Abstract
The chemical composition of hyssop oil from Bulgaria was determined by gas chromatography with flame ionization detection and gas chromatography–mass spectrometry on two different chromatographic columns. The quantity of identified compounds was shown correspond to 97.2% and 98% of the total oil content. Among the detected compounds, cis-pinocamphone (48.98%–50.77%), β-pinene (13.38%–13.54%), trans-pinocamphone (5.78%–5.94%) and β-phellandrene (4.44%–5.17%) were the major compounds. Hyssop oil demonstrated antifungal activity against 52 clinical isolates and reference strains of Candida albicans, Candida glabrata, Candida tropicalis, Candida parapsilosis and Candida krusei. The essential oil characterized with stronger antifungal activity in comparison with pure cis- and trans-pinocamphone, α- and β-pinene and β-phellandrene. Essential oil of Hyssopus officinalis L. from Bulgaria inhibited both fluconazol-sensitive and fluconazol-resistant strains.
Introduction
Genus Candida comprises about 280 species and is listed in order Cryptococcales. Candida species are causative microorganisms of vulvovaginal, oropharyngeal and skin infections.[Citation1–3] They are responsible for some of the most common nosocomial bloodstream infections with a high mortality rate. The possibility to grow in two forms, unicellular and filamentous, to adhere to the mucosal tissues and to produce extracellular enzymes such as lipases and proteases are the major virulence factors of the most prominent species, Candida albicans.[Citation4–8] During the last decades, a constantly increasing number of fungal diseases caused by resistant strains of different Candida species, especially non-albicans Candida species (NAC), is observed.[Citation9–14] Insufficient effectiveness of some azole preparations and higher toxicity of polyene antibiotics stimulates the search for new natural antifungal compounds.
One of the most promising natural alternatives to traditional antifungal preparations are essential oils from medicinal plants because they fulfill to a larger extent of the requirements for specific mechanism of action at relatively lower concentrations without induction of resistance. A traditional medicinal plant widely distributed in the East Mediterranean to Central Asia is Hyssopus officinalis L.[Citation15,Citation16] The extracts and essential oil from hyssop demonstrate antimicrobial, antiviral, antitumor, antioxidant and other biological activities.[Citation17–19]
Analysis of hyssop oil from Italy, Spain, Himalaya, Egypt, Turkey, Serbia, Romania, France and Iran by gas chromatography (GC) and gas chromatography–mass spectrometry (GC/MS) led to the identification of pinocamphone, iso-pinocamphone, β-pinene, 1,8-cineole, camphor, β-caryophyllene, linalool and myrtenyl acetate as major constituents of the oil.[Citation17,Citation19–27] There are, however, notable differences in the qualitative and quantitative composition of hyssop oil from different geographic regions with seasonal and technological fluctuations.[Citation27–30]
Among the biological activities of hyssop essential oil, its antimicrobial activity against pathogenic and spoilage bacteria has been most intensively studied.[Citation17–19,Citation31,Citation32] Most reports about the antifungal activity of hyssop oil are focused on its inhibitory effect against phytopathogenic and mycotoxin-producing fungi.[Citation20,Citation33–35] In contrast, there are only scarce studies about its anticandidal activity. To the best of our knowledge, the anticandidal activity of hyssop oil has mostly been determined against reference strains of C. albicans, but less is known about its activity against clinical isolates, especially against NAC species. For example, Mazzanti et al. [Citation31] and Kizil et al. [Citation19] reported strong antimicrobial activity of essential oil from H. officinalis L. against C. albicans, C. tropicalis and C. krusei. On the contrary, according to other authors, hyssop oil demonstrates only moderate to weak antifungal activity.[Citation26,Citation36,Citation37]
In general, the available data about the antimicrobial activity of essential oil from H. officinalis L., and particularly its anticandidal activity, are to some extent controversial. Furthermore, little is known about the probable mechanism of antimicrobial action of hyssop oil against clinical isolates of Candida species.
The aim of present study was to determine the chemical composition and antifungal activity of essential oil of H. officinalis L. from Bulgaria against clinical isolates of different Candida species and to attempt to elucidate the probable mechanism of its anticandidal action.
Materials and methods
Essential oil sample
The essential oil of H. officinalis L. used in this study was a commercial sample produced by steam distillation in industrial conditions and was purchased from Vigalex Ltd. (Gurkovo, Bulgaria).
