Abstract
Staphylococcus aureus causes serious hospital-acquired (HA) and community-acquired (CA) infections. Skin and soft-tissue infections especially are sometimes caused by strains harbouring Panton-Valentine leukocidin (PVL). PVL belongs to a family of bi-component leukocidal toxins produced by staphylococci. It is a pore-forming toxin encoded by lukF-PV and lukS-PV. A total of 70 S. aureus strains: 38 (54%) methicillin-resistant (MRSA) and 32 (46%) methicillin-susceptible (MSSA), were isolated from patients admitted to Dicle University Hospital (Turkey). Identification of S. aureus and antibiotics-susceptibility testing were performed with PHOENIX 100. PVL genes and mecA genes were detected by polymerase chain reaction. Of the 70 studied strains, 36 ones (51%) were community acquired and 34 ones (49%) were hospital acquired . A total of 38 (54%) strains were positive for mecA (mecA+), of which 32 ones (84%) were HA. Of the mecA− strains, 30 (94%) were CA. Of the 70 studied strains, 12 (17%) strains were PVL+: 8 (22%) of the 36 CA strains and 4 (12%) of the 34 HA strains. Of the 12 PVL+ strains, 4 strains were mecA+. The PVL positivity rate was 25% in MSSA, whereas 10.5% in MRSA. Of the overall PVL+ strains, seven strains were obtained from wounds; four ones from skin abscess; and one from blood culture. Taken together, the obtained results showed a substantial level of PVL genes in the studied region. Although PVL is known as a common virulence factor of CA MRSA, HA MRSA isolates in our study showed a considerable rate of PVL positivity.
Introduction
Staphylococcus aureus can cause infections ranging from local skin and soft-tissue infections to life-threatening diseases, such as bacteremia and necrotizing pneumonia. S. aureus is one of the most prevalent pathogens that causes serious hospital-acquired (HA) and community-acquired (CA) infections.[Citation1]
Methicillin-resistant S. aureus (MRSA) was first described in 1961 in England [Citation2] and has become endemic and epidemic in hospitals worldwide.[Citation3] MRSA isolation rates increased in the United States, Asia and some European countries and Turkey.[Citation1,Citation3,Citation4] In S. Aureus, methicillin resistance caused by the production of a low affinity penicillin-binding protein (PBP2a) is encoded by the mecA gene, located on a large mobile genetic element – the staphylococcal chromosomal cassette mec (SCCmec).[Citation5,6] Whereas CA MRSA strains usually harbour SCCmec type IV and V, HA MRSA strains are mainly associated with SCCmec-I, II and III throughout the world.[Citation7,8]
S. aureus infections, especially skin and soft-tissue infections, are sometimes caused by strains harbouring Panton-Valentine leukocidin (PVL), which belongs to a family of bi-component leukocidal toxins produced by staphylococci. PVL is a pore-forming toxin encoded by lukF-PV and lukS-PV [Citation9] and is known as a virulence factor sometimes associated with tissue necrosis.[Citation7] PVL can trigger neutrophil lysis or apoptosis and tissue necrosis by the release of cytotoxic lysosomal granule contents from lysed neutrophils.[Citation10,11] Both methicillin-susceptible S. aureus (MSSA) and CA-MRSA can express PVL.[Citation9]
We aimed to determine the rates of PVL in CA or HA S. aureus strains isolated from different clinical samples in our hospital.
Subjects and methods
Patients
Seventy patients admitted to Dicle University Hospital in 2012 took part in our study. They were from 0 to 79 years of age. Thirty-seven (53%) of the patients were female and 33 (47%) were male. Informed consent was obtained from all patients or their parents/legal guardians.
Strains
A total of 70 S. aureus strains: 38 (54%) MRSA and 32 (46%) MSSA were isolated from clinical samples obtained from the patients. The strains were stored at −80 °C until use. Identification and antibiotics-susceptibility testing were performed with PHOENIX 100 (Becton Dickinson, Franklin Lakes, NJ) and methicillin resistance of strains was confirmed by the cefoxitin disk diffusion test (Oxoid, Hampshire, England), based on recommendations of the Clinical Laboratory Standards Institute. For quality control, reference strains ATCC 43300 and ATCC 29213 and a PVL+ strain were used in the study.
