Abstract
Blast, caused by the ascomycete fungus Magnaporthe oryzae, is one of the most devastating diseases of rice due to the high variation of the pathogen. Breeding high-yielding rice cultivar with durable resistance to rice blast is a priority in Southern China and in places where rice cultivation is an important branch in farming. Effectiveness and accuracy of resistant cultivar breeding largely depend on the development of markers specific to the target gene. In silico prediction of the resistance (R) Pi39 gene content of the interval was made and hence a candidate gene was identified to develop a perfect insertion–deletion (InDel) based marker for Pi39 gene selection. The Pi39 gene was successfully introgressed in two elite cultivars using both foreground (the InDel) and background (genome-wide microsatellites) genotypic and phenotypic selection. Five selected BC3F3 progeny lines were recovered and showed a high level of blast resistance. At least 97.5% of their genome was inherited from their recurrent parent. The agronomic performance of four lines (D94, D98, D112 and D113) was at least as good as that of their recurrent parent.
Introduction
Rice blast is caused by the fungus Magnaporthe oryzae and remains one of the most destructive rice crops diseases in China.[Citation1,Citation2] As deployment of the host plant's resistance is considered to be the safest management option, the development of resistant cultivars is a continuing priority in rice breeding programmes, particularly in Southern China.[Citation3,Citation4] Genetic resistance, based on single genes, has a history of rapid breakdown, caused by pathogen adaptation.[Citation5] One strategy for improving the durability of genetic resistance is to stack several genes into a single cultivar,[Citation6–8] whereas another is to exploit genes which confer a broad resistance spectrum.[Citation9–11] Nearly 100 distinct blast resistance genes have been identified, of which at least 14 (Pi1, Pi2, Pi9, Pi20(t), Pi33, Pi39, Pi40(t), Pi47, Pi48, Pi54rh, Pi56, Piz, Piz-t, and Pigm) have been described as conferring broad spectrum resistance.[Citation4,Citation12–22] The broad spectrum resistance gene Pi39, carried by the Yunnanense cultivar (cv.) Q15, is a homonym of the one carried by the cultivar Chubu 111 [Citation23] and is also a particularly promising introgression target for rice that grows in the Guangdong Province, where the climate is favourable for the development of rice blast epidemics.[Citation2,Citation17]
Table 1. Details of the PCR primers used in this study.
DNA-based markers have an increasing impact on conventional breeding practices.[Citation24] The most effective markers are those which lie within the sequence of the target gene itself, since otherwise there is always a risk of miss-selection when a recombination event has separated the marker from the target site.[Citation25,Citation26] Such ‘perfect’ markers have been developed for blast resistance genes, allowing them to be used as highly reliable selective aids. DNA markers specific for Pita and Pib genes have been used to follow their introgression into advanced breeding lines.[Citation27,Citation28] Allele-specific and insertion–deletion (InDel) marker sets are available for nine blast resistance genes, providing an efficient marker system for marker-assisted selection (MAS).[Citation29,Citation30] Recently, a functional marker for the blast resistance gene Pit has been developed and employed in the mining of this gene in diverse rice varieties or landraces.[Citation16] In the case of Pik gene locus in rice, markers, which reflect allele-specific blast resistance, were identified. This allows the differentiation of Pik alleles from each other by applying MAS during rice breeding processes.[Citation4,Citation31,Citation32] The present study was focused on developing a ‘perfect’ genetic marker for Pi39 and using it to introgress the gene into two high yielding, but blast susceptible cultivars – ‘Yuexiangzhan’ (YXZ) and ‘Yueyinsimiao’ (YYSM).