Gas chromatography analysis
The essential oil sample was subjected to GC analysis, with a 0.5 μL plunger-in-needle syringe at a very high split rate. GC with flame ionization detection (GC/FID) and GC/MS analysis were carried out simultaneously using a Finnigan Thermo Quest Trace GC with a dual split/splitless injector, a FID detector and a Finnigan Automass quadrupole mass spectrometer (Thermo Quest, Manchester, UK). One inlet was connected to a 50 m × 0.25 mm × 1.0 μm SE-54 (5% diphenyl, 1% vinyl-, 94% dimethylpolysiloxane) fused silica column (CS Chromatographie Service, Germany), the other injector was coupled to a 60 m × 0.25 mm × 0.25 μm Carbowax 20M (polyethylene glycol) column (J&W Scientific, USA). The two columns were connected at the outlet with a quartz Y connector and the combined effluents of the columns were split simultaneously to the FID and MS detector with a short (ca. 50 cm) 0.1 mm internal diameter (ID) fused silica restrictor column as a GS/MS interface. The carrier gas was helium 5.0 with a constant flow rate of 1.5 mL·min−1; the injector temperature was 230 °C; the FID detector temperature, 250 °C; GC/MS interface heating, 250 °C; ion source at 150 °C; EI mode at 70 eV; scan range 40–300 amu. The applied temperature program was as follows: 46 °C for 1 min to 100 °C at a rate of 5 °C·min−1; 100–230 °C at 2 °C·min−1; 230 °C for 13.2 min. Identification was performed using Finnigan XCalibur 1.2 software (Thermo Scientific Inc.) with MS correlations through the US National Institute of Standards and Technology, [Citation38] Adams essential oil, [Citation39] MassFinder, literature data [Citation40–42] and our own library. Retention indices of reference compounds and those from literature data were used to confirm peak data. Quantification was achieved through peak area calculations of the FID chromatogram.
Test micro-organisms
To evaluate the antifungal activity of hyssop essential oil, the following strains were used as test cultures: 28 strains of C. albicans (reference strain ATCC 10231 and 27 clinical isolates); eight strains of C. glabrata (reference strain ATCC 90030 and seven clinical isolates); six strains of C. tropicalis (reference strain NBIMCC 23 and five clinical isolates); six strains of C. parapsilosis (reference strain ATCC 22019 and five clinical isolates) and four C. krusei clinical isolates. The strains were purchased from National Reference Laboratory of Mycology at the National Center of Infectious and Parasitic Diseases, Sofia; Department of Microbiology and Immunology at Medical University of Plovdiv and Clinical laboratory Chronolab Ltd., Plovdiv. All the clinical isolates were identified to the level of species in these laboratories and were kindly provided to the Department of Biochemistry and Microbiology, Faculty of Biology at the University of Plovdiv (Bulgaria). The strains were maintained on Sabouraud Dextrose Agar with chloramphenicol (SDA; HiMedia, India) at 4 °C and were deposited in the Department's microbial collection.
Antimicrobial testing
Antimicrobial testing of essential oil was performed according to Clinical Laboratory Standard Institute (CLSI) M27-A3 Reference Serial Broth Microdilution Method.[Citation43] A stock solution was prepared by diluting the essential oil sample in dimethyl sulfoxide (DMSO; Sigma-Aldrcih, Co). Serial twofold dilutions of the stock solution were prepared in RPMI1640 broth medium buffered to pH 7.0 with 0.165 mol·L−1 3-N-morpholinopropanesulfonic acid (MOPS buffer, Sigma-Aldrich, Co) to reach final concentrations of the oil ranging from 2048 to 1 µg·mL−1 and were distributed in 96 wells microtitration plates. The final concentration of DMSO did not exceed 1% (v/v) and did not influence the growth of yeasts. Control samples of inoculated broth medium with and without solvent were also incubated under the same conditions. Each well was inoculated with 0.1 mL inoculum suspension (0.5 × 103–2.5 × 103 cfu·mL−1) prepared according to CLSI M27-A3.[Citation43] After 48 h incubation at 35 °C, microbial growth was evaluated visually and the minimal inhibitory concentration (MIC) was determined. MIC was defined as the lowest concentration at which total inhibition of microbial growth was detected. MIC was presented as an average value of the MICs detected for separate strains within the species. MICs of fluconazole (FLC strip, 0.016–256 μg·mL−1) and amphotericin B (AP strip, 0.002–32 μg·mL−1) were also determined by HiComb™ MIC Test (HiMedia, India), according to manufacturer's instructions. To determine the minimal fungicidal concentration (MFC) of the essential oil, 0.1 mL of each dilution showing no growth was spread on Potato Dextrose Agar (PDA, HiMedia, India). The inoculated Petri dishes were incubated at 35 °C for 48 h. The colony-forming units were counted and compared with control samples. MFC was defined as the lowest concentration that killed more than 99.9% of the initial inoculum. MFC was presented as an average value of the MFCs detected for different strains within the species.
