?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
An alkaliphilic-thermotolerant Bacillus cereus N1 isolated from Bani Salama Lake, Wadi El-Natron, Egypt, was proved to produce mannanase enzyme. Optimization of the fermentation medium components using Plackett–Burman design was applied. Glucose and inoculum size were found to be the most significant factors enhancing the production of the enzyme. On applying optimized medium in the fermentation process, an enzyme productivity of 42.2 UmL−1 was achieved with 6.4 fold increase compared to the basal one. Mannanase was also extracted and purified using chromatography such as ion-exchange chromatographic and gel filtration methods. It was indicated that, the mannanase activity extracted and purified from the isolate B. cereus N1 was reduced to 321.6 U (about 36% of the whole mannanase in the culture filtrate) in comparison with the initial mannanase activity (900 U) and the total protein content reduced to 52 mg (the initial total protein content was 220 mg). However, the specific activity for the mannanase from B. cereus N1 at the end of the purification steps was found to be about 628 Umg−1 compared to 4.2 Umg−1 at the initial culture filtrate. It was also indicated that the mannanase enzyme was purified almost 149-fold.
Introduction
Alkaliphiles thermophilic–thermotolerants are reported to be a rich source of alkaline thermotolerant active enzymes, that have numerous applications in many industrial processes due to an interest in their physiological adaptation to high pH and temperature.[Citation1–3]
Of the enzymes now available for the industry, enzymes such as proteases, cellulases, lipases, mannanases and pullulanases are by far the most widely employed and they still remain the target biomolecules.[Citation2] Many of the alkaliphilic Bacillus spp. were proven to be one of the main producers of those enzymes.[Citation4,Citation5]
Mannanase participates in the degradation of hemicellulose and similar polysaccharides by hydrolyzing the β-1, 4-glycosidic linkages within the main chain. The hemicelluloses are the second richest renewable energy substances on Earth. Mannan, glucomannan, galactomannan and galactoglucomannan are the major polysaccharides that constitute hemicelluloses.[Citation6] β-1,4-Mannanases produced through biotechnology have become ubiquitous in industrial applications. They are widely applied in drug, printing and dyeing, textile, oil exploitation, production of animal feed, food industry, instant coffee processing, paper and biological research.[Citation7] Furthermore, they are employed for the preparation of mannooligosaccharides used as non-nutritional food additives for selective growth of human beneficial intestinal microflora (bifidobacteria and lactobacilli). Moreover, they have shown to be effective in laundry detergents.[Citation8] Most of these applications of β-mannanase can only be completed under extreme conditions.
Unlike conventional optimization, statistical optimization methods present a more balanced alternative to the OVAT (one-variable-at-a-time) approach, since it takes into account the interaction of variables in generating the process response.[Citation9] Statistical experimental designs have been used for many decades and can be adopted on several steps of an optimization strategy, such as for screening experiments or searching for the optimal conditions of a targeted response.[Citation9] Recently, the results analyzed by a statistical planned experiment are better acknowledged than those carried out by the traditional OVAT method. Some of the popular choices, applying statistical designs to bioprocessing, include the Plackett–Burman design.[Citation10]
In this work, we report the optimization, purification and characterization of β-mannanse from an alkaliphilic-thermotolerant Bacillus cereus N1 isolated from Bani Salama Lake in Wadi El-Natron.
Materials and methods
Bacterial strains
Bacillus cereus N1 used in this study is an alkaliphilic-thermotolerant bacterium isolated from Bani Salama Lake, Wadi El-Natron, Egypt, previously identified by El-Sharouny et al. [Citation11] using 16S rDNA with accession number KF164288.
Media
Luria Broth medium (LB) [Citation12] was used for maintenance of bacteria and seed culture. pH was adjusted at 10 ± 0.2.
Horikoshi-I medium [Citation13] was used for fermentation experiments. pH was adjusted at 10 ± 0.2.