Materials and methods
Candidate gene annotation and sequence analysis
The gene annotation program RiceGAAS (http://ricegaas.dna.affrc.go.jp/rgadb/) was used to identify candidates for Pi39 within the Nipponbare genomic region defined by the closest flanking markers (39M11 and 39M22) ((A) and 1(B)).[Citation17] The full length of the chosen candidate, including its promoter and terminator, was amplified from the genomic DNA of the Pi39 donor Q15 by using long-range PCR (Takara, Dalian, China) with primer pair 39-LF/R, as described by Liu X et al.[Citation17] The amplicon was inserted into the AscI restriction site of the vector pCAMBIA1300AscI and then it was sequenced (). RACE (Rapid Amplification of cDNA Ends) PCR was conducted using a GeneRacer Kit (Invitrogen, Groningen, The Netherlands), following the manufacturer's instructions. The Pi39 5′ RACE product was then amplified using nested PCR. The first reaction used reversed primer 39-5RACE1 and the GeneRacer 5′ primer, provided by the kit. The second round used nest reversed primer 39-5RACE2 in combination with the GeneRacer 5′ nest primer. The 3′ RACE employed forward 39-3RACE1 along with the GeneRacer 3′ primer. A mediate RT-PCR fragment was obtained by a pair of primers 39-RTF/R (). Sequencing of amplicons derived from the cDNA of the blast susceptible cultivars Q1063, Kasalath and 93-11 was carried out using the primer pair 39-CDSF/R. All PCRs were based on a high-fidelity Taq polymerase (NEB, England). After an A-tailing procedure, the PCR amplicons were inserted into the pMD20 T-vector (TaKaRa, Dalian, China) and sequenced by Invitrogen (Guangzhou, China). All primers' sequences are given in . Sequences alignments were performed using the DNAstar 7.10 (DNASTAR, Inc) software package.
Figure 1. The genomic region surrounding Pi39. Note: Physical map of the Pi39 locus on chromosome 12 based on the ‘Nipponbare’ sequence (adapted from [Citation17], Springer license number: 3551371059670 [Liu X, Yang Q, Lin F, Hua L, Wang C, Wang L, Pan Q. Identification and fine mapping of Pi39(t), a major gene conferring the broad-spectrum resistance to Magnaporthe oryzae. Mol Genet Genomics. 2007;278:403–410.]) (A); according to RiceGAAS (http://ricegaas.dna.affrc.go.jp/), the Pi39 region harbours seven predicted genes, two of which have an NB-ARC domain (filled arrow) (B); the structure of OJ1115-G02 Autopredgene22 and OJ1115-G02 Autopredgene23, as predicted by RiceGAAS. The numbers shown at the top refer to cv. Nipponbare genomic sequence positions along chromosome 12 (C); the structure of Pi39, as determined by a comparison between its cDNA and gDNA sequence. Exons are shown as boxes and introns are shown as horizontal lines, connecting the exons. The positions of the Pi39-specific InDel and SNP markers are indicated by triangles (D).
![Figure 1. The genomic region surrounding Pi39. Note: Physical map of the Pi39 locus on chromosome 12 based on the ‘Nipponbare’ sequence (adapted from [Citation17], Springer license number: 3551371059670 [Liu X, Yang Q, Lin F, Hua L, Wang C, Wang L, Pan Q. Identification and fine mapping of Pi39(t), a major gene conferring the broad-spectrum resistance to Magnaporthe oryzae. Mol Genet Genomics. 2007;278:403–410.]) (A); according to RiceGAAS (http://ricegaas.dna.affrc.go.jp/), the Pi39 region harbours seven predicted genes, two of which have an NB-ARC domain (filled arrow) (B); the structure of OJ1115-G02 Autopredgene22 and OJ1115-G02 Autopredgene23, as predicted by RiceGAAS. The numbers shown at the top refer to cv. Nipponbare genomic sequence positions along chromosome 12 (C); the structure of Pi39, as determined by a comparison between its cDNA and gDNA sequence. Exons are shown as boxes and introns are shown as horizontal lines, connecting the exons. The positions of the Pi39-specific InDel and SNP markers are indicated by triangles (D).](/cms/asset/c55b2e2b-f7ff-4771-8c2a-8e99f0e13033/tbeq_a_1011894_f0001_b.gif)
Marker development
For target Pi39, a primer pair (39SM) was chosen, which targeted an InDel differentiating the resistant from the non-resistant allele ((D)). The amplification was performed in T100 machine (Bio-Rad Laboratories, Inc) and the programme was 94 ℃ for 3 min, followed by 35 cycles at 94 ℃ for 40 s, 58 ℃ for 1 min and 72 ℃ for 1 min, with a final extensional at 72 ℃ for 5 min. The PCR products were separated on 2.0% agarose gel, which contained ethidium bromide and was visualized by ultraviolet light.