Time–kill test
Hyssop oil treatments were prepared in 1 mL volumes at twice the desired final concentration in phosphate buffered saline (PBS) by using stock oil solution. Controls contained PBS with relevant concentration of DMSO without essential oil. Test solutions and controls were inoculated with 1 mL yeast working suspension (2×106–4 × 106 cfu·mL−1) and 0.1 mL sample was taken immediately from the controls for viability counts. Test solutions and controls were incubated at 35 °C with shaking at 120 r·min−1 on a rotary laboratory shaker. Samples were taken at 2, 4, 6, 8 and 10 h for viability scoring.
Methylene blue dye inclusion test
Hyssop oil treatments were prepared in 1 mL volumes at twice the desired final concentration in PBS by using stock oil solution. Controls contained PBS with relevant concentration of DMSO without essential oil. Test solutions and controls were inoculated with 1 mL yeast working suspension (2 × 106–4 × 106 cfu·mL−1) and were incubated at 35 °C with shaking at 120 r·min−1 on a laboratory rotary shaker. Samples of 0.08 mL were taken at 0, 2, 4, 6, 8 and 10 h and mixed by vortexing with 0.02 mL 0.05% methylene blue solution. The samples were left for 5 min at room temperature. A native mount was prepared and the number of cells stained in blue was scored by observing about 200 cells in at least 10 different visual fields with an optical microscope (Olympus CX21). The percentage of blue stained cells was calculated.
Acidification of the external medium
The external medium acidification after addition of glucose in the presence of hyssop oil was performed as described by Lunde and Kubo [Citation44] with minor modifications. Amounts of essential oil stock solution were added to aliquots of yeast working suspension (2 × 106–4 × 106 cfu·mL−1) to reach the desired final oil concentrations. Controls containing PBS with relevant concentration of DMSO without essential oil were also prepared. The samples were incubated for 10 min at room temperature and then 1 mL of 20% glucose solution was added to the samples to a final glucose concentration of 2%. After glucose addition, the samples were mixed by vortexing for 20 s. The treatments and control samples were incubated at room temperature and the pH values of the samples were determined potentiometrically (pH-meter, VWR) at 10, 30, 60, 90 and 120 min.
Data analysis
Each test was performed in triplicate and the results are expressed as means ± standard deviation
Analysis of variance was performed with Statistica V10 (StatSoft).
Results and discussion
Chemical composition
The chemical composition of essential oil from H. officinalis L. growing in Bulgaria was analysed using GC/FID and GC/MS. For better separation and identification of the chemical constituents of the oil two different columns were used. Sixty-six constituents were identified by the first column (SE-54), representing 97.2% of the total oil content. Fifty-five constituents were identified by the second column (CW20M), representing 98% of the total oil content. Between 21 and 46 different compounds have been identified in hyssop essential oils from various geographic regions and reported by other authors.[Citation17–20,Citation24–27] Thus, in comparison with other hyssop oils, the studied oil sample from Bulgaria was characterized with a highly varied and complex chemical composition ().
Table 1. Chemical composition of hyssop essential oil from Bulgaria.
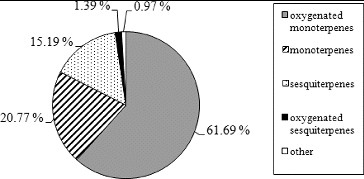
The major components detected by both columns were as follows: cis-pinocamphone (48.98%–50.77%), β-pinene (13.38%–13.54%), trans-pinocamphone (5.78%–5.94%) and β-phellandrene (4.44%–5.17%). In the literature, cis-pinocamphone, trans-pinocamphone, β-pinene, β-phellandrene and pinocarvone are reported as the most abundant constituents of hyssop oil.[Citation17,Citation19,Citation21–27] The obtained results show that the oil sample analyzed in this study belongs to the group of hyssop oils rich in cis-pinocamphone. The distribution of identified chemical compounds into groups as mean percentages of both columns is shown in .
