Abstract
Antimicrobial activity and antibiotic susceptibility were tested for 23 Lactobacillus and three Bifidobacterium strains isolated from different ecological niches. Agar-well diffusion method was used to test the antagonistic effect (against Staphylococcus aureus, Escherichia coli, Bacillus cereus and Candida albicans) of acid and neutralized (pH 5.5) lyophilized concentrated supernatants (cell-free supernatant; CFS) and whey (cell-free whey fractions; CFW) from de Man–Rogosa–Sharpe/trypticase-phytone-yeast broth and skim milk. Acid CFS and CFW showed high acidification rate-dependent bacterial inhibition; five strains were active against C. albicans. Neutralized CFS/CFW assays showed six strains active against S. aureus (L. acidophilus L-1, L. brevis 1, L. fermentum 1, B. animalis subsp. lactis L-3), E. coli (L. bulgaricus 6) or B. cereus (L. plantarum 24-4В). Inhibition of two pathogens with neutralized CFS (L. bulgaricus 6, L. helveticus 3, L. plantarum 24-2L, L. fermentum 1)/CFW (L. plantarum 24-5D, L. plantarum 24-4В) was detected. Some strains maintained activity after pH neutralization, indicating presence of active substances. The antibiotics minimum inhibitory concentrations (MICs) were determined by the Epsilometer test method. All strains were susceptible to ampicillin, gentamicin, erythromycin and tetracycline. Four lactobacilli were resistant to one antibiotic (L. rhamnosus Lio 1 to streptomycin) or two antibiotics (L. acidophilus L-1 and L. brevis 1 to kanamycin and clindamycin; L. casei L-4 to clindamycin and chloramphenicol). Vancomycin MICs > 256 μg/mL indicated intrinsic resistance for all heterofermentative lactobacilli. The antimicrobially active strains do not cause concerns about antibiotic resistance transfer and could be used as natural biopreservatives in food and therapeutic formulations.
Introduction
Foods are now considered not only in terms of taste and immediate nutritional needs, but also in terms of their ability to improve the health and well-being of consumers.[Citation1] Hence, the increased interest in food ingredients with valuable bioactive properties and, consequently, in lactic acid bacteria (LAB) and bifidobacteria with antagonistic activity against pathogenic micro-organisms. There are different mechanisms for control and inhibition of other microbes, e.g. nutrient competition, production of inhibitory compounds, immunostimulation and competition for binding sites. Among these activities, the production of organic acids (such as lactic acid), which results in lowered pH, is the most important. Additionally, certain strains are also capable of producing bioactive molecules, such as ethanol, formic acid, fatty acids, hydrogen peroxide and bacteriocins, that have antimicrobial activity.[Citation2] Lactobacillus and Bifidobacterium spp. and their by-products have been shown to be effective in several aspects. One of the most important advantages is the extended shelf life and safety of minimally processed foods, because these antimicrobial substances are safe and effective natural inhibitors of pathogenic and food spoilage bacteria in various foods. Additionally, the consumption of viable bacteria in the form of probiotics and functional foods is widely used for improvement of the balance and activity of the advantageous intestinal microflora, which has prophylactic benefit.[Citation1]
The close contact with native microbiota in the human intestine is an excellent precondition for horizontal transfer of antimicrobial resistance genes with the aid of mobile genetic elements.[Citation3] Therefore, the safety of cultures intended for use as food additives should be carefully re-assessed, even though most strains of the Lactobacillus and Bifidobacterium group are classified as ‘generally recognized as safe’ bacteria due to their long history of safe use and proven health benefits. Thus, antibiotic-resistance screening for starter and probiotic cultures now tends to become systematic. In order to eliminate the possibility of acquired resistance, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) of the European Food Safety Authority (EFSA) requires the determination of the minimum inhibitory concentrations (MICs) of the most relevant antibiotics for each bacterial strain that is used as a feed additive.[Citation4]
In this study, Lactobacillus and Bifidobacterium spp. were screened for their antagonistic activity against four food-borne and human pathogens and antibiotic susceptibility for development of probiotics and food biopreservatives.