Plant materials
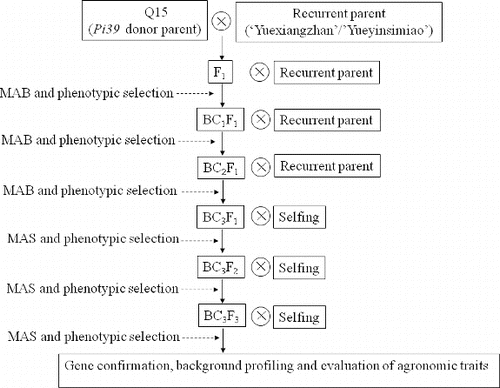
The donor of Pi39 was the native upland rice cv. Q15, while the recipient cvs. were YXZ and YYSM. Each of the F1 hybrids Q15 × YXZ and Q15 × YYSM was backcrossed with its respective recurrent parent () and advanced lines were developed by following a backcrossing strategy using both genotypic and phenotypic selection. The presence of Pi39 was ensured by the use of marker 39SM. BC3F1 generation was allowed to self-fertilize, and a combination of genotypic and phenotypic selections were applied to identify the individuals carrying Pi39 in a genetic background as close as possible to that of the recurrent parent. In field screening, where blast resistance was being monitored, the cv. Yueluzhan was included as a susceptible spreader. Four Pi39 susceptible cultivars, Tsuyuake, Q61, Kasalath and 93-11 were subjected to Pi39 candidate gene sequence. A panel of 121 accessions (16 landrace, 11 modern cultivars and 94 wild rice entries) (Table S1 in the Online Supplementary Appendix) were scanned to evaluate the specificity of the Pi39 marker.
Background genotyping
A set of 187 simple sequence repeat (SSR) marker assays, marking loci of known genomic location on each of the 12 chromosomes, was applied for background genotyping (Figure S1(A) and S1(B) in the Online Supplementary Appendix). Their location was based on the rice genetic map [Citation33] as depicted in Gramene (http://www.gramene.org). PCR amplification and marker detection were done in the same way as previously described.[Citation34]
Blast resistance in the field
Field screening for response to blast infection was carried out in a known disease hot spot in the Lütian county in the north Guangdong Province.[Citation35] Around 20 grains of each BC3F2 and BC3F3 selections were sown in a line of 67 cm row, with an inter-row spacing of 23 cm, along with Q15 and the parental cultivar (YXZ and YYSM). The set of rows was surrounded by Yueluzhan plants. Disease reaction was scored on a 0–9 scale 10–15 days after the Yueluzhan plants showed severe signs of infection. The lines with a score of 0–3 were considered as resistant, 4–5 as moderately resistant, 6 as moderately susceptible and 7–9 as susceptible.[Citation36]
Agronomic performance
A set of 20 plants of each selected advanced backcross lines were transplanted to a 20 cm × 17 cm space in a randomized complete block design with three replications during the late season of 2012 at the Guangdong Agriculture Academic Research Institution Dafeng research farm. The following traits were recorded for 10 plants per line: number of panicles per plant, panicle length (cm), grains per panicle, filled grains (%), 1000-grain weight (g) and yield per plant (g). The least significant difference (LSD) statistic analysis was used to distinguish between mean values, and was obtained by using the software package SPSSv16.0 for Windows (SPSS Inc. Chicago, ILL, USA).
Results and discussion
Identification of Pi39 candidate
The fine-scale mapping of Pi39 succeeded in locating the gene within a 37 kb region, which harbours seven predicted genes according to RiceGAAS analysis ((A) and 1(B)).[Citation17] Two of these putative genes (OJ1115-G02 Autopredgene22 and OJ1115-G02 Autopredgene23) have an NB-ARC domain which is diagnostic for a major class of plant disease resistance genes ((B) and 1(C)). Both the genomic DNA and the cDNA versions of OJ1115-G02 Autopredgene23 were sequenced and compared to identify the gene's size and structure. Since there was an overlap between the 5′ and 3′ RACE products and the RT-PCR fragment, the complete transcribed region could be recognized ((D)). The result of the gene structure identification indicated that OJ1115-G02 Autopredgene22 and OJ1115-G02 Autopredgene23 are essential in one and the same transcription ((D)), which provided strong evidence that Autopredgene22 and Autopredgene23 had been split due to software miss annotation. Thus, Autopredgene22 and Autopredgene23 form a single gene, which was considered as a strong candidate gene for the Pi39 resistance. Based on the sequence analysis results, the Pi39 candidate gene contained a 3195-bp coding region, interrupted by one intron of 5949 bp and flanked by a 49-bp 5′-untranslated region (UTR) and a 344-bp 3′-UTR. A 1562-bp intron was present within the 3′-UTR ((D)).