DNA extraction
A boiling technique was used for rapid DNA extraction. Briefly, a fresh passage of the strains was performed and whole colonies were suspended into 500 μL of sterile distilled water and then vortexed. The suspension was incubated in a dry heating block at 100 °C for 15 min and centrifugated at 15000 × g for 20 min at 4 °C. Then, 200 μL of supernatant [Citation12] was collected and the DNA concentration was measured by a NanoDrop 1000 spectrophotometer (Thermo, USA). The extracted DNA samples were stored at −20 °C until use.
MecA and PVL polymerase chain reaction (PCR)
Primer sequences for the PVL genes [Citation13] were as follows: forward for luk-PV-1, 5′-ATC ATT AGG TAA AAT GTC TGG ACA TGA TCC A-3′; reverse for luk-PV-2, 5′-GCA TCA ACT GTA TTG GAT AGC AAA AGC-3′; and for mecA [Citation14]: forward 5′-AAA ATC GAT GGT AAA GGT TGG C-3′, reverse 5′-AGT TCT GCA GTA CCG GAT TTG C-3′.
Amplification was performed with a Veriti™ 96-Well Thermal Cycler (Applied Biosystems, CA, USA). PCR conditions were as follows: 5 min at 94 °C, followed by 30 amplification cycles, each consisting of 30 s of denaturation at 94 °C, 30 s of annealing at 55 °C and 1 min of extension at 72 °C. Final extension was performed at 72 °C for 10 min. PCR products were separated electrophoretically in a 1.5% agarose gel in 0.5× TBE (Tris–Borate–Ethylene diamine tetra acetic acid) buffer and photographed (BIO-RAD, Italy). Analyses were done by comparison with a 100 bp GeneRuler (Thermo, Lithuania). DNA fragments of 433 and 310 bp were considered as positive for the lukS/F-PV and mecA gene, respectively, by comparing with the positive controls.
Results and discussion
PVL is a biocomponent synergohymenotropic cytotoxin associated with furunculosis, severe necrotizing hemorrhagic pneumonia, necrotizing fasciitis and other lesions involving the skin and mucosa.[Citation15] The gene encoding PVL is frequently found in CA-MRSA strains carrying SCCmec-IV.[Citation16] Since the PVL gene is known to be associated with virulence,[Citation17] determining the presence of the PVL gene in MRSA strains might be important to early and proper therapy for serious MRSA infections.[Citation18,19]
In our study, the mean age of the patients was 40.96 years and their median age was 43.00 with a standard deviation of ±20.77. Out of the 70 studied strains, 36 strains (51%) were CA and 34 strains (49%) were HA. A total of 30 strains were isolated from wounds, 14 from blood, 12 from sputum, 9 strains were isolated from abscess, 2 from vaginal sample, 2 from urine and 1 from a nasal swap. Of the 70 strains, 38 ones (54%) were positive for mecA (mecA+), while 32 ones (46%) were negative for mecA. Eight (22%) of the CA strains and 30 (88%) of the HA strains were mecA+. Of the 38 mecA+ strains, 30 (79%) were HA. Of the 32 mecA− strains, 30 (94%) were CA. Twelve (17%) of the 70 studied strains were PVL+: 8 (22%) of the 36 CA strains and 4 (12%) of the 34 HA strains (8 CA, 4 HA). Of the 12 PVL+ strains (), 4 strains were mecA+ (). Among the methicillin susceptible strains, the PVL positivity rate was 25% (8/32) and among the methicillin resistant ones, 10.5% (4/38). Of the PVL+ strains, seven strains were obtained from wounds, four from skin abscess and one from blood culture. The characteristics of the PVL+ and mecA+ strains are presented in .
Table 1. Characteristics of PVL + S. aureus strains isolated in Dicle University Hospital (Diyarbakır, Turkey).
Figure 1. Agarose gel imaging of amplified lukS/F-PV gene. Lane pk is positive control. DNA fragments of 433 bp (isolates 2, 3, 8, 9, 11, 12, 14, 19, 20, 25, 26 and 27) were considered positive.
