?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The growing demand of plasmid DNA for gene therapy and third generation vaccines has encouraged the development of simple, robust and scalable production bioprocesses. Bearing in mind the purification step, the membrane separation processes may be a viable and cost-effective option that may be used without compromising the plasmid integrity. In this work, an operation of tangential flow ultrafiltration in a hollow-fiber module was used for the pre-purification of plasmid pVAX1-NH36 from clarified lysates of Escherichia coli DH5α culture. The ultrafiltration system was characterized using model solutions to determine the membrane resistance, plasmid concentration on the membrane wall and the system mass-transfer coefficient. In the analysis, a model based on Darcy's law and the stagnant film layer was used. These results allowed to estimate an optimal flux of 0.0016 cm/s and an optimal bulk concentration for the diafiltration process of 5.50 µg/mL. The concentration of lysates containing the plasmid of interest was performed by constant flow in batch mode achieving a 41% of impurities removal. These results suggest the use of a combination of concentration--diafiltration steps for the pre-purification of the plasmid lysates processed. The electrophoretic and high-performance liquid chromatography by hydrophobic interaction analysis showed that the plasmid supercoiled isoform maintained its integrity and no appreciable plasmid loss was present.
Nomenclature
| = | transmembrane pressure gradient, kg/cm2 | |
| = | inlet pressure, kg/cm2 | |
| = | outlet pressure, kg/cm2 | |
| = | filtrate pressure, kg/cm2 | |
| = | filtrate flux, cm3/(cm2·s) | |
| = | viscosity, kg/(m·s) | |
| = | membrane resistance, cm−1 | |
| = | solute resistance, cm−1 | |
| = | mass transfer coefficient, (cm3/cm2·s) | |
| = | gel polarization layer concentration, µg/mL | |
| = | bulk solution concentration, µg/mL | |
| = | maximum filtrate flux, cm3/(cm2·s) | |
| = | value of |
Introduction
Recently, a new generation of vaccines and plasmid DNA (pDNA) based treatments which are considered simpler and easier to use and produce have been developed.[Citation1] The growing demand for pDNA has led manufacturers to re-evaluate their production methodologies, obtaining downstream processes that satisfy the regulatory agencies' demands. Since the purification step is a key cost in the production of pDNA, it is necessary to reduce the volume and remove most impurities during the initial stages of the process to avoid overloads during the high-resolution step.
Currently, there are some options for pre-purification of pDNA at laboratory scale, such as aqueous two-phase systems and solvent precipitation which have shown good results for impurities removal and high yield recovery of pDNA. However, large amounts of solvents, alcohols or salts are used to accomplish these operations, increasing the production cost and the environmental impact. Recently, a new bioprocess to obtain purified pDNA from E. coli ferments was developed using hollow-fiber tangential flow filtration (TFF) and tandem anion-exchange membrane chromatography operating in frontal mode [Citation2]; the results of the study encourage a deeper analysis of the TFF operation.
Tangential flow filtration is a novel and promising technique for the recovery of pDNA from alkaline lysates since it is recognized as scalable, cost-effective and highly selective operation. TFF takes advantage of the size difference between pDNA and molecules in the lysate such as proteins, endotoxins and low molecular weight RNA. The feed flow during TFF is directed parallel to the membrane and thus perpendicular to the filtrate flow. This allows retentate species to be swept along the membrane surface and out of the device, significantly increasing the process flux compared to that obtained in normal flow filtration.[Citation3–5]
A hollow fibre module operating in TFF mode was used in this work for the pre-purification of plasmid pVAX1-NH36 from clarified lysates of Escherichia coli DH5α cultures. The system was characterized using model liquid solutions and the experimental data were analysed using phenomenological models in order to estimate the operation parameters. The pre-purification of lysates harbouring the plasmid of interest was conducted in batch-concentration mode using a constant permeate flow. The quality analysis of the plasmid was performed by gel electrophoresis and by hydrophobic interaction high-performance liquid chromatography (HIC-HPLC).