Materials and methods
Bacteria and source of isolation
Twenty-three Lactobacillus strains (13 homofermentative and 10 heterofermentative) and three Bifidobacterium strains, part of the laboratory collection of Lactina Ltd. (Bankya, Bulgaria), were selected for this study. In a preliminary (unpublished) study, the strains were identified using biochemical (API 50 CHL) and molecular tests (species-specific polymerase chain reaction or sequence analysis). The source of isolation for each strain is presented in . All cultures were stored at −65 °C in appropriate broth media supplemented with glycerol (20% v/v). Before the assay, the strains were pre-cultivated twice in MRS (de Man–Rogosa–Sharpe) broth (Hi-Media Pvt. Ltd., India) for lactobacilli or TPY broth (trypticase-phytone-yeast) for bifidobacteria at 37 °C for 24 h.
Table 1. Lactobacillus and Bifidobacterium strains included in this study and source of isolation.
Test micro-organisms
Three bacterial food-borne pathogens and one yeast culture were selected as test micro-organisms and were obtained from the National Bank for Industrial Micro-organisms and Cell Cultures (Bulgaria): Staphylococcus aureus NBIMCC 3703, Escherichia coli NBIMCC 3702, Bacillus cereus NBIMCC 1085 and Candida albicans NBIMCC 74. The cultures of S. aureus and E. сoli were propagated in nutrient broth (NB, HiMedia), B. cereus in tryptic soy broth (TSB, Merck, Germany) and C. аlbicans in Sabouraud dextrose broth (HiMedia).
Antimicrobial activity assay
Two model systems for antimicrobial production were applied: cultivation in MRS or TPY broth (for Lactobacillus and Bifidobacterium spp., respectively) and cultivation in 10% (w/v) skim milk (Fude + Serrahn Milchprodukte GmbH & Co, Germany). The media were inoculated with 10% (v/v) previously activated Lactobacillus or Bifidobacterium culture. After incubation at 37 °C for 28 h, the cultures were centrifuged (5000 g for 20 min at 5 °C) for removal of bacterial cells. Part of the cell-free supernatants (CFS) and the cell-free whey fractions (CFW) were left with their initial acid pH. The rest of the samples were buffered with 5 mol/L NaOH at рН 5.5 ± 0.1 in order to eliminate the putative effect of produced organic acids. The pH values of the neutralized samples were consistent with the pH of LAB cultures before freeze drying in the real technological process. After filtration (0.22 μm pore size; Millipore), the acid and neutralized CFS (aCFS and nCFS) and CFW (aCFW and nCFW) were lyophilized (Martin Christ GmbH, Germany) in Petri dishes (10 mL) at the following conditions: freezing at −45 °С for 2 h, heating at 32 °C, vacuum 0.370 mbar, duration 40 h. The obtained dry samples were dissolved in 2 mL of sterile distilled water (resulting in 5× concentration increase as compared to the initial culture prior to lyophilization) and stored at −65 °C until later use in the screening procedures.
Аgar-well diffusion method was used to determine the inhibitory effect.[Citation5] Exponential cultures of the test micro-organisms were diluted to a suitable turbidity and used to inoculate a melted and cooled Mueller–Hinton Agar (MHA, HiMedia) to a final concentration of ∼106–107 CFU/mL. Only C. albicans was plated on Sabouraud dextrose agar (HiMedia) by spreading the cell suspension with a sterile cotton swab. Wells, 8 mm in diameter, were punched in the agar plates and 100 μL of CFS and CFW were added to the wells. After incubation overnight at 37 °C, the antimicrobial activity was expressed as the diameter of the inhibition zones (mm) around the wells. Zones of inhibition ≥10 mm were regarded as positive.
Antibiotic susceptibility
For selected Lactobacillus and Bifidobacterium strains, the MICs (μg/mL) of nine antibiotics were determined using commercial E-test® (Epsilometer test, bioMerieux, France): ampicillin, vancomycin, gentamicin, kanamycin, streptomycin, erythromycin, clindamycin, tetracycline and chloramphenicol. The concentration on the strips was from 0.016 to 256 μg/mL with the exception of streptomycin (0.064–1024 μg/mL). Bacterial cultures in the exponential growth phase were diluted to a suitable turbidity and used to inoculate a melted and cooled iso-sensitest agar (90% w/v, Oxoid, UK) supplemented with MRS or TPY agar (10% w/v) [Citation6] to a final concentration of ∼106–107 CFU/mL. E-test strips were placed on the surface of the inoculated agar and incubated at 37 °C for 24 h. The MIC was interpreted as the point at which the ellipse intersected the E-test strip as described in the E-test technical guide.
