenotype compared with the wild type as the starting point.[Citation16] In our study, UV and ultrasonic treatment were used as mutagenizing agents to improve the volumetric productivity of the wild-type strain. and show the mortality rate of B. anomalus PSY-001 cells after treatment with UV light and ultrasound, respectively. As shown in the figures, cells of the wild-type strain were found to be sensitive to UV irradiation and ultrasound. The mortality rate of the cells increased the longer the exposure to the mutagenizing agents was. Cell suspensions with about 80% mortality rate, which had been exposed to UV light irradiation for 40 s or to ultrasonic treatment for 40 min, were used in the subsequent strain screening. Six mutants, V-1, V-2, V-3, U-1, U-2 and U-3, were obtained by the first series of experiments with UV irradiation and ultrasonic treatment from 100 colonies. These mutants showed a large increase in β-glucosidase enzyme activity from 598 to 3072 U L−1 in comparison with B. anomalus PSY-001 ().
Genome shuffling of improved mutant population
The efficiency of genome shuffling is known to be a function of the efficiencies of generation and fusion of protoplasts, of recovery of protoplasts after fusion.[Citation17] In our experiments, protoplasts were generated by treatment with snailase to digest the yeast cell wall. Initial experiments to optimize conditions for formation and regeneration of protoplasts were carried out with the three strains of B. anomalus PSY-001 that were to be used for protoplast fusion experiments.
Three successive rounds of protoplast fusion were carried out, and after each round, the pH of the plates used for selection was decreased. Screening was done at the end of each fusion and colonies having both traits selected for the next round. After the first fusion, the best five colonies (F1-2, F1-16, F1-25, F1-32 and F1-46) were selected from the first shuffled library and used for the second fusion. Five colonies (F2-3, F2-9, F2-14, F2-18 and F2-42) were obtained from the second shuffled library and used for the next fusion. After the third fusion, five colonies (F3-5, F3-8, F3-16, F3-25 and F3-47) were obtained from the third shuffled library. The results are shown in .
Among the five strains, two selected isolates (F3-16 and F3-25) showed β-glucosidase activities of 4610 and 4790 U L−1, respectively, which were nearly eightfold higher as compared to B. anomalus PSY-001, and were selected for the subsequent studies.
The methods commonly used for improvement of industrial microorganisms range from classical strain improvement techniques to metabolic engineering. Although classical strain improvement is effective, it is very time- and labour-consuming. Protoplast-related techniques have extensive application in the fermentation industry as well as in genetic engineering and molecular biology. These techniques can provide stable changes in the genome of Brettanomyces spp. In the present study, genome shuffling was successfully applied as an effective whole-cell engineering strategy for the rapid improvement of industrially important microbial phenotypes. Our results support the assertion that genome shuffling offers more advantages than classical strain improvement strategies and rational genetic methods for strain improvement (i.e. metabolic engineering).[Citation18]
Genetic stability of F3-25
To check the genetic stability of F3-25, we cultured the shuffled mutant from three successive rounds of protoplast fusion for eight generations and measured the β-glucosidase activities of every other generation. All the generations showed similar activity as the initial strain, suggesting that F3-25 was genetically stable and suitable for the next steps of investigation and industrial production. This makes the strain promising for use in food flavour production.
Conclusions
In the present work, a recombinant strain, F3-25, was obtained after three rounds of genome shuffling and its production of β-glucosidase activity reached 4790 U L−1, which was a nearly eightfold increase compared to the original strain B. anomalus PSY-001.
Additional information
Funding
References
- Wedral D, Shewfelt R, Frank J. The challenge of Brettanomyces in wine. LWT Food Sci Technol. 2010;43(10):1474–1479.
- Smith MT. Brettanomyces Kufferath & van Laer (1921). In: Kurtzman CP, Fell JW, Boekhout T, editors. The yeasts: a taxonomic study. 5th ed. Vol. 2. Amsterdam: Elsevier; 2011. p. 983–986.