Materials and methods
Theoretical background
During TFF, the fluid to be filtered flows parallel to the membrane surface and permeates through the membrane due to a pressure difference known as transmembrane pressure gradient (∆TMP) given by:
(1)
(1) where Pi is the inlet pressure to the module, Po is the outlet pressure of the module and Pf is the filtrate pressure.
The resistance model based on Darcy's law describes the relation between ΔTMP and the permeate flux J.[Citation6] For macromolecules in solution, the relation is expressed as
(2)
(2) where µ is the viscosity of the solution, Rm is the membrane resistance and Rs is the solute polarization layer resistance.
A common model used for ultrafiltration systems is the gel layer model, which states that in macromolecular solutions the increase of the solute concentration on the membrane wall due to has a limit concentration where the solution forms a gel layer. The flux variation in this region is pressure independent and is described by the equation
(3)
(3) where ks is the mass-transfer coefficient and Cg is the limit gel layer concentration that is constant for a given system. It must be determined by the extrapolation to J = 0 on a plot of J in function of the natural logarithm of the bulk concentration, Cb.[Citation7]
Experimental system
The TFF system used was the MidJet Benchtop System (GE Healthcare, USA) with a tangential flow ultrafiltration membrane of 300 kDa, which has a max ΔTMP capacity of 1.5 kg/cm2, according to the manufacturer. The Pressure Display Unit (GE Healthcare, USA) monitored the pressure. Cole Palmer Flow Controller 600–6000 r/min (Cole Palmer, USA) maintained the feed flow rate constant during all experiments.
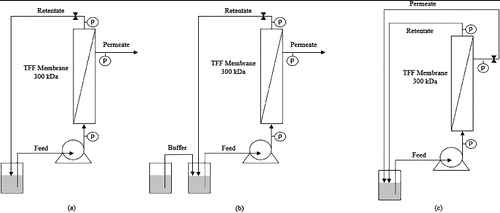
Experiments were performed using different configurations. In batch mode (), the entire volume of feed is contained within the recycle tank and the permeate flows out of the system. This batch configuration was used to determine the relationship between the permeate flux and ΔTMP during hydraulic filtration and to concentrate lysate solutions. Diafiltration () is an operation in which a buffer is added to the recycle tank simultaneously with the filtration. This mode is useful to perform a buffer exchange during the preparation of clarified lysates. In the total recirculation mode (), both the retentate and the permeate solutions are recycled to the feed tank, in order to maintain a constant concentration in the system. This mode was used to study the effect of process variables at constant plasmid concentration.
Membrane resistance determination
An experiment using deionized water was conducted at a constant feed rate of 25 mL/min at different ΔTMP in the range of 0–1.0 kg/cm2 for the determination of membrane resistance. The ΔTMP was fixed by a pinch valve on the retentate flow line. Different ΔTMP values within the range span permitted by the membrane were set and the corresponding permeate flux was calculated.
Clarified lysates preparation
E. coli DH5α cells harbouring the plasmid pVAX1-NH36 were fermented in a 2.0 L bioreactor (Applikon, the Netherlands) using defined medium with glycerol as the carbon source.[Citation8] Cells were harvested and disruption was performed by alkaline lysis based on the method of Birnboim and Doly [Citation3,Citation9]; briefly, cells were resuspended in 25 mmol/L tris(hydroxymethyl)aminomethane (Tris)–HCl, 50 mmol/L glucose and 10 mmol/L ethylenediaminetetraacetic acid (EDTA), (pH 8.0), at a ratio of 8 mL of buffer per 1 g of biomass. Alkaline lysates were prepared by adding 8 mL/g biomass of a 200 mmol/L NaOH, 1% (w/v) sodium dodecyl sulphate (SDS) solution on ice. Lysis was halted after 10 min by adding 8 mL/g biomass of a pre-chilled 3 mol/L potassium–5 mol/L acetate (pH 5.0) solution prepared with glacial acetic acid, potassium acetate and water. Cell debris, precipitated proteins and precipitated genomic DNA (gDNA) were removed by centrifugation at 6000 g on a Biofuge Stratos centrifuge (ThermoScientific, USA) and the resulting supernatant (≈24 mL/g biomass at 1.7 mol/L acetate with pH ∼ 5.2) was vacuum filtrated through a 0.45 µm membrane (Pall, USA) and used for further processing.