Results and discussion
Antimicrobial activity is a very important criterion for selection of starter and probiotic culture as natural antagonists of potentially harmful bacteria. Therefore, 23 Lactobacillus and three Bifidobacterium strains from the Lactina Ltd. collection were screened for their activity against four food-borne and human pathogens: Staphylococcus aureus, Escherichia coli, Bacillus cereus and Candida albicans. Lyophilized and concentrated, acid and neutralized cell-free filtrates obtained after cultivation of the selected lactobacilli and bifidobacteria in MRS or TPY broth (CFS) and skim milk (CFW) were tested for activity. Skim milk was chosen as a second model system because it is a natural medium for the growth of most LAB and bifidobacteria and is commonly used for production of freeze-dried cultures. At the same time, it is an excellent medium for development of many pathogens.
Acid CFSs and CFWs of all tested cultures showed activity against S. aureus, B. cereus and E. coli (). Correlation between the rate of acidification (pH) and the diameter of inhibitory zone was observed for most strains. The aCFWs of two strains, L. brevis 1 (рН 4.87) and L. fermentum 1 (рН 4.79), were inhibitory only for S. aureus. C. albicans was less affected. Acid CFSs of only five strains (L. bulgaricus 1, L. bulgaricus 2, L. rhamnosus Lio1, L. paracasei 4K, L. plantarum 24-4В) were active against the yeast. None of the acid CFWs inhibited C. albicans (data not shown).
Figure 1. Antimicrobial activity of Lactobacillus and Bifidobacterium strains against Staphylococcus aureus NBIMCC 3703: (A) aCFS and nCFS; (B) aCFW and nCFW. *pH values of acid CFSs and acid CFWs.
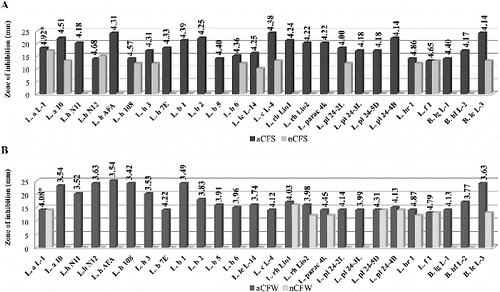
Figure 2. Antimicrobial activity of Lactobacillus and Bifidobacterium strains against Bacillus cereus NBIMCC 1085: (A) aCFS and nCFS; (B) aCFW and nCFW. *pH values of acid CFSs and acid CFWs.
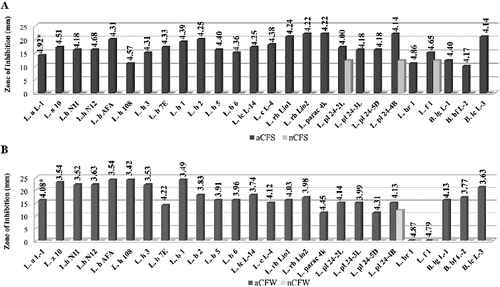
Figure 3. Antimicrobial activity of Lactobacillus and Bifidobacterium strains against Escherichia coli NBIMCC 3702: (A) aCFS and nCFS; (B) aCFW and nCFW. *pH values of acid CFSs and acid CFWs.