The development of a Pi39-specific marker
The comparison between the Pi39 candidate cDNA sequences of Q15 and Tsuyuake () revealed the presence of a 90 bp InDel in the 3′-UTR, along with eight single nucleotide polymorphisms (SNPs) which affected the predicted peptide sequence. However, seven of the eight SNPs were simultaneously present in both Q15 and one (or more) of the susceptible Q1063, Kasalath and 93-11, so they cannot be diagnostic for the resistant allele. Thus either the leucine for arginine shift at position 171 and/or the 90 bp InDel are likely to be responsible for the Pi39-mediated resistance. The latter was convenient, because the target for marker development and its detection were very straightforward. The use of major resistance genes in breeding for blast resistant rice is complicated as many Pi genes confer resistance to overlapping spectra of blast phenotypes. Also they are often organized as clusters, which makes it difficult to monitor for the presence of individual resistance genes and their introgression in breeding lines.[Citation4,Citation37] The elaboration of gene-specific DNA markers simplifies and accelerates the selection of lines carrying multiple resistance genes, since it avoids to carry out a complex set of progeny to test the presence of the resistance gene in each host.[Citation29] The widely used Pik locus is multi-allelic and each known allele has been tagged with a specific marker. These assays have been effectively used for resistance breeding and searching for novel alleles in large germplasm sets.[Citation4,Citation29,Citation30] Pi39, along with the genes Pi20(t), Pita, Pita-2, Pi4, Pi6, Pi12, Pi19(t), Pi21, Pi24, Pi31, Pi32, Pi57, Pitq-6, are all clustered to the pericentromeric region of chromosome 12, which is a part of the chromosome where recombination is suppressed.[Citation17] Recombination suppression near the centromere region of the rice chromosome may inhibit the ability to separate these resistance (R) genes/alleles.[Citation28,Citation38] Thus the identification of gene-specific (as opposed to a R gene block-specific) DNA markers, distinguishing each of these R genes near the centromere region, is technically challenging. The Pi39-specific InDel marker, which is described in the present research, has the potential to greatly reduce the time and costs, required for disease screening, in order to distinguish the Pi39 from other Pi genes in this cluster.
Table 2. Comparison of putative amino acid sequences among the Pi39 resistance and susceptible allele.
A screen of a 121 entry diversity panel showed that the InDel was not present in any of the landraces or modern cultivars, but was present in 11 wild rice accessions in heterozygosis state and in one wild rice accession (W067) in homozygous state (Table S1 in the Online Supplementary Appendix). An essential requirement for a molecular marker and its large-scale implementation in breeding programme is that it should be informative over a wide range of genetic backgrounds, so that the marker can be applied to a wide range of crosses.[Citation39,Citation40] A demonstration of the robustness of the Pi39-specific marker was given by the screen of the 121 accession diversity panel. Furthermore, the finding that the Pi39-specific marker was restricted to Q15 and a few wild rice accessions indicated that the gene has not yet been widely exploited in Chinese rice improvement programmes. Therefore, it is a valuable and potentially durable allele in contemporary breeding programmes.
Introgression of Pi39 resistance
A selection of five F1 plants was made from the cross Q15 × YXZ and the heterozygosity of the Pi39 loci confirmed their hybridity. After pollination by the recurrent parent YXZ, 150 BC1F1 progeny were generated, from which four Pi39 heterozygotes plants were selected for the next round of backcrossing. A set of 150 BC3F1 plants was obtained from 15 BC2F1 selections, and eight of these were self-fertilized to generate 500 BC3F2 plants; 11 selected BC3F2 plants were self-fertilized once more to produce a population of 800 BC3F3 individuals. Finally, two BC3F3 blast resistant (Pi39 homozygous) selections were made (D94 and D98) (). Phenotypic selection at each backcross and selfing generation was conducted to eliminate partially sterile, tall and/or late flowering plants.[Citation41] A similar strategy was applied for the introgression of Pi39 into YYSM, producing the three BC3F3 selections (D112, D113 and D114). Field blast resistance analysis of all five selected BC3F3 lines suggested that they show resistance to the rice blast in field and the resistance phenotypes are perfectly associated with the Pi39 genotypes in selection ().
