?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The aim of this study was to analyse different model redox mediatiors: anthraquinone-2,7-disulphonate (2,7-AQDS), anthraquinone-1-sulphonate (α-AQS), anthraquinone-2-sulphonate (AQS) and anthraquinone-2,6-disulphonate (AQDS), with regard to the structure–activity relationship indicated by their cyclic voltammograms, their chemical structure, their oxidation–reduction potential and their capacity to accelerate the denitrification reaction during denitrification. Compared to the control, the nitrate removal after 21 h of denitrification was enhanced by a factor of 1.94, 1.56, 1.56 and 1.3 by 2,7-AQDS, AQDS, AQS and α-AQS, respectively. The ranking of the mediators based on their reduction potential ( ) was 2,7-AQDS > AQDS ≥ AQS > α-AQS.
was positively correlated with accelerating the denitrification in experiments using 0.024 mmol·L−1 redox mediator, 360 mg L−1 NO3−–N and 5.0 g L−1 of dry cell weight. Practical computational approaches (the frontier orbital theory and the atoms-in-molecules theory) and inductive and resonance effects were also used to explain and assess how the molecular structure and stability of the redox mediators affect the accelerating capacity during denitrification.
Introduction
Compounds derived from naphthoquinone and anthraquinone play an important role in anaerobic biotransformation of pollutants.[Citation1–3] During the previous 20 years, considerable evidence has accumulated that many quinone moieties used as artificial redox mediators can contribute to the reduction of such contaminants as azo dyes,[Citation1,Citation4–8] polyhalogenated compounds,[Citation9–11] nitro-aromatics,[Citation12,Citation13] nitrate [Citation3,Citation14,Citation15] and metals.[Citation1,Citation16–20] To understand the role of artificial redox mediators in the anaerobic biotransformation of pollutants in greater detail, the redox properties, molecular structure and transformation pathways (stability) of quinone redox mediators have been studied with regard to their acceleration capacity during pollutant biotransformation.[Citation6,Citation14–16] For example, the thermodynamics and kinetics of electron transfer reactions with both the electron donor (e.g., hydrogen sulphide as a bulk reductant) and the terminal electron acceptor (e.g., nitro aromatic explosives in contaminant degradation) and the stability toward irreversible side reactions are key factors in determining anaerobic biotransformation or biodegradation.[Citation1,Citation21,Citation22] Aeschbacher et al. [Citation23] investigated two electrochemical methods to assess the redox properties of humic substances. A key question in the successful application of redox mediators is the manner of assessing, predicting and establishing the relationship between characteristics of redox mediators and their catalytic capabilities in various reactions. However, to the best of our knowledge, there are no reports regarding the structure–activity relationship of redox mediators during denitrification, which is the critical question regarding their acceleration/inhibitory effects and is the bottleneck in the rapid development and extensive application of redox mediators. The structure–activity relationship of redox mediators will, thus, be expected to become the focus of anaerobic biotransformation of pollutants in the future.
In this study, four similar dissolved quinones were selected as redox mediators to explore the structure–activity relationship of redox mediators during denitrification. Their cyclic voltammograms (CVs) were sufficiently well defined for quantitative characterization of such parameters as the peak potentials (Ep), half-peak potentials (Ep/2), peak currents (ip), peak separations (∆Ep) and peak current ratios (). The structure–activity relationships of the redox mediators were also analysed based on practical computational approaches [the frontier orbital theory and the atoms-in-molecules (AIM) theory] as were their inductive and resonance effects, which other researchers have not used to explain the accelerating effect of redox mediators in the denitrification process.
Materials and methods
Chemicals
The chemicals used in this study were of analytical grade and were purchased from Xiandai Ltd. (Shijiazhuang, China). Anthraquinone-2,7-disulphonic acid (2,7-AQDS), anthraquinone-1-sulphonic acid (α-AQS), anthraquinone-2-sulphonic acid (AQS) and anthraquinone-2,6-disulphonic acid (AQDS) were selected as redox mediators. Their chemical structures are shown in Supplemental data (Figure S1).
Bacterial strains and culture conditions
Paracoccus versutus strain GW1 (1416 bp, GU111570) was cultivated in a mineral medium containing 0.025 g L−1 KH2PO4, 1.25 g L−1 KHCO3, 0.2 g L−1 MgSO4·7H2O, 0.3 g L−1 CaCl2·2H2O, 0.00625 g L−1 FeSO4, 0.00625 g L−1 ethylenediaminetetraacetic acid (EDTA) and 1 ml L−1 of trace element solution.[Citation3] The trace element solution contained 15 g L−1 EDTA, 0.43 g L−1 ZnSO4·7H2O, 0.24 g L−1 CoCl2·6H2O, 0.99 g L−1 MnCl2·4H2O, 0.25 g L−1 CuSO4·5H2O, 0.19 g L−1 NiCl2·6H2O, 0.22 g L−1 NaMoO4·2H2O, 0.014 g L−1 H3BO4, 0.21 g L−1 NaSeO4·10H2O and 0.05 g L−1 NaWO4·2H2O.[Citation3] The growth conditions of strain GW1 were as follows: pH 7.0, temperature of 35 °C, sodium acetate as a carbon source and a chemical oxygen demand/NO3–N ratio of 3:1. The biomass concentration was determined by measuring the optical density at 660 nm, and the biomass concentration of strain GW1 was controlled at a dry cell weight of 5.0 g L−1 in all of the experiments.