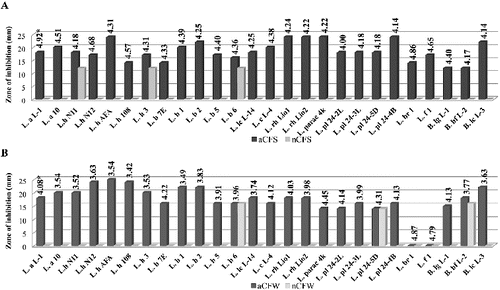
After pH neutralization, 18 strains were determined as active due to the observed ability to inhibit the growth of at least one target strain. In most cases, a bacteriostatic zone of inhibition was observed. The highest activity with nCFSs and nCFWs was registered against S. Aureus: 46.2% and 35.6% of the strains, respectively. Higher activity with nCFSs was observed among the strains of L. acidophilus, L. bulgaricus, L. helveticus and L. lactis ((A)). Conversely, nCFWs of L. plantarum, L. paracasei, L. rhamnosus and L. fermentum strains with antistaphylococcal activity were predominant ((B)). Three strains with nCFS and one strain with nCFW were active against B. cereus (). Аlso three strains with nCFS and nCFW inhibited E. coli (). Six strains were active against S. aureus (L. acidophilus L-1, L. brevis 1, L. fermentum 1 and B. animalis subsp. lactis L-3), E. coli (L. bulgaricus 6) or B. cereus (L. plantarum 24-4В) in both model systems (broth and milk). Inhibition of two pathogens was also observed. Activity against both Gram-positive micro-organisms S. aureus and B. cereus showed nCFS of L. plantarum 24-2L and L. fermentum 1, and nCFW of L. plantarum 24-4В. Activity against S. aureus and E. coli showed nCFS of L. bulgaricus 6 and L. helveticus 3, and nCFW of L. plantarum 24-5D. Activity of nCFSs and nCFWs against the three bacterial test micro-organisms was not registered. None of the tested nCFSs and nCFWs was active against C. аlbicans (data not shown).
The obtained results clearly show the role of acidity and pH for the antagonistic activity of Lactobacillus and Bifidobacterium spp. in vitro. The increased production of lactic acid through fermentation reduces pH of the media, which is known to inhibit the growth of most food-borne pathogens. The antimicrobial effect is also due to the undissociated form of the acid and its capacity to reduce the intracellular pH, leading to inhibition of vital cell functions.[Citation7] Different sensitivity of the test micro-organisms determines different zone of inhibition at the same pH. The lack of activity against E. coli for two strains with aCFW could be explained with the results obtained by Goel et al.[Citation8] for increased survival of E. coli in a fermented milk product with pH over 4.6.
The observed inhibition for some strains after elimination of the putative effects of lactic acid raised the question for possible production of other inhibitor substances, such as hydrogen peroxide, bacteriocin and bacteriocin-like substances. The greater activity against Gram-positive micro-organisms than against Gram-negative ones that was observed in our work is in accordance with the previous studies.[Citation7] The activity against Gram-positive pathogens is mostly due to the bactericidal effect of protease sensitive bacteriocins,[Citation2,Citation9] while the antagonistic effects towards Gram-negative pathogens could be related to the production of organic acids and hydrogen peroxide.[Citation10,11] However, a few bacteriocins of LAB active against E. coli and Salmonella typhimurium have also been reported.[Citation12,13] On the other hand, the antibacterial activity of six strains (L. acidophilus L-1, L. bulgaricus 6, L. plantarum 24-4В, L. fermentum 1, L. brevis 1 and B. animalis subsp. lactis L-3) in both system (broth and milk) suggests a mechanism of action different from that mentioned above. The application of such strains gives a potential advantage in the food preservation strategy.
Regardless of the nature of the antibacterial substances produced by the neutralized variants, the ability to retain this activity after lyophilization would allow production of active dry starter and probiotic cultures. Strains L. plantarum 24-2L and 24-4B and L. fermentum 1 could be used as starter organisms in the production of bread and bakery products due to their activity against B. cereus. The inhibitory effect of Lactobacillus strains used as starters against rope-forming Bacillus has been previously reported.[Citation14,15] Lactobacilli have also been shown to be effective in preventing the recurrence of urinary tract infection in women,[Citation16] and traveler's diarrhea.[Citation17] E. coli is the most common cause of these diseases. In this aspect, L. bulgaricus 6 and L. helveticus N11 exhibiting activity against this pathogen are good candidates for alleviating the symptoms and prophylaxis of such conditions. In our previous study,[Citation18] L. helveticus N11 was proved to be active against the uropathogenic E. coli strain 536 and enteropathogenic E. coli strain E2348. Use of strains inhibiting S. aureus and E. coli as antimicrobial agents may provide a safe alternative in food preservation. A few studies reported Lactobacillus spp. with strong anti-Candida activity.[Citation9,Citation19] Although there are some clinical trials that support the effectiveness of lactobacilli for prevention or treatment of vaginal yeast infections (C. albicans), evidence regarding potential benefit still remains inconclusive.[Citation20] The presence of active strains with potential application as natural biopreservatives or as probiotic cultures in specific therapeutic formulas determined our next steps towards more profound examination of the nature of the antimicrobial substances produced by selected Lactobacillus and Bifidobacterium strains.