Oxygenated monoterpenes were the major group, representing 61.69% of the total oil content, followed by monoterpene hydrocarbons (20.77%), sesquiterpene hydrocarbons (15.19%), oxygenated sesquiterpenes (1.39%), phenylpropanoids and other compounds (0.97%). These results are in accordance with the chemical composition of hyssop oil from the Balkan Peninsula reported by Glamočlija et al. [Citation22]
Antifungal activity
MIC and MFC values
The antifungal activity of Bulgarian hyssop oil was tested against 52 strains belonging to five species of genus Candida by the serial broth dilution method. The results from the MIC and MFC tests are shown in .
Table 2. Antifungal activity of hyssop essential oil from Bulgaria and of fluconazole and amphotericin B against clinical isolates of Candida species.
Five C. albicans strains, three C. glabrata strains, one C. tropicalis strain, two C. parapsilosis strains and four C. krusei strains were resistant to fluconazole, which corresponds to 28.8% of the tested strains and 32.7% of the strains were sensitive in a dose-dependent manner. All of the resistant strains were from the group of clinical isolates. None of the 52 tested strains was resistant to amphotericin B, but 40.4% of the strains were sensitive (dose dependent). The relatively high percentage of fluconazole resistance and some disadvantages of polyene antibiotics [Citation45,Citation46] illustrate the need for new alternative preparations with different mechanisms of anticandidal activity without causing resistance.
The studied hyssop essential oil demonstrated antifungal activity against all of the clinical isolates and reference strains from genus Candida. The strains of C. albicans were most sensitive to the essential oil, followed by these belonging to C. krusei, C. parapsilosis and C. tropicalis. The C. glabrata strains were least sensitive to hyssop oil of all the studied strains.
The MIC values of hyssop oil from Bulgaria (128–1024 μg·mL−1) were much higher in comparison with the MIC values of fluconazole (0.5–128 μg·mL−1) and amphotericin B (0.06–2 μg·mL−1). The major advantage of hyssop oil is that it is active against fluconazole-resistant strains, which suggests that its mechanism of anticandidal action probably differs from that of azoles.
In comparison with the MIC values of hyssop oils from various geographic regions,[Citation12,Citation26,Citation31] the studied Bulgarian hyssop oil demonstrated stronger inhibitory activity against medically important representatives of C. albicans, C. tropicalis and C. krusei. This fact could be explained by the more complex chemical composition of the hyssop oil from Bulgaria.
To clarify the influence of the chemical composition of hyssop oil on its anticandidal activity, additional experiments are a must. For this purpose, the inhibitory action of the major oil constituents and their isomers, such as cis- and trans-pinocamphone, α- and β-pinene and β-phellandrene, were tested against 52 strains of five species of genus Candida. The obtained results for the anticandidal action of pure compounds are shown in .
Figure 2. Antifungal activity of hyssop oil and some major oil constituents against clinical isolates of Candida species.
Note: 1 – hyssop oil, 2 – α-pinene, 3 – β-pinene, 4 – trans-pinocamphone, 5 – cis-pinocamphone, 6 – β-phellandrene.
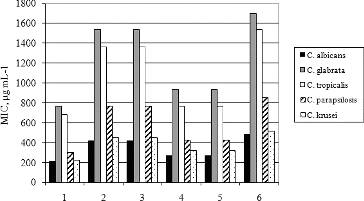
The hyssop oil demonstrated stronger anticandidal activity in comparison with pure compounds. The MIC values of essential oil varied between 210.3 ± 62.3 μg·mL−1 against the most sensitive species, C. albicans, and 768 ± 280.4 μg mL−1 against the most resistant species, C. glabrata. The MIC values of α- and β-pinene were approximately two times higher than the MIC values of the essential oil against the same Candida species. The MIC values of β-phellandrene against C. albicans and C. glabrata were about 2.3 times higher in comparison with those of the hyssop oil. The most active constituent of the essential oil among the tested pure compounds was pinocamphone. Its MIC values were 28% and 21% higher than those of hyssop oil against C. albicans and C. glabrata, respectively. Taken together, the obtained results suggest that the overall anticandidal activity of the studied hyssop oil from Bulgaria is probably due to the synergic action of more than one compound. The MIC values of cis- and trans-pinocamphone against the used microbial strains were the same, as well as those of α- and β-pinene. These equal MIC values indicate that isomerization does not influence the anticandidal activity of monoterpenes.
Time–kill curves
Another criterion for estimation of the antimicrobial activity of essential oils is the time–kill curve. The time–kill curves of the most sensitive clinical isolate of C. albicans and the most resistant strain of C. glabrata treated with hyssop essential oil at concentrations equal to MIC (256 and 1024 μg·mL−1, respectively) are presented in .