Culture condition
Fermentation was carried out in 250 mL Erlenmeyer flask containing 100 mL of the Horikoshi-I media, inoculated with one mL inoculum of seed culture in LB medium and incubated at 37 °C, pH 10 and 180 rpm for 24 h.[Citation4,Citation14]
Application of Plackett–Burman design for optimization of mannanase production
Application of a complete factorial design would require 2n experiments if n factors have to be investigated. In the present case, seven variables would lead to 128 trials, which is a very large number. Using a fraction of the factorial design without losing information about the main effects of variables can reduce the number of experiments.[Citation15]
The Plackett–Burman experimental design, a fractional factorial design,[Citation16] was used in this research to reflect the relative importance of various growth media component factors on mannanase activity in liquid cultures. In mannanase assay experiment, seven independent variables were screened in eight combinations organized according to the Plackett–Burman design matrix ( and ). For each variable, the high (+) and low (−) levels were tested. Medium components are given in gL−1 and inoculum size was added in ml with culture (A550 = 1.0).
Table 1. Factors examined as independent variables affecting mannanase enzyme activity and their levels in the Plackett–Burman experimental design.
Table 2. The Plackett–Burman experimental design matrix for seven factors.
All trials were performed in duplicates and the average of mannanase activity results were treated as the responses. The main effect of each variable was determined by the following equation:where Exi is the variable main effect, Mi+ and Mi− are mannanase production in trials where the independent variable (xi) was present in high and low concentrations, respectively, and N is the number of trials divided by 2.
A main effect with a positive sign indicates that the high concentration of this variable is nearer to optimum and a negative sign indicates that the low concentration of this variable is nearer to optimum. Using Microsoft Excel, statistical t-values for equal unpaired samples were calculated for determination of variable significance.
Analytical methods
Determination of mannanase activity
Mannanase activity was assayed in the culture supernatant after cell removal at 50 °C and pH 10, by measuring the reducing sugars liberated during the hydrolysis of galactomannan where the developed red brown colour was measured spectrophotometrically at 575 nm.[Citation14,Citation17] One unit of activity was defined as the amount of enzyme catalyzing the production of 1 μmol of the reducing sugar per minute, using mannose as the standard.
Estimation of total protein content
The total protein content of the samples was determined according to Lowry et al. [Citation18].
Purification of mannanase enzyme
The bacterium was grown in a batch culture to obtain the crude enzyme for purification. All purification steps were carried out at 4 °C, unless otherwise stated. Ion-exchange chromatographic methods and gel filtration methods were used to get a purified mannanase from bacterial strains.
Separation using anion-exchange column chromatography
The diethylaminoethanol (DEAE)-sepharose CL6B as an example for anion exchange column chromatography [Citation19] was used to purify the mannanase enzyme from the isolate B. cereus N1. The column equilibrated with 20 mmol/L tris-acetate buffer, pH 7, and the suspension was filled into a column (3.5 × 20 cm). The column was washed with 300 mL of equilibration buffer and then the bound proteins were eluted with a linear gradient from 0 to 1.0 mol/L NaCl (100 mL) in 20 mmol/L tris-acetate buffer, pH 7. The flow rate used was 1 mL min−1 and fractions collected were examined for total protein content and for mannanase activity. The fractions which contained the highest mannanase activity were pooled and dialyzed twice against 5 L of 20 mmol/L tris-acetate buffer, pH 7.
Separation using gel filtration column chromatography
Gel filtration column chromatographic methods were used as one of the steps to discard the foreign proteins depending on the molecular mass principles and purify the mannanase enzymes in polishing form from B. cereus N1. This separation was carried out through fast protein liquid chromatography.
Separation using the 32/60 Sephacryl S-100 high resolution (HR) column chromatography
Gel filtration, as final step of purification for mannanase enzyme, was conducted on a 2.5 × 60 cm column pre-packed with Sephacryl S-100 HR. The column was equilibrated with 50 mmol/L sodium acetate buffer, pH 6, containing 0.2 mol/L NaCl. The elution rate was 60 mLh−1.
All the fractions (30 fractions, 4 mL each) collected were tested for total protein content and for mannanase activity. Fractions containing mannanase activities were pooled and dialyzed twice against 5 L of 20 mmol/L sodium acetate buffer, pH 6.
Concentrating the purified protein
The purified mannanase was concentrated using the ultrafiltration tubes at a speed of 5000 rpm for 30 min at 4 °C in Centricon 10 (Amicon, USA) ultrafiltration concentrators (membrane cut-off of 10 kDa). The used ultrafiltration tubes were cleaned by centrifuging first with 2 mL bidistilled water to ensure the cleanliness of the ultrafiltration membrane tubes.