In addition to antimicrobial activity, the MICs of nine antimicrobials of human and veterinary importance were determined for all strains. Lack of transferable resistance against therapeutic antibiotics is an important criterion for selection of an appropriate functional strain.[Citation4] Two groups of antibiotics are generally recommended: inhibitors of cell-wall synthesis (ampicillin and vancomycin) and inhibitors of protein synthesis (chloramphenicol, gentamicin, streptomycin, kanamycin, tetracycline, erythromycin and clindamycin). The obtained results and reference microbiological breakpoints are presented in . A micro-organism inhibited at breakpoint level to a specific antimicrobial is defined as susceptible. When the MIC is higher than the breakpoint, the micro-organism is considered resistant.[Citation4] For the analysis, E-test was chosen in our study, as it is a simple quantitative method that is commonly used for antimicrobial susceptibility testing of different micro-organisms.[Citation21–23]
Table 2. MIC (μg/mL) of antimicrobials for Lactobacillus and Bifidobacterium strains determined by E-test®.
In this study, all tested Lactobacillus and Bifidobacterium strains were susceptible toward ampicillin, gentamicin, erythromycin and tetracycline (). For most of the strains kanamycin, clindamycin, streptomycin and chloramphenicol were effective inhibitors. Only four lactobacilli could be considered resistant to one antibiotic (L. rhamnosus Lio 1 to streptomycin) or two antibiotics (L. acidophilus L-1 and L. brevis 1 to kanamycin and clindamycin, L. casei L-4 to clindamycin and chloramphenicol) with MICs higher than the breakpoints recently proposed by the FEEDAP Panel.[Citation4]
The obtained results are in accordance with previously reported data for lactobacilli and bifidobacteria. Generally, they are sensitive to the Gram-positive spectrum antibiotic erythromycin, the broad-spectrum antibiotics tetracycline and chloramphenicol and the beta-lactam antibiotic ampicillin.[Citation21,Citation23,Citation24] Nevertheless, acquired genes which are potentially transferable have been detected in lactobacilli.[Citation25] Among the most commonly observed resistance genes, there are two genes coding for tetracycline and erythromycin resistance, followed by genes for chloramphenicol resistance.[Citation26,27] Thus, the chloramphenicol resistance of one of the Lactobacillus strains tested in our study deserves special attention in order to avoid potential risk. By contrast, the resistance against Gram-negative spectrum antibiotics kanamycin and streptomycin is frequently observed in lactobacilli and bifidobacteria.[Citation6,Citation21–23] It may be explained by the high rate of spontaneous chromosomal mutations conveying resistance to these antibiotics.[Citation23,Citation28] Strains with this type of acquired resistance have a low potential for horizontal spread and may be used as feed additives.[Citation4] Among the aminoglycosides, lower MIC for gentamicin compared to kanamycin and streptomycin was observed as previously reported by Danielsen and Wind.[Citation23] Although clindamycin is one of the most effective antibiotics against Gram-positive micro-organisms, three of the tested lactobacilli (L. casei L-4, L. acidophilus L-1 and L. brevis 1) were shown to be resistant according to the microbiological breakpoint of this drug. Clindamycin is used for treatment of bacterial vaginosis and resistant strains could be used to restore the normal vaginal microflora together with antimicrobial bacterial vaginosis treatment.[Citation29]
L. acidophilus, L. helveticus, L. bulgaricus, L. lactis and Bifidobacterium proved to be very susceptible to vancomycin, as reported by other authors.[Citation6,Citation21] However, the highest concentration of this antibiotic was not inhibiting for all heterofermentative lactobacilli (). This resistance was previously documented as intrinsic or ‘natural’.[Citation6] According to EFSA,[Citation4] bacterial strains carrying intrinsic resistance present a minimal risk for horizontal spread and thus, may be used as a feed additive.