Figure 3. Time–kill curves of hyssop oil against clinical isolates of C. albicans and C. glabrata
Note: C – controls.
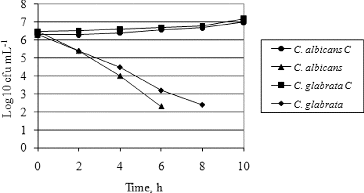
The results showed that on the sixth hour of oil treatment the total count of viable cells of C. albicans was between 1 × 102 and 3.2 × 102 cfu·mL−1 and after the sixth hour no viable cells were detected. The C. glabrata strain was more resistant and it took 8 h of treatment for the total count of viable cells to decrease to the level of C. albicans (2.5 × 102 and 3.2 × 102 cfu·mL−1).
Methylene blue dye inclusion
Methods based on the absorption of methylene blue and fluorescent dyes are among the most widely used techniques for studying the influence of different compounds on the cell membrane permeability.[Citation47–49] In our efforts to clarify the probable mechanism of antifungal action of hyssop oil, methylene blue absorption by fluconazol-sensitive and fluconazole-resistant strains of C. albicans and C. glabrata treated with essential oil at concentrations equal to MIC, 50% of MIC and 25% of MIC were carried out. The obtained results are shown in .
Figure 4. Absorption of methylene blue by fluconazole-sensitive (FS) and fluconazole-resistant (FR) strains of C. albicans (a) and C. glabrata (b) treated with hyssop oil.
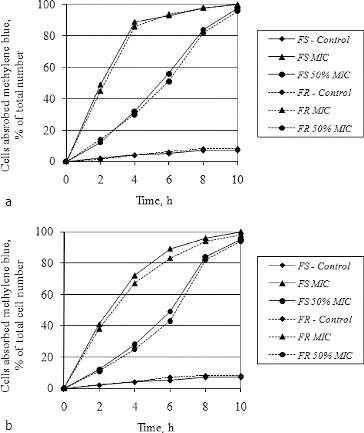
When treated with essential oil at a concentration equal to MIC (256 μg·mL−1), about 98% of the yeast cells from both C. albicans strains absorbed methylene blue on the eighth hour of treatment ((a)). This indicates that the hyssop oil affected the yeast membrane permeability of both the fluconasole-sensitive and the fluconazole-resistant strain. When C. albicans cells were treated with essential oil at a concentration equal to 50% of MIC (128 μg·mL−1), 97% of the cells absorbed methylene blue solution, but on the 10th hour. These results indicate that the hyssop oil affected the permeability of the yeast membrane in a dose-dependent mechanism. The same trends were observed when both strains of C. glabrata were treated with essential oil at a concentration equal to MIC (1024 μg mL−1) ((b)). In both species, the difference between the total percentages of stained cells in the fluconazol-sensitive and the fluconazol-resistant strains was non-significant, suggesting that the mechanism of anticandidal action of hyssop oil differs from that of azoles.
Effect on membrane ATPase activity
The transport of low molecule substances like glucose from the nutritive medium through the cell membrane is carried out by membrane ATPase (adenosine triphosphatase), which effluxes protons from the microbial cell and in this way decreases the pH of the cultural medium.[Citation43] According to Chambel et al. [Citation50], lipophilic compounds inhibit membrane ATPase and disrupt the normal transport through the cell membrane. To verify whether the hyssop oil can attack the membrane ATPase of Candida yeasts, the dynamics of pH variation of microbial suspensions of fluconazole-resistant strains of C. albicans and C. glabrata was studied in the presence of 2% of glucose (). These experiments were carried out only with fluconazole-resistant strains, since there were no significant differences between the antifungal action of hyssop oil against fluconazole-sensitive and fluconazole-resistant strains (see above).
Figure 5. Dynamics of pH changes in the cultural broth of C. albicans and C. glabrata treated with hyssop oil.