The samples were collected from the ultrafiltration-membrane tubes to other small tubes by attaching the tubes to each other and reverse the position of the ultafiltration-membrane tubes in the centrifuge and starting centrifugation at a speed of 1500 rpm for 15 min to avoid crashing of the ultrafiltration membrane.
Polyacrylamide gel electrophoresis (PAGE)
The denaturing SDS-PAGE (SDS, sodium dodecyl sulfate) has been used for detecting the mannanase enzyme homogeneity and estimation of its molecular mass using protein marker. The gels were prepared according to the method of Laemmli [Citation20] with some modifications using 10% polyacrylamide gels and in the help of using a molecular mass marker containing wide range of proteins ranging from 14.2 to 205 kDa.
Results and discussion
The thermoalkaliphiles and alkaliphiles are promising in terms of production of biomolecules suited for industrial applications.[Citation5] Alkaliphilic Bacillus species are the most characterized organisms among alkaliphiles. They produce so many extracellular alkaline-adapted enzymes that they are often good sources for industrial enzymes.[Citation1] Vijayalaxmi et al. [Citation21] reported the production of alkaline β-mannanase by alkaliphilic Bacillus sp. N16-5 isolated previously from sediment of Wudunur Soda Lake in Inner Mongolia, China.
Screening of significant variables affecting the production of mannanase enzyme from Bacillus cereus N1 using Plackett–Burman design
Plackett–Burman design, an efficient technique for medium component optimization,[Citation22] was employed to identify significant variables that enhance mannanase production and to find out their probable optimal levels in a limited number of experiments. In this study, seven variables were analyzed with regard to their effects on enzyme production using a Plackett–Burman design.()
The selected variables included nutritional factors such as carbon source (glucose), nitrogen source (yeast extract and peptone), source of phosphates (K2HPO4), other complementary salts (MgSO4.7H2O and NaCl) and finally, inoculum size. Environmental factors were kept constant with basal values of pH 10 and culture volume of 100 mL. The seven variables were evaluated by eight experiments and the levels of each variable were determined based on prior experience with the system. The independent variables were examined and their settings are shown in .
The responses in show a wide variation in mannanase activity, ranging from 0 to 32 UmL−1 corresponding to the combined effect of the seven parameters in their specific ranges. For determination of variable significance, statistical t-values for equal unpaired samples were calculated with respect to observations. The necessary statistical analyses of this experiment are shown in . The main effect of each variable upon mannanase was estimated and presented graphically in .
Table 3. Statistical analysis of the Plackett–Burman experimental result for B. cereus N1.
Figure 1. Elucidation of cultivation factors affecting mannanase production by B. cereus N1 using Plackett–Burman experimental design.
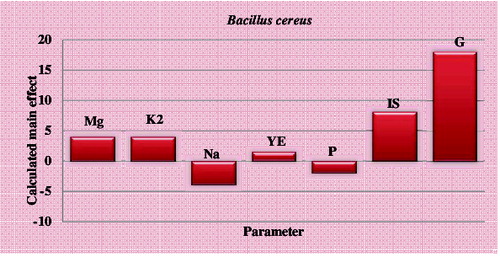
As shown in , the main effect results of strain B. cereus N1 point out that high level of MgSO4.7H2O, K2HPO4, inoculum size and glucose in the growth medium affects mannanase activity positively. This figure also suggests that low concentration levels of NaCl, yeast extract and peptone would result in high mannanase activity. Therefore, one could state that the enzyme production by B. cereus N1 was mainly dependent on glucose and NaCl, i.e. the high concentration of NaCl had the most significant negative effect on mannanase production, while the high concentration of glucose had the most significant positive effect on mannanase production. Therefore, decreasing NaCl concentration and increasing glucose concentration in the culture medium will enhance the extracellular mannanase production.
Lin et al. [Citation4] used a 22 factorial design, which was employed to optimize the medium compositions for the production of alkaline β-mannanase by alkaliphilic Bacillus sp. N16-5 isolated previously from sediment of Wudunur Soda Lake in Inner Mongolia, China. They found that the production of alkaline β-mannanase by strain N16-5 was induced by addition of some substrates containing β-mannan into the medium. The most suitable carbon source was locust bean gum and the nitrogen sources were peptone and yeast extract.