Table 3. Reaction of the five selected BC3F3 lines to rice blast in field.
SSR-based genetic background profiling of BC3F3 lines
Out of 187 SSRs, 96 were informative between Q15 and YXZ and 95 between Q15 and YYSM (Figure S1(A) and S1(B) in the Online Supplementary Appendix). Background selection was applied to the BC3F3 generation, in order to achieve an average recovery of 99.3% of the YXZ and 98.3% of the YYSM genome (). Donor DNA was restricted to small regions in chromosome 3, 4, 6, 9 and 10, as well as in the vicinity of Pi39, which lies in chromosome 12.[Citation17] The D94 background was 99.5% recipient, that of D98 was 99.0%, that of both D113 and D114 was 98.7% and that of D112 was 97.6% (). Without any background selection, simulations assumed a 50% reduction in donor genome with each backcross in a standard backcross strategy.[Citation41] On this basis, six backcrosses are needed to cover an average of 99.2% of the recurrent parent genome.[Citation41,Citation42] The advantage of deploying background selection is that the number of the required backcross generations can be decreased, which represents a substantial reduction of the time to complete the breeding task.[Citation43] A combined foreground and background selection strategy enabled the selection of a three gene introgression line (xa5, xa13 and Xa21) having a 97% level of the recurrent parent background by the fourth backcross generation.[Citation44] In the present study, the recovery had already reached 99% by the third backcross, thanks to the effective deployment of background phenotypic and SSR-based selection.
Table 4. Substituted chromosome segments from recurrent parent in advanced backcross breeding lines of rice.
Agronomic performance of improved selections
The field performance of the five selected backcross lines D94, D98, D112, D113 and D114 was evaluated during the late season of 2012 (). The YXZ-derived selection D94 produced more panicles per plant, a greater percentage of filled grains and a higher yield per plant than YXZ, whereas plants of the other selection, D98, formed longer panicles, set more grains per panicle and also produced a greater yield per plant than YXZ. All of the three YYSM-derived lines were characterized by longer panicles than those of recurrent parent YYSM. Plants D112 formed more panicles per plant and had a better yield than YYSM, but there was no significant variation with respect to 1000 grain weight among the improved lines and their recurrent parents. The presence of Pi39 in these improved lines can be expected to provide a level of resistance against most of the rice blast strains present in the Guangdong region, so this should have a positive impact on yield, stability and sustainability of the local rice crop. Grain yield and quality of the four Pi39 breeding lines (D94, D98, D112 and D113) are at least as good as, if not better than, those of the respective recurrent parent, which confirms the absence of any unfavourable linkage drag associated with the Pi39 gene. Therefore, these lines can be used directly as improved cultivars or as donors of Pi39 in ongoing breeding programmes.
Table 5. Comparison of principal agronomic traits between introgression lines and their recurrent parents.
Conclusions
In this study, a ‘perfect’ InDel-based marker for Pi39 gene selection was developed. Robustness of the Pi39-specific marker was verified by the screen of the 121 accession diversity panel. The Pi39 gene was successfully introgressed in two elite cultivars using both foreground (the InDel) and background (genome-wide microsatellites) genotypic and phenotypic selection. Five selected BC3F3 progeny lines were recovered and showed a high level of blast resistance. At least 97.5% of their genome was inherited from their recurrent parent. The agronomic performance of four lines (D94, D98, D112 and D113) was at least as good as that of their recurrent parent.
Acknowledgements
The authors greatly appreciate the kind help of Dr Xiaoyuan Zhu at the Plant Protection Research Institute of the Guangdong Academy of Agricultural Sciences, in providing the laboratory equipment.
Supplemental data
Supplemental data for this article can be accessed online http://dx.doi.org/10.1080/13102818.2015.1011894.