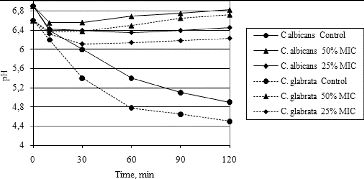
The pH of the culture medium in the control (untreated) samples of C. albicans and C. glabrata () decreased for 120 min from 6.9 to 4.9 and from 6.6 to 4.5, respectively. The final pH value of the culture broth of both strains treated with hyssop oil at concentrations equal to 50% of MIC (128 and 512 μg·mL−1 for C. albicans and C. glabrata, respectively) was 6.82 for C. albicans and 6.72 for C. glabrata. Treatment of both strains with hyssop oil at concentrations equal to 25% of MIC (64 μg·mL−1) also caused an increase in the final pH value in comparison with the untreated samples, but to a lesser extent. Thus, the effect of essential oil on pH variation was dose-dependent. The obtained results indirectly support the assumption that hyssop oil not only increases membrane permeability, but also inhibits membrane ATPase. To prove the exact mechanism of anticandidal action of hyssop oil, additional experiments need to be carried out. Our results could be explained by the fact that essential oils could cause a decrease in cell ATP and in this way could disrupt the normal function of membrane ATPase.[Citation46] This suggestion is also in agreement with other reports that essential oils, such as oregano and tea tree oil, pure geraniol and thymol, increase the membrane permeability and inhibit membrane enzymes.[Citation51–54]
Conclusions
The studied Bulgarian hyssop oil demonstrated antifungal activity against clinical isolates of five different species of genus Candida. The obtained results indicated that the inhibitory action of the oil is due to its complex chemical composition and synergic action of compounds such as cis- and trans-pinocamphone and α- and β-pinene. The major advantage of hyssop oil from Bulgaria in comparison with azoles is that it is active against both fluconazole-sensitive and fluconazole-resistant clinical Candida spp. isolates. The mechanism of anticandidal action of hyssop oil could possibly be due to causing an increase in yeast membrane permeability and disrupting the normal membrane transport by affecting membrane ATPase.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ostrosky-Zeichner L, Rex JH, Pappas PG, Hamill RJ, Larsen RA, Horowitz HW, Powderly WG, Hyslop N, Kauffman CA, Cleary J, Mangino JE, Lee J. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob Agents Chemother. 2003;47:3149–3154.
- Posteraro B, Torelli R, De Carlos E, Posteraro P, Sanduinetti M. Antifungal susceptibility testing: current role from the clinical laboratory perspective. Mediterr J Hematol Infect Dis. 2014;6:e2014030.
- Vázquez-Conzález D, Persguía-Ortiz AM, Hundeiker M, Bonifaz A. Opportunistic yeast in infections: candidiasis, cryptococcosis, trichosporonosis and geotrichosis. J Dtsch Dermatol Ges. 2013;11:381–395.
- De Azevedo I, Semprebom AM, Baboni FB, Rosa RT, MacHado MAN, Samaranayake LP, Rosa EAR. Low virulent oral Candida albicans strains isolated from smokers. Arch Oral Biol. 2012;57:148–153.
- Fu Y, Luo G, Spellberg B, Edwards J, Ibrahim A. Gene overexpression/suppression analysis of candidate virulence factors of Candida albicans. Eukar Cell. 2008;7:483–492.
- Kosalec I, Pepeljnjak S, Matica B, Jarža-Davila N. Virulence factors of yeast Candida albicans. Farm Glas. 2005;61:381–396.
- Ramesh N, Priyadharsini M, Sumathi CS, Balasubramanian V, Hemapriya J, Kannan R. Virulence factors and antifungal sensitivity pattern of Candida spp. isolated from HIV and TB patients. Indian J Microbiol. 2011;51:273–278.
- Yang YL. Virulence factors of Candida species. J Microbiol Immunol Infect. 2003;36:223–228.
- Amar CS, Ashish J, Hajare V, Sreekantha, Yogesh B, Vinod Ch. Study of prevalence and antifungal susceptibility of Candida. Int J Pharma Bio Sci. 2013;4:361–381.
- Carvalhinho S, Costa AM, Coelho AC, Martins E, Sampaio A. Susceptibilities of Candida albicans mouth isolates to antifungal agents, essential oils and mouth rinses. Mycopathologia. 2012;174:69–76.
- Jerez-Puebla E, Fernández M, Illnait T, Perurena R, Rodríguez I, Martínez G. In vitro susceptilidad de Cаndida spp. аisladas de la cavidad oral de pacientes VIH/sida a itraconazol, clotrimazol y ketoconazol [In vitro susceptibility of Candida spp. isolated from the oral cavity of HIV/AIDS patients to itraconazole, clotrimazole and ketoconazole]. Arch Venez Farmacol Terap. 2012;31:80–84. Spanish.
- Mahmoudabadi AZ, Zarrin M, Fard MB. Antifungal susceptibility of Candida species isolated from Candiduria. Jundishapur J Microbiol. 2013;6:24–48.