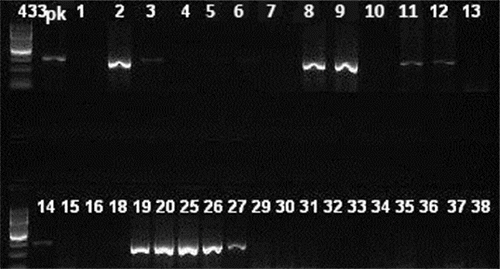
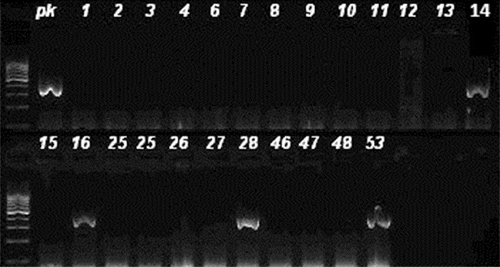
Figure 2. Agarose gel imaging of amplified mecA gene. Lane pk is positive control. DNA fragments of 310 bp (isolates 14, 16, 28 and 53) were considered positive.
The analysis of our results showed that the rates of PVL genes in the studied region are at a substantial level. Although PVL is known as a common virulence factor of CA MRSA, HA-MRSA isolates in our hospital had a considerable rate of PVL positivity.
Van der Meeren et al. [Citation20] reported 15.1% prevalence of HA-MRSA infection among inpatients as compared to 1% prevalence of CA-MRSA infection among outpatients. In their study, the PVL toxin gene was detected in 81.1% of MSSA and in 11.1% of MRSA. Montagnani et al. [Citation21] reported three cases of severe infections in infants; they were caused by PVL+ S. aureus and evolved with a positive outcome. Wang et al. [Citation22] observed that the positivity rates of mecA, ermA, ermB and ermC in the S. aureus isolates were 13/60. Among the 60 isolates, 30 harboured enterotoxin genes, with sea being the most frequent toxin gene (33%), followed by sec (15%), sed (12%) and seb (5%). The PVL gene was detected in four strains. Eleven MRSA isolates were of the SCCmec type III. Haider et al. [Citation23] reported a case of severe necrotizing haemorrhagic pneumonia in a 12-year-old boy, who needed full ventilatory support and died despite all efforts. In their case presentation, post-mortem examination of lung swabs confirmed the presence of PVL-associated S. aureus. Mariem et al. [Citation24] characterized 69 MRSA strains isolated from two Tunisian hospitals and reported that 79% of CA-MRSA strains and 51% of HA-MRSA strains were PVL-positive. According to AlFouzan et al. [Citation25], out of 291 S. aureus isolates, 30.6% were MRSA. Genes for PVL were detected in 14.6% and 12.0% of the MRSA and MSSA isolates, respectively. The majority of the PVL-producing MRSA and MSSA were isolated from cases of skin (30.7%) and soft tissue (21.8%) infections. Both MRSA types carried SCCmec type III, IV, IVc and V genetic elements.[Citation25] In another meta-analysis including 76 studies from 31 countries, PVL strains were strongly associated with skin and soft-tissue infections, while comparatively rarely with pneumonia, musculoskeletal infections, bacteremias and colonizing strains.[Citation26]
In Turkey, the carriage rate of the PVL gene by MRSA isolates appears to be very low. Karahan et al. [Citation27] reported that out of 304 studied S. aureus strains (230 HA and 74 CA), 261 were MRSA and 43 were MSSA. PVL positivity was determined in 12 (11 CA) strains. Eight were MRSA, and four were MSSA. Their results indicated that PVL-positive strains were able to cause infection in nearly every system, without the need for additional risk factors.