Lin_Fei_Online_Suppl_Appendix.pdf
Download PDF (612 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This research was supported by the Chinese National Natural Science Foundation [grant number 30800711]; the National Transgenic Research Projects [grant number 2014ZX08009004B]; and the Agricultural Key Projects of the Department of Science and Technology of the Government of the Guangdong Province [grant number 2007A020100002].
References
- Shen MG, Lin JY. The economic impact of rice blast disease in China. In: Zeigler RS, Leong SA, Teng PS, editors. Rice blast disease. England, UK: CAB International; 1994. p. 321–331.
- Chen HL, Chen BT, Zhang DP, Xie YF, Zhang Q. Pathotypes of Pyricularia grisea in rice fields of central and southern China. Plant Dis. 2001;85:843–850.
- Khush GS, Jena KK. Current status and future prospects for research on blast resistance in rice (Oryza sativa L.). In: Wang GL, Barbara V, editors. Advances in genetics, genomics and control of rice blast disease. Netherlands, NL: Springer; 2009. p. 1–10.
- Hua L, Wu J, Chen C, Wu W, He X, Lin F, Wang L, Ashikawa I, Matsumoto T, Wang L, Pan Q. The isolation of Pi1, an allele at the Pik locus which confers broad spectrum resistance to rice blast. Theor Appl Genet. 2012;125:1047–1055.
- Correa-Victoria FJ, Zeigler RS. Pathogenic variability in Pyricularia grisea at a rice blast ‘host spot’ breeding site in eastern Colombia. Plant Dis. 1993;77:1029–1035.
- Hittalmani S, Parco A, Mew TV, Zeigler RS, Huang N. Fine mapping and DNA marker-assisted pyramiding of the three major genes for blast resistance in rice. Theor Appl Genet. 2000;100:1121–1128.
- Koide Y, Telebanco-Yanoria MJ, Fukuta Y, Kobayashi N. Detection of novel blast resistance genes, Pi58(t) and Pi59(t), in a Myanmar rice landrace based on a standard differential system. Mol Breed. 2013;32:241–252.
- Singh VK, Singh A, Singh SP, Ellur RK, Singh D, Gopala Krishnan S, Bhowmick PK, Nagarajan M, Vinod KK, Singh UD, Mohapatra T, Prabhu KV, Singh AK, Balyan H. Marker-assisted simultaneous but stepwise backcross breeding for pyramiding blast resistance genes Piz5 and Pi54 into an elite Basmati rice restorer line ‘PRR78’. Plant Breed. 2013;132:486–495.
- Buschges R, Hollricher K, Panstruga R, Simons G, Wolter M, Frijters A, van Daelen R, van der Lee T, Diergaarde P, Groenendijk J, Topsch S, Vos P, Salamini F, Schulze-Lefert P. The barley mlo gene: a novel control element of plant pathogen resistance. Cell. 1997;88:695–705.
- Wang GL, Mackill DJ, Bonman JM, McCouch SR, Champoux MC, Nelson RJ. RFLP mapping of genes conferring complete and partial resistance to blast in a durably resistant rice cultivar. Genetics. 1994;136:1421–1434.
- Qu S, Liu G, Zhou B, Bellizzi M, Zeng L, Dai L, Han B, Wang GL. The broad-spectrum blast resistance gene Pi9 encodes a nucleotide-binding site-leucine-rich repeat protein and is a member of a multigene family in rice. Genetics. 2006;172:1901–1914.
- Liu G, Lu G, Zeng L, Wang GL. Two broad-spectrum blast resistance genes, Pi9(t) and Pi2(t), are physically linked on rice chromosome 6. Mol Genet Genomics. 2002;267:472–480.
- Zhou B, Qu S, Liu G, Dolan M, Sakai H, Lu G, Bellizzi M, Wang GL. The eight amino-acid differences within three leucine-rich repeats between Pi2 and Piz-t resistance proteins determine the resistance specificity to Magnaporthe grisea. Mol Plant Microbe Interact. 2006;19:1216–1228.
- Deng Y, Zhu X, Shen Y, He Z. Genetic characterization and fine mapping of the blast resistance locus Pigm(t) tightly linked to Pi2 and Pi9 in a broad-spectrum resistant Chinese variety. Theor Appl Genet. 2006;113:705–713.