Conclusions
This study tested the antimicrobial activity and antibiotic susceptibility of 26 Lactobacillus and Bifidobacterium strains. The results obtained at a laboratory scale allowed selection of active strains. Ten strains with antimicrobial activity against two pathogens or in both model systems (broth and milk) appeared to be most promising: L. acidophilus L-1; L. bulgaricus 6; L. helveticus N11; L. helveticus 3; L. plantarum 24-2L; L. plantarum 24-4В; L. plantarum 24-5D; L. fermentum 1; L. brevis 1 and B. animalis subsp. lactis L-3. They may play an important role in the food industry as starter cultures, co-cultures or bioprotective cultures, to improve food quality and safety or as probiotic therapeutics appropriate for clinical practice. In addition, sensitivity or intrinsic resistance of the majority of the strains to a recommended set of antibiotics make them safe for use in different products for human or animal consumption.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Saarela M, Lähteenmäki L, Crittenden R, Salminen S, Mattila-Sandholm T. Gut bacteria and health foods – the European perspective. Int J Food Microbiol. 2002;78(1–2):99–117.
- De Vuyst L, Leroy F. Bacteriocins from lactic acid bacteria: production, purification, and food applications. J Mol Microbiol Biotechnol. 2007;13(4):194–199.
- Teuber M, Meile L, Schwarz F. Acquired antibiotic resistance in lactic acid bacteria from food. Antonie van Leeuwenhoek. 1999;769(1–4):115–137.
- Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012;10(6):2740–2750.
- Tagg JR, McGiven AR. Assay system for bacteriocins. Appl Microbiol. 1971;21(5):943–949.
- Klare I, Konstabel C, Werner G, Huys G, Vankerckhoven V, Kahlmeter G, Hildebrandt B, Müller-Bertling S, Witte W, Goossens H. Antimicrobial susceptibilities of Lactobacillus, Pediococcus and Lactococcus human isolates and cultures intended for probiotic or nutritional use. J Antimicrob Chemother. 2007;59:900–912.
- Kivanç M, Yilmaz M, Çakir E. Isolation and identification of lactic acid bacteria from boza, and their microbial activity against several reporter strains. Turkish J Biol. 2011;35(3):313–324.
- Goel MC, Kulshrestha DC, Marth EH, Francis DW, Bradshaw JG, Read RB. Fate of coliforms in yogurt, buttermilk, sour cream and cottage cheese during refrigerated storage. J Milk Food Technol. 1971;34:54–58.
- Atanassova M, Choiset Y, Dalgalarrondo M, Chobert JM, Dousset X, Ivanova I, Haertlé T. Isolation and partial biochemical characterization of a proteinaceous anti-bacteria and anti-yeast compound produced by Lactobacillus paracasei subsp. paracasei strain M3. Int J Food Microbiol. 2003;87(1-2):63–73.
- Makras L, De Vuyst L. The in vitro inhibition of Gram-negative pathogenic bacteria by bifidobacteria is caused by the production of organic acids. Int Dairy J. 2006;16(9):1049–1057.
- Makras L, Triantafyllou V, Fayol-Messaoudi D, Adriany T, Zoumpopoulou G, Tsakalidou E, Servin A, De Vuyst L. Kinetic analysis of the antibacterial activity of probiotic lactobacilli towards Salmonella enterica serovar typhimurium reveals a role for lactic acid and other inhibitory compounds. Res Microbiol. 2006;157(3):241–247.
- Gong HS, Meng XC, Wang H. Plantaricin MG active against Gram-negative bacteria produced by Lactobacillus plantarum KLDS1.0391 isolated from “Jiaoke”, a traditional fermented cream from China. Food Control. 2010;21(1):89–96.