Plasmid concentration effect on TFF
A clarified lysate containing 4.96 µg/mL of plasmid was washed using a buffer solution of Tris 20 mmol/L pH 7.5 HCl in diafiltration mode (). The diafiltrated solution was used to obtain the solutions with plasmid concentration of 1.04, 3.81 and 4.96 µg/mL. In order to determine the effect of plasmid concentration on the permeate flux, 100 mL of each dilution were fed to the TFF system, using a total recirculation mode () with a constant recirculation rate of 25 mL/min. Different ΔTMP values within the range span permitted by the membrane were set and the corresponding permeate flux was calculated.
Batch concentration of clarified lysates at constant flux by TFF
Samples of 100 mL of clarified lysate were fed to the TFF system at a constant recirculation rate of 25 mL/min using a constant permeate rate of 0.5 mL/min. The solution was concentrated to a final volume of 50 mL. The performance of the operation was followed by determinations of plasmid concentration and electrophoretic analysis of samples of the feed, permeate and retentate solutions collected during the operation.
Agarose gel electrophoresis
Samples obtained during the process were analysed by horizontal electrophoresis in 0.8% agarose gels. Electrophoresis was carried out for 3 h at 60 V using tris–acetate–EDTA buffer (TAE: 40 mmol/L Tris base, 20 mmol/L acetic acid and 1 mmol/L EDTA, pH 8.0) as the running buffer. Gels were stained with ethidium bromide (0.5 µg/mL) after running and visualized under ultraviolet light.
Hydrophobic interaction HPLC
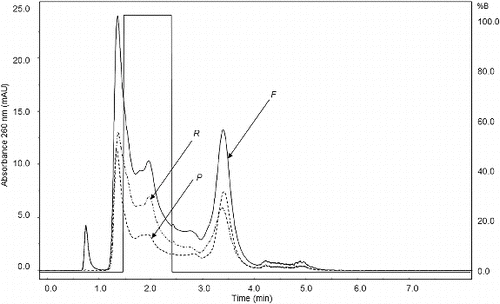
Plasmid concentration was determined by HIC-HPLC, as described previously [Citation10]. Briefly, a 10 cm × 4.6 cm HIC Source 15 PHE PE column (phenyl ligand, polystyrene/divinylbenzene matrix) was connected to an Akta Purifier 10UPC system (GE Healthcare, USA) and equilibrated with 1.5 mol/L ammonium sulphate in Tris–HCl 10 mmol/L, pH 8.0. Samples (30 µL volume of diluted sample in equilibration buffer) were injected and eluted at 1 mL/min. After injecting the sample, the column was washed for 1.4 min with the same buffer used for equilibration. The concentration of ammonium sulphate was then instantaneously decreased to zero (no ammonium sulphate) by eluting the column with 10 mmol/L Tris–HCl (pH 8.0). This condition was maintained during the next 0.7 min in order to elute bound species. After this period, the column was re-equilibrated with 1.5 mol/L ammonium sulphate in 10 mmol/L Tris (pH 8.0) for 5.5 min. All pDNA isoforms (supercoiled, open circular and linear) were eluted in the flow through, with a retention time around 0.7 min, whereas bound impurities were eluted as a series of peaks with retention times between 1.0 and 4.5 min. The absorbance at 260 nm and the conductivity (which is directly related to the ammonium concentration) of the eluate were continuously recorded. The plasmid concentration was quantified using a calibration curve constructed with standards of the plasmid prepared in the 0–50 µg/mL concentration range. HPLC purity degree was defined as the percentage of the plasmid peak area when compared with the total area of all peaks on the chromatogram. The percentage of impurities absorbing at 260 nm that were not removed by the TFF procedure was also estimated on the basis of the non-pDNA peaks in the HIC-HPLC chromatogram, by calculating the ratio of the corresponding areas after and before TFF.