- Daenen L, Saison D, Sterck F. Screening and evaluation of the glucoside hydrolase activity in Saccharomyces and Brettanomyces brewing yeasts. J Appl Microbiol. 2008;104(2):478–488.
- Vanderhaegen B, Neven H, Daenen L, Verstrepen KJ, Verachtert H, Derdelinckx G. Furfuryl ethyl ether: important aging flavor and a new marker for the storage conditions of beer. J Agric Food Chem. 2004;52(6):1661–1668.
- Rajasree KP, Mathew GM, Pandey A, Sukumaran RK. Highly glucose tolerant beta-glucosidase from Aspergillus unguis: NII 08123 for enhanced hydrolysis of biomass. J Ind Microbiol Biotechnol. 2013;40(9):967–975.
- Hopwood DA, editor. Natural product biosynthesis by microorganisms and plants, Part A. Vol. 515. Methods in enzymology. Amsterdam: Elsevier; 2012. p. xv–xx.
- Palmeri R, Spagna G. Beta-glucosidase in cellular and acellular form for winemaking application. Enzym Microb Technol. 2007;40(3):382–389.
- Pyo YH, Lee TC, Lee YC. Enrichment of bioactive isoflavones in soymilk fermented with beta-glucosidase-producing lactic acid bacteria. Food Res Int. 2005;38(5):551–559.
- Chakraborty U, Sikdar SR. Intergeneric protoplast fusion between Calocybe indica (milky mushroom) and Pleurotus florida aids in the qualitative and quantitative improvement of sporophore of the milky mushroom. World J Microbiol Biotechnol. 2010;26(2):213–225.
- Chen XY, Wei PL, Fan LM, Yang D, Zhu XC, Shen WH, Xu ZN, Cen PL. Generation of high-yield rapamycin-producing strains through protoplasts-related techniques. Appl Microbiol Biotechnol. 2009;83(3):507–512.
- Stephanopoulos G. Metabolic engineering by genome shuffling – two reports on whole-genome shuffling demonstrate the application of combinatorial methods for phenotypic improvement in bacteria. Nat Biotechnol. 2002;20(7):666–668.
- Wang HK, Sun Y, Chen C, Sun Z, Zhou YC, Sun FD, Zhang HP, Dai YJ. Genome shuffling of Lactobacillus plantarum for improving antifungal activity. Food Control. 2013;32(2):341–347.
- Zhang YX, Perry K, Vlctor A, Powell K, Stemmer WP, Cardayre DS. Genome shuffling leads to rapid phenotypic improvement in bacteria. Nature. 2002;415(6872):644–646.
- Xu ZN, Shen WH, Chen XY, Lin JP, Cen PL. A high-throughput method for screening of rapamycin-producing strains of Streptomyces hygroscopicus by cultivation in 96-well microtiter plates. Biotechnol Lett. 2005;27(15):1135–1140.
- Lin J, Pillay B, Singh S. Purification and biochemical characteristics of beta-D-glucosidase from a thermophilic fungus, Thermomyces lanuginosus – SSBP. Biotechnol Appl Biochem. 1999;30:81–87.
- Yu L, Pei XL, Lei T, Wang YH, Feng Y. Genome shuffling enhanced L-lactic acid production by improving glucose tolerance of Lactobacillus rhamnosus. J Biotechnol. 2008;134(1–2):154–159.
- John RP, Gangadharan D, Nampoothiri KM. Genome shuffling of Lactobacillus delbrueckii mutant and Bacillus amyloliquefaciens through protoplasmic fusion for L-lactic acid production from starchy wastes. Bioresour Technol. 2008;99(17):8008–8015.
- Gong J, Zheng H, Wu Z, Chen T. Genome shuffling: progress and applications for phenotype improvement. Biotechnol Adv. 2009;27(6):996–1005.