The significant variables were identified by statistical analysis of the Plackett–Burman experiment using the t-test supported by Excel Microsoft Office to determine the statistical significance of the measured response and calculated main effects for Bacillus cereus N1 (). Significance of coefficients has been reported to be directly proportional to t-test and inversely to P-value. The smaller the P-values, the bigger the significance of the corresponding coefficient.[Citation23]
Some researchers employed confidence levels greater than 70% as significant effect levels.[Citation24] Other investigators find that, confidence levels greater than 90% are acceptable [Citation25] or even levels greater than 95% are considered to have a significant effect on the response.[Citation26] However, an intermediate value of P = 0.2 corresponded to a statistical confidence of 80% was chosen in this study, and hence, any component showing a statistical confidence equal and/or higher than 80% was considered significant.
According to the data obtained from the Plackett–Burman experimental results and all calculations related to this experimental design, it can be predicted that high microbial mannanase production could be obtained using a medium formula of the following composition (gL−1): glucose, 15; yeast extract, 2; K2HPO4, 1.5; NaCl, 5; peptone, 7; inoculum size (mL),1.5 and MgSO4.7H2O, 0.5. The pH was constant at 10 and was adjusted by adding 2 mL of NaHCO3 after the sterilization process aseptically. Also galactomannan was added to the media at a constant weight of 4 gL−1.
Verification experiment
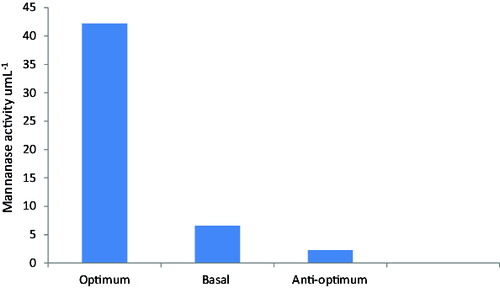
In order to validate the obtained data and to evaluate the accuracy of the applied Plackett–Burman statistical design, a verification experiment was carried out in duplicates to predict the near optimum levels of independent variables. The data were examined and compared to the basal and anti-optimized medium in , where one could notice that during the verification experiment applied on B. cereus N1, the mannanase production is raised 6.4 fold when growing in optimized medium.
The results of this experiment suggested that the most effective variables, concerning mannanase activity are the concentrations of glucose in addition to the inoculum size in mL. Among those, statistical analyses of the data (t-test) demonstrated the significance of glucose and inoculums size in mL.
Inoculum size has significant effect on the mannanase production. Therefore, a suitable inoculum size is needed to have the highest mannanse production as lower inoculum size was able to slow down the biomass proliferation. Thus, the degradation of the substrates by the microbes is slower and affects the metabolite production.[Citation27]
On the other hand, regarding the effect of glucose concentration, Youssef et al. [Citation28] studied the effect of carbon sources on the production of β-mannanase using the basal culture medium supplemented with 2% mannan as a control. In other trials, mannan was replaced by equal amounts of different carbon sources, as a sole carbon source. It was found that the lowest mannanase activity was obtained in cultures containing glucose as a carbon source, which was found to promote mannanase activity but in a much lower level rather than that achieved if replaced by coconut. However, still few investigators used glucose or any other readily metabolizable carbohydrates as a sole carbon source with significant production of mannanase.[Citation29] Generally, the synthesis of mannanase enzyme is inducible in microbial cells and appears to be controlled by carbon repression when more easily metabolizable carbon sources, e.g. glucose, are present in the culture medium together with a substrate suitable for inducing enzyme synthesis. Hence, the enzyme formation in the organism starts only when the repressing glucose is completely metabolized, although moderate levels of enzymes were produced when glucose was used as the only carbon source.[Citation29]
Extraction and purification of mannanase enzyme
A scheme of extracted and purified mannanases enzyme for B. cereus N1 was shown in . It was indicated that the mannanase activity extracted and purified from the isolate B. cereus N1 was reduced to 321.6 U (about 36% of the whole mannanase in the culture filtrate) in comparison with the initial mannanase activity (900 U) and the total protein content was reduced to 52 mg in comparison with the initial total protein content of 220 mg. However, the specific activity for the enzyme at the end of the purification steps was found to be about 628 Umg−1 comparing to 4.2 Umg−1 at the initial culture filtrate. It was also indicated that at the end of the extraction steps, the mannanase enzyme was purified almost 149-fold.