- Marchaim D, Lemanek L, Bheemreddy S, Kaye KS, Sobel JD. Fluconazol-resistent Candida albicans vulvovaginitis. Obstet Gynecol. 2012;120:1407–1414.
- Mulu A, Kassu A, Anagaw B, Moges B, Gelaw B, Alemayehu M, Belyhun Y, Biadglegne F, Hurissa Z, Moges F, Isogai E. Frequent detection of ´azole´ resistant Candida species among late presenting AIDS patients in northwest Ethiopia. BMC Infect Dis. 2013;13:82.
- Sharopov FS, Kukaniev MA, Thompson RM, Satyal P, Setzer WN. Composition and antimicrobial activity of the essential oil of Hyssopus seravshanicus growing wild in Tajikistan. Der Pharma Chemica. 2012;4:961–966.
- Asadi-Pooya AA, Nikseresht AR, Yaghoubi E. Old remedies for epilepsy: Avicenna's medicine. Iran Red Cres Med J. 2012;14:174–177.
- Dehghanzadeh N, Ketabchi S, Alizadeh A. Essential oil composition and antibacterial activity of Hyssopus officinalis L. growning in Iran. Asian J Exp Biol Sci. 2012;3:767–771.
- Fathiazad F, Hamedeyazdan S. A review on Hysssopus officinaliis L.: Composition and biological activities. J Pharm Pharmacol. 2011;5:1959–1966.
- Kizil S, Haşimi N, Tolan V, Kilini E., Karataş H. Chemical composition, antimicrobial and antioxidant activities of hyssop (Hyssopus officinalis L.) essential oil. Not Bot Hort Agrobot Cluj-Napoca. 2010;38:99–10.
- Fraternale D, Ricci D, Epifano F, Curini M. Composition and antifungal activity of two essential oils of hyssop (Hyssopus officinalis L.). J Essent Oil Res. 2004;16:617–622.
- Garcia-Vallejo MC, Guijarro-Herraiz J, Perez-Alonso MJ, Velasco-Negueruela A. Volatile oil of Hyssopus officinalis L. from Spain. J Essent Oil Res. 1995;7:567–568.
- Glamočlija J, Soković M, Vukojevic J, Milencovic I, Brkic D, van Griensven LJLD. Antifungal activity of essential oil Hyssopus officinalis L. against mycopathogen Mycogone perniciosa (MANG). Matica Srpska J Nat Sci. 2005;109:123–128.
- Manitto P, Hadjieva B, Hadjieva P, Zlatkovska E, Tzvetkova A. Gas chromatography-mass spectral analysis of Bulgarian and Italian essential oils from Hyssopus officinalis L. Bulg Chem Ind. 2004;75:89–95.
- Mitić V, Dordevic S. Essential oil composition of Hyssopus officinalis L. cultivated in Serbia. Phys Chem Technol. 2000;2:105–108.
- Mohan M, Seth R, Singh P, Lohani H, Gupta S. Composition of the volatiles of Hyssopus officinalis (L.) from Uttarakhand Himalaya. Nat Acad Sci Lett. 2012;35:445–448.
- Özer H, Sokmen M, Gulluce M, Adiguzel A, Kilic A, Sahin F, Sokmen A, Baris O. In vitro antimicrobial and antioxidant activities of the essential oils and methanol extracts of Hyssopus officinalis L. spp angustifolius. Ital J Food Sci. 2006;18:73–83.
- Wesolowska A, Jadczak D, Grezeszuk M. Essential oil composition of hyssop (Hyssopus officinalis L.) cultivated in north-western Poland. Herba Pol. 2010;56:57–65.
- Kazazi H, Rezaei K, Ghotbsharif S, Emamdjomeh Z, Yamini Y. Supercritical fluid extraction of flavors and fragrances from Hyssopus officinalis L. cultivated in Iran. Food Chem. 2007;105:805–811.
- Langa E, Cacho J, Palavra AMF, Burillo J, Mainar AM, Urieta JS. The evolution of hyssop oil composition in the supercritical extraction curve modelling of the oil extraction process. J Supercrit Fluids. 2009;49:37–44.
- Moro A, Zalacain A, De Mendoza JH, Carmona M. Effects of agronomic practices on volatile composition of Hysssopus officinalis L. essential oils. Molecules. 2011;16:4131–4139.