Kilic et al. [Citation28] collected 385 clinical MRSA isolates and overall, SCCmec types I, II, III, IV, V, nontypeable and PVL occurrence were detected in 11 (2.8%), 3 (0.8%), 316 (82.1%), 20 (5.1%), 20 (5.1%), 15 (3.9%) and 5 (1.3%) isolates, respectively. The PVL gene was detected in 10% of SCCmec-IV/V isolates, in contrast to 0.3% in SCCmec-I/II/III (χ2 = 25.164, p < 0.001). Baykam et al. [Citation29] isolated S. aureus from anterior nares of 121 patients. MRSA was isolated from 1.2% of these patients and all of the MRSA isolates were positive for the mecA and PVL genes. In the study of Tekeli et al. [Citation30], among 100 MRSA bloodstream isolates, the dominant MRSA clone had SCCmec type III, agr type 1 and revealed sequence type (ST) 239. Alp et al. [Citation31] reported that the rate of MRSA in patients with apparent infections (sepsis, meningitis, lung abscess or septic arthritis) ranged from 12% to 75%, within the seven participating centres. None of the isolates contained the PVL genes. Sesli Çetin et al. [Citation32] obtained nasal and throat swabs from subjects who did not have prior history of any health care exposure. Genotyping of 5 PVL+ isolates by pulse field gel electrophoresis revealed that one child and a teacher from the same class were colonized with the clonally related strains, suggesting that close contact with colonized people could be considered a risk factor for being colonized. In the study of Baran et al. [Citation33], 30 strains were phenotypically identified as MRSA and after assessing the risk factors, 28 (93.3%) of them were classified as HA and 2 (6.7%) of them as CA. PVL gene positivity was detected only in CA-MRSA isolates (2/2; 100%). In the report of Akoğlu et al. [Citation34], all the MRSA strains isolated from patients at intensive care units and surgical wards were positive for the mecA gene. Of the isolates, 61.8% harboured SCCmec type III, 34.5% SCCmec variant IIIB and 2.7% SCCmec type IV. PVL was positive in 12.7% of the isolates.[Citation34] Demir et al. [Citation35] analysed 92 CA and 150 HA isolates and identified 77 strains as mecA positive. PVL was not observed among the MRSA isolates, but 8 (5.3%) HA-MSSA and 14 (15.2%) CA-MSSA, mostly isolated from furuncles (71.4%), were positive for PVL.
Taken together, the results from our study indicated that PVL is an important virulence factor for skin and soft tissue infection caused by S. aureus. A limitation of our study was the low number of studied strains. We did not determine the relationship between the PVL positivity and the SCCmec types, and also the antibiotic susceptibility rates. We could suggest that large-scale molecular studies associated with SCCmec typing of S. aureus strains and determining the virulence factors of S. aureus strains are necessary for the management of S. aureus infections in the studied region.
Conclusions
In our study, 17% of the analysed S. aureus strains were PVL+. PVL prevalance was 22% among CA strains and 12% among HA strains. PVL positivity rate was 25% in MSSA, and 10.5% in MRSA isolates. Most of the PVL positive strains were isolated from cutaneous infections, except one blood culture isolate, indicating that PVL could be considered an important virulence factor for skin and soft tissue infection caused by S. aureus. The rates of PVL genes in the studied region were shown to be substantial. Although PVL is known as a common virulence factor of CA MRSA, HA-MRSA isolates in our hospital had a considerable rate of PVL positivity. We could suggest that larger scale molecular studies associated with SCCmec typing of S. aureus strains and determining the virulence factors of S. aureus strains are necessary for management of S. aureus infections in the studied region.
Additional information
Funding
References
- Chambers HF. Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clin Microbiol Rev. 1997;10:781–791.
- Jevons MP. “Celbenin”-resistant staphylococci. BMJ. 1961;1:124–125.
- Deresinski S. Methicillin-resistant Staphylococcus aureus: an evolutionary, epidemiologic and therapeutic odyssey. Clin Infect Dis. 1961;40:562–573.
- Oztop AY, Pinarbasi H, Kocagoz S, Bakici MZ, Bakir M. Molecular genotyping of methicillin-resistant Staphylococcus aureus strains in a teaching hospital in Turkey. Microb Drug Resist. 2004;10:154–159.
- Baba T, Takeuchi F, Kuroda M, Yuzawa H, Aoki K, Oguchi A, Nagai Y, Iwama N, Asano K, Naimi T, Kuroda H, Cui L, Yamamoto K, Hiramatsu K. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet. 2001;359:1819–1827.