- Berruyer R, Adreit H, Milazzo J, Gaillard S, Berger A, Dioh W, Lebrun MH, Tharreau D. Identification and fine mapping of Pi33, the rice resistance gene corresponding to the Magnaporthe grisea avirulence gene ACE1. Theor Appl Genet. 2003;107:1139–1147.
- Hayashi K, Yasuda N, Fujita Y, Koizumi S, Yoshida H. Identification of the blast resistance gene Pit in rice cultivars using functional markers. Theor Appl Genet. 2010;121:1357–1367.
- Liu X, Yang Q, Lin F, Hua L, Wang C, Wang L, Pan Q. Identification and fine mapping of Pi39(t), a major gene conferring the broad-spectrum resistance to Magnaporthe oryzae. Mol Genet Genomics. 2007;278:403–410.
- Huang H, Huang L, Feng G, Wang S, Wang Y, Liu J, Jiang N, Yan W, Xu L, Sun P, Li Z, Pan S, Liu X, Xiao Y, Liu E, Dai L, Wang GL. Molecular mapping of the new blast resistance genes Pi47 and Pi48 in the durably resistant local rice cultivar Xiangzi 3150. Phytopathology. 2011;101:620–626.
- Li W, Lei CL, Cheng ZJ, Jia YL, Huang DY, Wang JL, Wang JK, Zhang X, Su N, Guo XP, Zhai HQ, Wan JM. Identification of SSR markers for a broad-spectrum blast resistance gene Pi20(t) for marker-assisted breeding. Mol Breed. 2008;22:141–149.
- Das A, Soubam D, Singh PK, Thakur S, Singh NK, Sharma TR. A novel blast resistance gene, Pi54rh cloned from wild species of rice, Oryza rhizomatis confers broad spectrum resistance to Magnaporthe oryzae. Funct Integr Genomics. 2012;12:215–228.
- Jeung JU, Kim BR, Cho YC, Han SS, Moon HP, Lee YT, Jena KK. A novel gene, Pi40(t), linked to the DNA markers derived from NBS-LRR motifs confers broad spectrum of blast resistance in rice. Theor Appl Genet. 2007;115:1163–1177.
- Liu Y, Liu B, Zhu X, Yang J, Bordeos A, Wang G, Leach JE, Leung H. Fine-mapping and molecular marker development for Pi56(t), a NBS-LRR gene conferring broad-spectrum resistance to Magnaporthe oryzae in rice. Theor Appl Genet. 2013;126:985–998.
- Terashima T, Fukuoka S, Saka N, Kudo S. Mapping of a blast field resistance gene Pi39(t) of elite rice strain Chubu 111. Plant Breed. 2008;127:485–489.
- Mackill DJ. Molecular markers and marker-assisted selection in rice. In: Varshney RK, Tuberosa R, editors. Genomics applications in crop improvement. 2. New York: Springer; 2007. p. 147–168.
- Iyer-Pascuzzi AS, McCouch SR. Functional markers for xa5-mediated resistance in rice (Oryza sativa, L.). Mol Breed. 2007;19:291–296.
- Andersen JR, Lübberstedt T. Functional markers in plants. Trends Plant Sci. 2003;8:554–560.
- Fjellstrom RG, Conaway-Bormans CA, McClung AM, Marchetti MA, Shank AR, Park WD. Development of DNA markers suitable for marker assisted selection of three Pi genes conferring resistance to multiple Pyricularia grisea pathotypes. Crop Sci. 2004;44:1790–1798.
- Wang Z, Jia Y, Rutger JN, Xia Y. Rapid survey for presence of a blast resistance gene Pi-ta in rice cultivars using the dominant DNA markers derived from portions of the Pi-ta gene. Plant Breed. 2007;126:36–42.
- Hayashi K, Yoshida H, Ashikawa I. Development of PCR-based allele-specific and InDel marker sets for nine rice blast resistance genes. Theor Appl Genet. 2006;113:251–260.
- Kumar GS, Kumari KA, Rani CVD, Sundaram RM, Vanisree S, Jamaloddin M, Swathi G. Study of simple sequence repeat (SSR) polymorphism for biotic stress resistance in elite rice variety JGL 1798. Afr J Biotechnol. 2013;12(40):5833–5838.