Results and discussion
Membrane resistance determination
The flux variation with the ΔTMP using deionized water as a model solution showed a linear behaviour. The model given by EquationEquation (2)(2)
(2) with Rs = 0 was adjusted to the experimental data obtaining the expression
with R2 = 0.9854. The membrane resistance was determined from the resulting line slope Rm = 16.9 × 109 cm−1. This parameter is useful to keep track of cleaning methodologies and membrane life determination. In our work, before each new experiment, the correct membrane resistance was verified.
Plasmid concentration effect on TFF
The variation of the permeate flux with the ΔTMP at different plasmid solution concentrations is shown in . In order to analyse the experimental data, EquationEquation (2)(2)
(2) was modified to the following form:
(4)
(4) where
is the maximum flux for a given system,
is the value of
when
.
Figure 2. J variation as a function of ∆TMP. Plasmid concentrations: (■) 1.04 µg/mL, (▴) 3.81 µg/mL and (•) 4.69 µg/mL.
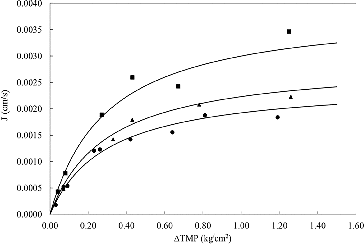
The continuous lines in represent the adjustment of the model to the experimental data and the model parameters obtained for each experiment are shown in . The parameters of the model were used to obtain where the variation of flux as a function of the natural logarithm of bulk concentration (Cb) is shown.
Table 1. Parameters for the ultrafiltration model.
Figure 3. J variation as a function of ln(Cb). (•) 0.50 kg/cm2, (■) 0.75 kg/cm2, (♦)1.00 kg/cm2, (▴) 1.25 kg/cm2 and (×) 1.50 kg/cm2.
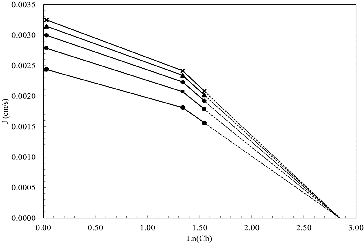
To determine the limit concentration on the membrane wall, it is necessary to extrapolate the value of on a graph as shown in . The slope of the line on the gel formation zone is equal to the mass transfer coefficient ks. When the correspondent analysis was performed, the expression obtained describes the flux variation on the pressure-independent zone as a function of the bulk concentration, according to EquationEquation (3)
(3)
(3) ,
.
Ng et al. [Citation11] demonstrated that the stagnant film model predicts an optimum bulk concentration and an optimal flux for diafiltration given, respectively, by: and
.
These results suggest that, for the experimental system studied, a concentration operation should be conducted prior to diafiltration. Using a bulk concentration of 5.50 µg/mL minimizes the diafiltration volumes needed to wash the clarified solution without increasing the time needed to perform the operation. The optimal can be calculated as the value of
when J = J*.
Batch concentration of clarified lysates at constant flux by TFF
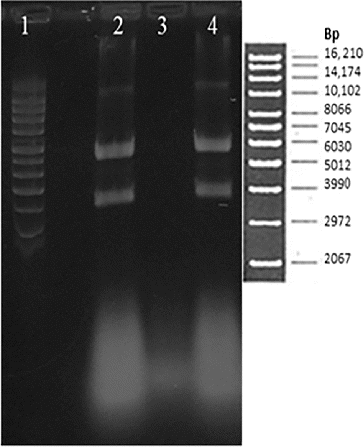
The analysis of the plasmid solutions by gel electrophoresis of the samples taken in the concentration operation is shown in . Lane 2 shows that the feed sample contains the supercoiled isoform of the plasmid of interest. In lane 3, it is observed that pDNA is not present in the permeate, suggesting that there was 100% plasmid rejection by the membrane and that part of RNA impurities permeated out of the system during the operation. Lane 4, which corresponds to the retentate solution, shows that the supercoiled isoform of the plasmid is maintained within the system; additionally, some of the RNA impurities are also maintained. It can also be observed in lanes 2 and 4 that a relaxed isoform of pDNA is present. However, the corresponding amounts of this isoform do not change with the pre-purification procedure.