- Okuma K, Iwakawa K, Turnidge JD, Grubb WB, Bell JM, O’Brien FG, Coombs GW, Pearman JW, Tenover FC, Kapi M, Tiensasitorn C, Ito T, Hiramatsu K. Dissemination of new methicillin-resistant Staphylococcus aureusclones in the community. J Clin Microbiol. 2002;40(11):4289–4294.
- Francis JS, Doherty MC, Lopatin U, Johnston CP, Sinha G, Ross T, Cai M, Hansel NN, Perl. T, Ticehurst JR, Carroll K, Thomas DL, Nuermberger E, Bartlett JG. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis. 2005;40:100–107.
- Gonzalez BE, Hulten KG, Dishop MK, Lamberth LB, Hammerman WA, Mason EO Jr, Kaplan SL. Pulmonary manifestations in children with invasive community-acquired Staphylococcus aureus infection. Clin Infect Dis. 2005;41:583–590.
- Mine Y, Nakasone I, Yamamoto Y, Utani A, Yamane N, Uezato H, Takahashi K. Dissemination of Panton–Valentine leukocidin-positive methicillin-resistant Staphylococcus aureus in Okinawa, Japan. J Dermatol. 2013;40:34–38.
- Kaneko J, Kamio Y. Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: structures, pore-forming mechanism, and organization of the genes. Biosci Biotechnol Biochem. 2004;68:981–1003.
- Boyle-Vavra S, Daum RS. Community-acquired methicillin-resistant Staphylococcus aureus: the role of Panton-Valentine leukocidin. Lab Invest. 2007;87:3–9.
- York MK, Gibbs L, Chehab F, Brooks GF. Comparison of PCR detection of mecA with standard susceptibility testing methods to determine methicillin resistance in coagulase-negative staphylococci. J Clin Microbiol. 1996;34:249–253.
- Lina G, Piémont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, Vandenesch F, Etienne J. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128–1132.
- Merlino J, Watson J, Rose B, Beard-Pegler M, Gottlieb T, Bradbury R, Harbour C. Detection and expression of methicillin/oxacillin resistance in multidrugresistantand non-multidrug-resistant Staphylococcus aureus in Central Sydney, Australia. J Antimicrob Chemother. 2002;49:793–801.
- Yu F, Liu Y, Xu Y, Shang Y, Lou D, Qin Z, Parsons C, Zhou W, Huang X, Li Y, Hu H, Wang L. Expression of Panton-Valentine leukocidin mRNA among Staphylococcus aureus isolates associates with specific clinical presentations. PLoS One [Internet]. 2013 Aug 12 [cited 2014 Apr 20]. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3861483/
- Salgado CD, Farr BM. Community-acquired methicillin-resistant. JAMA. 2004;291(16):1960–1961.
- Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, Heffernan H, Liassine N, Bes M, Greenland T, Reverdy ME, Etienne J. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerging Infect Dis. 2003;9(8):978–984.
- Gauduchon V, Cozon G, Vandenesch F, Genestier AL, Eyssade N, Peyrol S, Etienne J, Lina G. Neutralization of Staphylococcus aureus Panton Valentine leukocidin by intravenous immunoglobulin in vitro. J Infect Dis. 2004;189:346–353.
- Salliot C, Zeller V, Puechal X, Manceron V, Sire S, Varache N, Etienne J, Desplaces N, Ziza JM. Panton-Valentine leukocidin-producing Staphylococcus aureus infections: report of 4 French cases. Scand J Infect Dis. 2006;38:192–195.
- Van der Meeren BT, Millard PS, Scacchetti M, Hermans MH, Hilbink M, Concelho TB, Ferro JJ, Wever PC. Emergence of methicillin resistance and Panton-Valentine leukocidin positivity in hospital- and community-acquired Staphylococcus aureus infections in Beira, Mozambique. Trop Med Int Health. 2014;19(2):169–176.
- Montagnani C, Cocchi P, Bianchi L, Resti M, de Martino M, Galli L. Severe infections caused by Panton-Valentine leukocidin-positive Staphylococcus aureus in infants: report of three cases and review of literature. Acta Paediatr. 2013;102:284–287.