Table 4. Purification scheme of mannanase enzyme from the isolate B. cereus N1.
Anion-exchange column chromatography
The DEAE-sepharose chromatography, as an anion exchange chromatography, was used in order to separate and purify the mannanase enzyme from the isolate B. cereus N1.
The concentrated suspension by the ultrafiltration Amicon system containing about 188 mg of total foreign proteins and 880 total units of mannanase activity was filled into the DEAE-sepharose column at pH 7. About 88.9% of the total mannanase enzyme (602 U) and about 7.1 mg of the total protein were recovered (). A 20-fold purification indicated that DEAE-sepharose CL 6B is considered to be a good step for separating and purifying the mannanase enzyme from the isolate B. cereus N1.
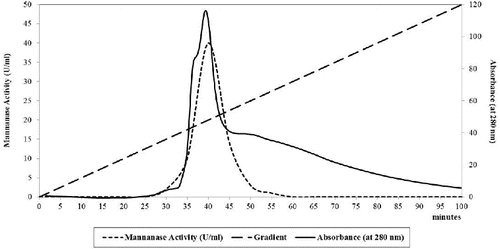
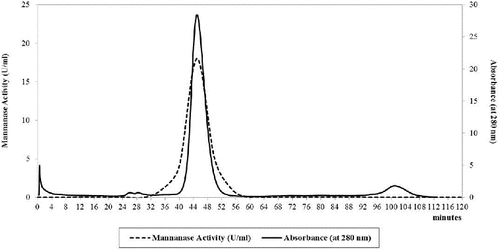
shows the elution diagram of the mannanase enzyme using DEAE-sepharose CL6B and it indicates a sharp peak of the mannanase enzyme elution, which gives the maximum activity after the gradient per cent reach about 45% from the sodium chloride.
Gel filtration chromatography
Gel filtration technique was used as a final step for mannanase purification. Chromatographic columns, such as 32/60 Sephacryl S-100 HR, were used in order to purify the mannanase enzyme in the final form.
32/60 Sephacryl S-100 HR chromatography
The dialyzed sample from the isolate B. cereus N1, which contains 7.1 mg of total proteins and 602 total units of mannanase activity, was filled into the 32/60 Sephacryl S-100 HR chromatographic column at pH value of 6.0.
The pooled collected fractions showed a recovery of about 35.7% of the total mannanase enzyme (321.6 U) and only 0.52 mg of the total protein (). About 149-fold purification showed the importance of using gel filtration as a method of separation and purifying the mannanase enzyme. illustrates the elution diagram of the mannanase enzyme using 32/60 Sephacryl S-100 HR chromatographic column and it indicates a sharp peak of the mannanase enzyme.
Figure 4. Sephacryl S-100 HR column as a gel filtration chromatography of B. cereus N1 mannanase preparation. Buffer: 50 mmol/L sodium acetate containing 0.2 mol/L NaCl, pH 6.
Chandra et al. [Citation30], who isolated and purified mannanase enzyme from Paenibacillus cookii and Bacillus sp., stated that about 6.4% and 17.2% mannanase were recovered, and 90.2- and 48.5-fold of purification were achieved at this stage. Moreover, mannanase from alkaliphilic Bacillus sp. N16-5 has been investigated by Ma et al. [Citation14]. The enzyme was purified with a yield of 8.8%. An approximately 15.8-fold purification to a specific activity of 5065 Umg−1 of protein was obtained for the mannanase activity when measured in 50 mM glycine–NaOH buffer (pH 9.5).
Testing of the enzyme purity and estimation of their molecular mass
In order to prove the purity of the mannanse enzyme extracted and purified from B. cereus N1, SDS gel electrophoresis was used. Using SDS gel electrophoresis is considered to be an indicator not only for of the purity of the mannanase enzyme, but also as a method for detecting the molecular mass of the purified enzymes.