Figure 4. Electrophoresis gel. Lane 1: molecular weight marker; Lane 2: feed solution 2X; Lane 3: permeate solution; and Lane 4: retentate solution.
The chromatograms of the analysed solutions shown in confirmed and extended the information obtained by the electrophoresis analysis. The solution purity increased approximately twofold and the results indicated total retention of the pDNA supercoiled isoform, since in the permeate solution chromatogram, no pDNA peak was present.
shows the peak integration area for each solution. During the operation, 41% of the impurities were removed by the membrane. In spite of the size difference of the pDNA and RNA molecules, the separation of these was not total. This might be attributed to the portion of RNA that is adsorbed to the gel layer on the membrane wall.[Citation3].
Table 2. Peak integration area for the concentration operation.
The obtained results offer a better understanding of membrane-based technology for the pre-purification of pDNA from clarified E. coli lysates. One of the main characteristics of the developed process is that it avoids the use of precipitating agents, frequently used in large quantities in the recovery steps of pDNA production processes. The technique can be coupled to a purification step using anion-exchange membrane chromatography, configured in ready-to-use devices, which can further simplify large-scale plasmid purification.
Conclusions
In this work, concentration and pre-purification by TFF of plasmid pVAX1-NH36 from E. coli DH5α lysates was achieved. The phenomenological models used to describe the variation of the permeate flux during ultrafiltration due to the TMP allowed to determine the system scaling parameters, the membrane resistance, plasmid concentration on the membrane wall and the system mass-transfer coefficient. These parameters permitted to establish the optimal operation conditions for diafiltration of lysate solutions. The optimal flux value obtained fitted within the range span permitted by the membrane and the pressure gradient was close to the boundary value between the gel formation and the pressure-independent zone. These results suggest that the solution of plasmid must be concentrated prior to its diafiltration. The quality analysis indicated that the TFF operation eliminates a considerable amount of impurities prior to high-resolution steps. To increase the production capacity, the process can be scaled using a larger membrane or multiple units in a combination of parallel and series flow configurations. This approach could, thus, allow efficient high-throughput capture of pDNA on the kilogram scale.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Prazeres DM, Monteiro GA, Ferreira GN, Diogo MM, Ribeiro SC, Cabral JM. Purification of plasmids for gene therapy and DNA vaccination. Biotechnol Annu Rev. 2001;7:1–30.
- Guerrero-Germán P, Prazeres DMF, Guzmán R, Montesinos-Cisneros RM, Tejeda-Mansir A. Purification of plasmid DNA using tangential flow filtration and tandem anion-exchange membrane chromatography. Bioprocess Biosyst Eng. 2009;32(5):615–623.
- Freitas S, Canario S, Santos JAL, Prazeres DMF. Alternatives for the intermediate recovery of plasmid DNA: performance, economic viability and environmental impact. Biotechol J. 2009;4:265–278.
- van Reis R, Zydney A. Bioprocess membrane technology. J Membrane Sci. 2007;297:16–50.
- Charcosset C. Membrane processes in biotechnologies and pharmaceutics. Amsterdam: Elsevier; 2012.
- Meireles M, Lavoute E, Bacchin P. Filtration of a bacterial fermentation broth: harvest conditions effects on cake hydraulic resistance. Bioprocess Biosyst Eng. 2003;25:309–314.
- Tejeda-Mansir A, Montesinos-Cisneros RM, Guzmán R. [Bioseparaciones]. 2nd ed. Hermosillo: Editorial UniSon-PEARSON; 2011. Spanish.
- Bohle K, Ross A. Plasmid DNA production for pharmaceutical use: role of specific growth rate and impact on process design. Biotechnol Bioeng. 2011;108:2099–2106.
- Birnboim HC, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523.
- Diogo MM, Queiroz JA, Prazeres DMF. Assessment of purity and quantification of plasmid DNA in process solutions using high-performance hydrophobic interaction chromatography. J Chromatogr A. 2003;998:109–117.
- Ng P, Lundblat J, Mitra G. Optimization of solute separation by diafiltration. Sep Sci Technol. 1976;11:499–502.