- Simova ED, Beshkova DB, Dimitrov ZhP. Characterization and antimicrobial spectrum of bacteriocins produced by lactic acid bacteria isolated from traditional Bulgarian dairy products. J Appl Microbiol. 2009;106(2):692–701.
- Mentes Ö, Ercan R, Akçelik M. Inhibitor activities of two Lactobacillus strains, isolated from sourdough, against rope-forming Bacillus strains. Food Control. 2007;18(4):359–363.
- Denkova R, Ilieva S, Denkova Z, Georgieva L, Krastanov A. Examination of the technological properties of newly isolated strains of the genus Lactobacillus and possibilities for their application in the composition of starters. Biotechnol Biotechnol Equipment. 2014;28(3):487–494.
- Stapleton AE, Au-Yeung M, Hooton TM, Fredricks DN, Roberts PL, Czaja CA, Yarova-Yarovaya Y, Fiedler T, Cox M, Stamm WE. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52(10):1212–1217.
- Oksanen PJ, Salminen S, Saxelin M, Hämäläinen P, Ihantola-Vormisto A, Muurasniemi-Isoviita L, Nikkari S, Oksanen T, Pörsti I, Salminen E, Siitonen S, Stuckey H, Toppila A, Vapaatalo H. Prevention of travelers diarrhea by Lactobacillus GG. Ann Med. 1990;22(1):53–56.
- Grozdanov L, Karaivanova E, Pimentel-Elardo S, Dobrindt U, Rumyan N. Taxonomy and in vitro probiotic potential of novel Lactobacillus isolates. Poster session presented at: The 9th International Symposium on Lactic Acid Bacteria: Health, Evolution and Systems Biology; 2008 Aug 31–Sep 5; Egmond aan Zee, Netherlands.
- Rönnqvist D, Forsgren-Brusk U, Husmark U, Grahn-Håkansson E. Lactobacillus fermentum Ess-1 with unique growth inhibition of vulvo-vaginal candidiasis pathogens. J Med Microbiol. 2007;56(11):1500–1504.
- Falagas ME, Betsi GI, Athanasiou S. Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. J Antimicrob Chemother. 2006;58(2):266–272.
- Ammor MS, Flórez AB, van Hoek AH, de Los Reyes-Gavilán CG, Aarts HJ, Margolles A, Mayo B. Molecular characterization of intrinsic and acquired antibiotic resistance in lactic acid bacteria and bifidobacteria. J Mol Microbiol Biotechnol. 2008;14(1–3):6–15.
- Charteris WP, Kelly PM, Morelli L, Collins JK. Gradient diffusion antibiotic susceptibility testing of potentially probiotic lactobacilli. J Food Prot. 2001;64(12):2007–2014.
- Danielsen M, Wind A. Susceptibility of Lactobacillus spp. to antimicrobial agents. Int J Food Microbiol. 2003;82(1):1–11.
- Zhou JS, Pillidge CJ, Gopal PK, Gill HS. Antibiotic susceptibility profiles of new probiotic Lactobacillus and Bifidobacterium strains. Int J Food Microbiol. 2005;98(2):211–217.
- Ammor MS, Florez AB, Mayo B. Antibiotic resistance in non-enterococcal lactic acid bacteria and bifidobacteria. Food Microbiol. 2007;24(6):559–570.
- Cataloluk O, Gogebakan B. Presence of drug resistance in intestinal lactobacilli of dairy and human origin in Turkey. FEMS Microbiol Lett. 2004;236(1):7–12.
- Lin CF, Fung ZF, Wu CL, Chung TC. Molecular characterization of a plasmid-borne (pTC82) chloramphenicol resistance determinant (cat-TC) from Lactobacillus reuteri G4. Plasmid. 1996;36(2):116–124.
- Curragh HJ, Collins MA. High levels of spontaneous drug resistance in Lactobacillus. J Appl Bacteriol. 1992;73(1):31–36.
- Ocaña V, Silva C, Nader-Macías ME. Antibiotic susceptibility of potentially probiotic vaginal lactobacilli. Infect Dis Obstet Gynecol. 2006;2006, ID 18182:1–6.