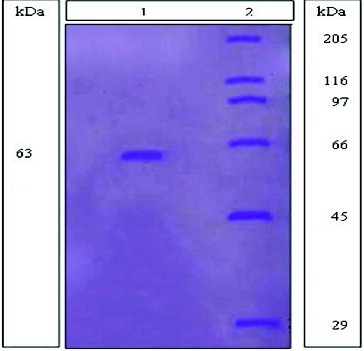
It showed on the gel in our case that only a single band appeared for mannanase from B. cereus N1 and no other bands appeared. This indicated the purity of the isolated mannanase enzyme with molecular mass estimated by comparing with the marker to be about 63 kDa (). This result is similar to that found in the literature for mannanase from other separate sources.[Citation31,Citation32]
Conclusion
In this work, an alkaliphilic-thermotolerant Bacillus cereus N1 was isolated from Bani Salama Lake, which is one of the famous soda lakes located in Wadi El-Natron, Egypt, that has also been rarely investigated for its microflora. Optimization of the fermentation medium components of the isolate was applied, using Plackett–Burman design, leading to 6.4 fold increase in enzyme activity. Mannanase was also extracted and purified using chromatography such as ion-exchange chromatographic and gel filtration methods, where the specific activity for the enzyme reached about 628 Umg−1 compared to 4.2 Umg−1 at the initial culture filtrate. It was also indicated that the mannanase enzyme at the end of the extraction steps was purified almost 149-fold and has a molecular mass of 63 kDa.
Acknowledgements
Our sincere thanks to Professor Soraya A. Sabry, Professor of Microbiology, Faculty of Science, Alexandria University for her great help and guidance.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Fujinami S, Fujisawa M. Industrial applications of alkaliphiles and their enzymes – past, present and future. Environ Technol. 2010;31(8–9):845–856.
- Asoodeh A, Lagzian M. Purification and characterization of a new glucoamylopullulanase from thermotolerant alkaliphilic Bacillus subtilis DR8806 of a hot mineral spring. Process Biochem. 2012;47:806–815.
- Abo-State MAM, Ghaly MF, Abdellah EM. Optimization of cellulase(s) and xylanase production by thermophilic and alkaliphilic Bacillus isolates. Am-Eurasian J Agric Environ Sci. 2013;13(4):553–564.
- Lin SS, Dou WF, Xu HY, Li HZ, Xu ZH, Ma YH. Optimization of medium composition for the production of alkaline beta-mannanase by alkaliphilic Bacillus sp. N16-5, using response surface methodology. Appl Microbiol Biotechnol. 2007;75(5):1015–1022.
- Sarethy IP, Saxena Y, Kapoor A, Sharma M, Sharma SK, Gupta S, Gupta V. Alkaliphilic bacteria: application in biotechnology. J Ind Microbiol Biotechnol. 2011;38:769–790.
- Yan X, An X, Gui L, Liang D. From structure to function: insights into the catalytic substrate specificity and thermostability displayed by Bacillus subtilis mannanase BCman. J Mol Biol. 2008;379(3):535–544.
- Abd-Rashid JI, Samat N, Yusoff WMW. Screening and optimization of medium composition for mannanase production by Aspergillus terreus SUK-1 in solid state fermentation using statistical experimental methods. Res J Microbiol. 2012;7(5):242–255.
- Bettiol JP, Showell MS. Detergent compositions comprising a mannanase and a protease. US Patent 6376445. 2002.
- Hao XC, Yui XB, Yan ZL. Optimization of the medium for the production of cellulase by the mutant Trichoderma reesei WX-112 using response surface methodology. Food Technol Biotechnol. 2006;44(1):89–94.
- Siala R, Frikha F, Mhamdi S, Nasri M, Sellami KA. Optimization of acid protease production by Aspergillus niger I1 on shrimp peptone using statistical experimental design. Scientific World J. 564932. Epub. 2012: 1–11.
- El-Sharouny EE, El-Sersy NA, El-Gayar AA. Investigation of some biotechnological aspects of two novel Bacillus species isolated from natural and man-made alkaline ecosystems in Egypt. JPAM. 2014;8(4):2701–2712.
- Moriyama H, Fukusaki E, Cabreracrespo J, Shinmyo A, Okada H. Structure and expression of genes coding for xylan-degrading enzymes of Bacillus pumilus. Eur J Biochem. 1987;166:539–545.