- Wang LX, Hu ZD, Hu YM, Tian B, Li J, Wang FX, Yang H, Xu HR, Li YC, Li J. Molecular analysis and frequency of Staphylococcus aureus virulence genes, isolated from bloodstream infections in a teaching hospital in Tianjin, China. Genet Mol Res. 2013;12:646–654.
- Haider S, Wright D. Panton-Valentine leukocidin Staphylococcus causing fatal necrotising pneumonia in a young boy. BMJ Case Rep. [Internet] 2013 Mar 14 [cited 2014 Apr 24]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23505079. doi:10.1136/bcr-2012-007655.
- Mariem BJ-J, Ito T, Zhang M, Jin J, Li S, Ilhem B-BB, Hammami A, Xiao H, Keiichi H. Molecular characterization of methicillin-resistant Panton-valentine leukocidin positive Staphylococcus aureus clones disseminating in Tunisian hospitals and in the community. BMC Microbiol. 2013;13:2.
- AlFouzan W, Al-Haddad A, Udo E, Mathew B, Dhar R. Frequency and clinical association of Panton-Valentine leukocidin-positive Staphylococcus aureus isolates: a study from Kuwait. Med Princ Pract. 2013;22:245–249.
- Shallcross LJ, Fragaszy E, Johnson AM, Hayward AC. The role of the Panton-Valentine leucocidin toxin in staphylococcal disease: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13:43–54.
- Karahan ZC, Tekeli A, Adaleti R, Koyuncu E, Dolapci I, Akan OA. Investigation of Panton-Valentine leukocidin genes and SCCmec types in clinical Staphylococcus aureus isolates from Turkey. Microb Drug Resist. 2008;14:203–210.
- Kilic A, Guclu AU, Senses Z, Bedir O, Aydogan H, Basustaoglu AC. Staphylococcal cassette chromosome mec (SCCmec) characterization and Panton-Valentine leukocidin gene occurrence for methicillin-resistant Staphylococcus aureus in Turkey, from 2003 to 2006. Antonie Van Leeuwenhoek. 2008;94:607–614.
- Baykam N, Esener H, Ergonul O, Kosker PZ, Cirkin T, Celikbas A, Eren S, Dokuzoguz B. Methicillin-resistant Staphylococcus aureus on hospital admission in Turkey. Am J Infect Control. 2009;37:247–249.
- Tekeli A, Koyuncu E, Dolapçi I, Akan OA, Karahan ZC. Molecular characteristics of methicillin-resistant Staphylococcus aureus strains isolated from blood cultures between 2002–2005 in Ankara University Hospital. Mikrobiyol Bul. 2009;43(1):1–10.
- Alp E, Klaassen CHW, Doganay M, Altoparlak U, Aydin K, Engin A, Kuzucu C, Ozakin C, Ozinel MA, Turhan O, Voss A. MRSA genotypes in Turkey: persistence over 10 years of a single clone of ST239. J Infect. 2009;58:433–438.
- Sesli Çetin E, Us E, Güneş H, Kaya S, Tekeli A, Demirci M. Investigation of Panton-Valentine leukocidin expressing Staphylococcus aureus colonization among children in a child care center. Am J Infect Control. 2010;38:565–567.
- Baran CB, Mutlu D, Baysan BO, Günseren F, Ergani A, Oğünç D, Colak D. Investigation of Panton-Valentine leukocidin gene, SCCmec gene cassette types and genotypes of methicillin-resistant Staphylococcus aureus strains isolated from outpatients. Mikrobiyol Bul. 2010;44:533–545.
- Akoğlu H, Zarakolu P, Altun B, Ünal S. Epidemiological and molecular characteristics of hospital-acquired methicillin-resistant Staphylococcus aureus strains isolated in Hacettepe University Adult Hospital in 2004–2005. Mikrobiyol Bul. 2010;44:343–355.
- Demir T, Coplu N, Bayrak H, Turan M, Buyukguclu T, Aksu N, Eksioglu M, Yalcin B, Atakan N, Kilic S, Karahan ZC, Esen B. Panton–Valentine leucocidin gene carriage among Staphylococcus aureus strains recovered from skin and soft tissue infections in Turkey. J Antimicrob Chemother. 2012;67:837–840.