- Yuan B, Zhai C, Wang W, Zeng X, Xu X, Hu H, Lin F, Wang L, Pan Q. The Pik-p resistance to Magnaporthe oryzae in rice is mediated by a pair of closely linked CC-NBS-LRR genes. Theor Appl Genet. 2010;122:1017–1028.
- Zhai C, Lin F, Dong Z, He X, Yuan B, Zeng X, Wang L, Pan Q. The isolation and characterization of Pik, a rice blast resistance gene which emerged after rice domestication. New Phytol. 2011;189:321–334.
- McCouch SR, Teytelman L, Xu YB, Lobos K, Clare K, Walton M, Fu BY, Maghirang R, Li ZK, Xing YZ, Zhang QF, Kono I, Yano M, Fjellstrom R, DeClerck G, Schneider D, Cartinhour S, Ware D, Stein L. Development and mapping of 2240 new SSR markers for rice (Oryza sativa L.) (Supplement). DNA Res. 2002;9:257–279.
- Zhu ML, Wang L, Pan QH. Identification and characterization of a new blast resistance gene located on rice chromsome 1 through linkage and differential analysis. Phytopathology. 2004;94:515–519.
- Zhu XY, Yang JY, Liu JM, SiTu ZM, Kang JP, Hu XY, Zhu MJ, Luo SH, Yang QY, Zeng LX, Jiang XY, Chen S. Evaluation on resistance of rice varieties in Guangdong to rice blast and strategy for its utilization. Guangdong Agric Sci. 2006;5:34–37.
- IRRI. Standard evaluation system (SES). For rice. Manila: International Rice Research Institute; 1996. p. 56.
- Bai J, Pennill LA, Ning J, Lee S, Ramalingam J, Webb C, Zhao B, Sun Q. Diversity in nucleotide binding site-leucine rich repeat genes in cereals. Genome Res. 2002;12:1871–1884.
- Chen M, Presting G, Barbazuk WB, Goicoechea JL, Blackmon B, Fang G, Kim H, Frisch D, Yu Y, Sun S, Higingbottom S, Phimphilai J, Phimphilai D, Thurmond S, Gaudette B, Li P, Liu J, Hatfield J, Main D, Farrar K, Henderson C, Barnett L, Costa R, Williams B, Walser S, Atkins M, Hall C, Budiman MA, Tomkins JP, Luo M, Bancroft I, Salse J, Regad F, Mohapatra T, Singh NK, Tyagi AK, Soderlund C, Dean RA, Wing RA. An integrated physical and genetic map of the rice genome. Plant Cell. 2002;14:537–545. Epub 2002/03/23.
- Mohan M, Nair S, Bhagwat A, Krishna TG, Yano M, Bhatia CR, Sasaki T. Genome mapping, molecular markers and marker-assisted selection in crop plants. Mol Breed. 1997;3:87–103.
- Yang H, Renshaw D, Thomas G, Buirchell BJ, Sweetingham MW. A strategy to develop molecular markers applicable to a wide range of crosses for marker assisted selection in plant breeding: a case study on anthracnose disease resistance in lupin (Lupinus angustifolius L.). Mol Breed. 2008;21:473–483.
- Randhawa HS, Mutti JS, Kidwell K, Morris CF, Chen X, Gill KS. Rapid and targeted Introgression of Genes into popular wheat cultivars using marker-assisted background selection. PLoS ONE. 2009;4:e5752.
- Hospital F. Size of donor chromosome segments around introgressed loci and reduction of linkage drag in marker-assisted backcross programs. Genetics. 2001;158:1363–1379.
- Suh JP, Jeung JU, Noh TH, Cho YC, Park SH, Park HS, Shin MS, Kim CK, Jena KK. Development of breeding lines with three pyramided resistance genes that confer broad-spectrum bacterial blight resistance and their molecular analysis in rice. Rice. 2013;6:5.
- Sundaram RM, Vishnupriya MR, Biradar SK, Laha GS, Reddy GA, Rani NS, Sarma NP, Sonti RV. Marker assisted introgression of bacterial blight resistance in Samba Mahsuri, an elite indica rice variety. Euphytica. 2008;160:411–422.