- Mazzanti G, Battinelli L, Salvatore G. Antimicrobial properties of the linalool-rich essential oil of Hyssopus officinalis L. var decumbens (Lamiaceae). Flavour Fragr J. 1998;13:289–294.
- Renzini G, Scazzocchio F, Lu M, Mazzanti G, Salvatore G. Antibacterial and cytotoxic activity of Hyssopus officinalis L. oils. J Essent Oil Res. 1991;11:649–654.
- Letessier MP, Svoboda KP, Walters DR. Antifungal activity of the essential oil of hyssop (Hyssopuss officinalis). J Phytopathol. 2001;149:673–678.
- Moro A, Librán CM, Berruga MI, Zalacain A, Carmona M. Mycotoxicogenic fungal inhibition by innovative cheese cover aromatic plants. J Sci Technol Agric. 2013;93:1112–1118.
- Mousavi SM, Raftos D. In viro antifungal activity of a new combination of essential oils against some filamentous fungi. Middle East J Sci Res. 2012;11:156–161.
- Marino M, Bersani C, Comi G. Impedance measurements to study the antimicrobial activity of essential oils from Lamiaceae and Compositae. Int J Food Microbiol. 2001;67:187–195.
- Mahboubi M, Kazempour N. In vitro antimicrobial activity of some essential oils from Labiatae Family. J Essent Oil Bear Pl. 2009;12:494–508.
- The National Institute of Standards and Technology/National Institute of Health/Environmental Protect Agency/ Mass Spectral Library (Upgrade). John Wiley & Sons; 2008.
- Adams RP. Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. 4th ed. Carol Stream, IL: Allured; 2007.
- Jennings W, Shibamoto T. Qualitative analysis of flavour and fragrance volatiles by glass capillary gas chromatography. New York, NY: Academic Press; 1980.
- Julian D, Konig WA. The Atlas of Spectral Data of Sesquiterpene Hydrocarbons. Hamburg: E.B. Verlag; 1988.
- Kondjoyan N, Berdagué JL. A compilation of relative retention indices for the analysis of aromatic compounds. Theix: Laboratoire Flavour; 1996.
- Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved Standard. 3rd ed. CLSI document M27-A3. Wayene, PA: CLSI; 2008.
- Lunde CS, Kubo I. Effect of polygodial on the Mitochondrial ATPase of Saccharomyces cerevisiae. Antimicrob Agents Chemother. 2000;44:1943–1953.
- Ostroumova OS, Efimova SS, Chulkov EG, Schagina LV. The interaction of dipole modifiers with polyene-sterol complexes. PLoS One. 2012;7(9):e45135. doi: 10.1371/journal.pone.0045135
- Yang YL, Cheng HH, Ho YA, Hsiao CF, Lo HJ. Fluconazole resistance rate of Candida species from different regions and hospital types in Taiwan. J Microbiol Immunol Infect. 2003;36:187–191.
- Cox SD, Mann CM, Markham JL. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J Appl Microbiol. 2000;88:170–175.
- Laroche C, Beney L, Marechal PA, Gervas P, The effect of osmotic pressure on the membrane fluidity of Saccharomyces cerevisiae at different physiological temperatures. Appl Microbiol Biotechnol. 2001;56:249–254.
- Pina-Vaz C, Rodrigues AG, Sansonetty F, Martinez-De-Oliveira J, Fonseca AF, Mårdh PA. Antifungal activity of local anesthetics against Candida species. Infect Dis Obstet Gynecol. 2000;8:124–137.
- Chambel A, Viegas CA, Sá-Correia I. Effect of cinnamic acid on the growth and on plasma membrane H+-ATPase activity of Saccharomyces cerevisiae. Int J Food Microbiol. 1999;50:173–179.
- Braga PC, Culici M, Alfieri M, Dal Sasso M. Thymol inhibits Candida albicans biofilm formation and mature biofilm. Int J Antimicrob Agents. 2008;31:472–477.
- Hammer KA, Carson CF, Riley TV. Melaleuca alternifolia (tee tree) oil inhibits germ tube formation by Candida albicans. Med Mycol. 2000;38:355–361.
- Tampieri MP, Galuppi R, Macchioni F, Carelle MS, Falcioni L, Cioni PL, Morelli I. The inhibition of Candida albicans by selected essential oils and their major components. Mycopathologia. 2005;159:339–345.
- Ultee A, Slump RA, Steging G, Smid EJ. Antimicrobial activity of carvacrol toward Bacillus cereus on rice. J Food Prot. 2000;63:620–624.