- Horikoshi K. Alkaliphiles – from an industrial point of view. FEMS Microbiol Rev. 1996;18:259–270.
- Ma YH, Xue YF, Dou YT, Xu ZH, Tao WY, Zhou PJ. Characterization and gene cloning of a novel β-mannanase from alkaliphilic Bacillus sp. N16-5. Extremophiles. 2004;8:447–454.
- Ooijkaas LP, Wilkinson EC, Tramper J, Buitelaar RM. Medium optimization for spore production of Conithyrium minitans using statistically-based experimental designs. Biotechnol Bioeng. 1998;64(1):92–100.
- Plackett RL, Burman JP. The design of optimum multifactorial experiments. Biometrica. 1946;33:305–325.
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428.
- Lowry OH, Rosenbrough NJ, Farr AL, Randal RJ. Protein measurement with the folin reagent. J Biol Chem. 1951;193:265–275.
- Junowicz E, Spencer JH. Rapid separation of nucleosides and nucleotides by cation-exchange column chromatography. J Chromatogr A. 1969;44:342–348.
- Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685.
- Vijayalaxmi S, Prakash P, Jayalakshmi SK, Mulimani VH, Sreeramulu K.. Production of extremely alkaliphilic, halotolerent, detergent, and thermostable mannanase by the free and immobilized cells of Bacillus halodurans PPKS-2. Purification and characterization. Appl Biochem Biotechnol. 2013;171(2):382–95.
- Hegde S, Bhadri G, Narsapur K, Koppal S, Oswal P, Turmuri N, Jumnal V, Hungund B. Statistical optimization of medium components by response surface methodology for enhanced production of bacterial cellulose by Gluconacetobacter persimmonis. J Bioprocess Biotech. 2013;4(1):1–5. doi:10.4172/2155-9821.1000142.
- Heck JX, Flores SH, Hertz PF, Ayub MAZ. Optimization of cellulase-free xylanase activity produced by Bacillus coagulans BL69 in solid-state cultivation. Process Biochem. 2005;40:107–112.
- Stowe RA, Mayer RP. Efficient screening of process variables. lnd Eng Chem. 1966;56:36–40.
- Abdel-Fattah YR, Olama ZA. L-asparaginase production by Pseudomonas aeruginosa in solid-state culture: evaluation and optimization of culture conditions using factorial designs. Process Biochem. 2002;38(1):115–122.
- Niladevi KN, Sukumaran RK, Jacob N, Anisha GS, Prema P. Optimization of laccase production from a novel strain-Streptomyces psammoticus using response surface methodology. Microbiol Res. 2009;164:105–113.
- Ramachandran S, Patel AK, Nampoothiri KM, Francis F, Nagy V, Szackacs G, Pandey A. Coconut oil cake – a potential material for production of α-amylase. Bioresour Technol. 2004;93(2):169–174.
- Youssef AS, El-Naggar MY, El-Aassar SA, Beltagy IA. Optimization of culture conditions for β-mannanase production by a local Aspergillus niger isolate. Int J Agric Biol. 2006;8(4):539–545.
- Sachslehner A, Haltrich D, Gübitz G, Nidetzky B, Kulbe KD. Efficient production of mannan-degrading enzymes by the basidiomycete Sclerotium rolfsii. Appl Biochem Biotech. 1998;70–72:939–953.
- Chandra MRS, Lee YS, Park IH, Zhou Y, Kim K, Choi YL. Isolation, purification and characterization of a thermostable β-mannanase from Paenibacillus sp. DZ3. J Korean Soc Appl Biol Chem. 2011;54(3):325–331.
- Duffaud GD, McCutchen CM, Leduc P, Parker KN, Kelly RM. Purification and characterization of extremely thermostable beta-mannanase, beta-mannosidase and alpha-galactosidase from the hyperthermophilic eubacterium Thermotoganea politana 5068. Appl Environ Microbiol. 1997;63(1):169–177.
- Puchart V, Vršanská M, Svoboda P, Pohl J, Ögel ZB, Biely P. Purification and characterization of two forms of endo-b-1,4-mannanase from a thermotolerant fungus, Aspergillus fumigatus IMI 385708. Biochimica Biophysica Acta. 2004;1674:239–250.