Effect of various redox mediators on the denitrification process
The denitrification experiments with the redox mediators were conducted under the following conditions: 0.024 mmol L−1 redox mediator, 360 mg L−1 NO3-N, 5.0 g L−1 of dry cell weight, pH 7.0 and a temperature of 35 °C. To assess the effect of the various redox mediators on the changes in the oxidation–reduction potential (ORP) during the denitrification process, the ORP values were also measured.
Electrochemical measurements
The cyclic voltammetric experiments were performed using a three-electrode configuration.[Citation4] The electrochemical measurements were performed using a Voltalab Powersuite/LK98BII (Tianjin Lanlike Electronics Technology Ltd., China) and controlled using the PowerSuite electrochemistry software (Tianjin Lanlike Electronics Technology Ltd., China). The glassy carbon electrode was successively polished using 5-, 1-, 0.3- and 0.05-mm diamond polishes and rinsed with 8 mol L−1 nitric acid and distilled water before use.
The experiments were scanned three times at a temperature of 35 °C, a redox mediator concentration of 5 μmol L−1, a 0.1 mol L−1 H2SO4 concentration, a voltage range of −700 to 700 mV and a scan rate of 10 mV s−1. All of the solutions were purged with N2 for 30 min prior to analysis. The vials were sealed with teflon-lined rubber stoppers and aluminium crimp caps.
Calculation methods
The orbital analysis was based on Fukui's frontier orbital theory.[Citation24] The geometries of the four quinones were fully optimized at the B3 LYP/ 6-311G(d) level using the Gaussian 03 set of code.[Citation25] The topological analyses of electron density, which were based on Bader's AIM theory,[Citation26] were performed using the AIM2000 program.[Citation27]
Analytical methods
The samples were centrifuged for 15 min at 8000 r min−1 to remove the insoluble particles from the supernatant.
The nitrate and nitrite concentration in the supernatant was measured by Ultraviolet–visible spectrophotometry (Tianmei, UV2600, UV/VIS spectrophotometer, China).[Citation28]
The ORP was measured using a digital pH meter [Delta-320, Far-Toledo Instruments (Shanghai) Ltd., China] and an ORP composite electrode (Leici-501, China).
The pH was determined using a digital pH meter (Delta-320, China).
All experimental analyses were performed in triplicate to ensure reproducibility, and the error bars in figures represent standard errors of the mean.
Results and discussion
Effects of redox mediator on the denitrification process
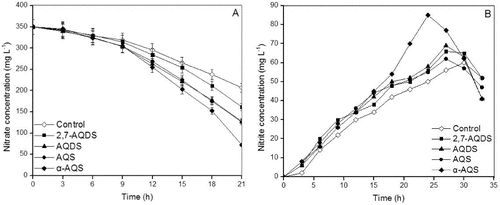
A) shows that 2,7-AQDS, α-AQS, AQS and AQDS all enhanced the denitrification compared to the control (without a redox mediator). With the use of 0.024 mol L−1 of each redox mediator, the nitrate concentration after 21 h of denitrification was approximately 207, 162, 127, 126 and 72 mg L−1 in the control and with the use of 2,7-AQDS, AQDS, AQS and α-AQS, respectively. That is, the nitrate removal efficiency after 21 h of denitrification was 53.7%, 63.7%, 63.7% and 79.4%, and was enhanced by a factor of 1.3, 1.56, 1.56 and 1.94 with the use of 2,7-AQDS, AQDS, AQS and α-AQS, respectively, as compared to the control. The various redox mediators differed in their capacity to accelerate the biodenitrification process: α-AQS showed the highest acceleration capacity and 2,7-AQDS, the lowest one, whereas AQS and AQDS had similar acceleration capacities.
During the traditional denitrification process, nitrate is converted into nitrite, nitrous oxide, nitric oxide and nitrogen gas. The concentration of accumulated nitrite is the limiting factor in the nitrogen removal. The highest nitrite concentration after 27 h of denitrification was approximately 85 mg L−1 with the use of 0.024 mol L−1 α-AQS (B)). The transformation from nitrate to nitrite varied among the redox mediators.
The denitrification process consists of sequential reactions, in which an intermediate metabolic product is the substrate for the next reaction. The concentration and activity of the NO2−reductase is induced and regulated to keep the free concentrations of NO2− below cytotoxic levels. The activity of NO2−reductase is considered to be enhanced with increasing NO2− concentrations.[Citation29]
Effect of redox mediator on the ORP change during the denitrification process
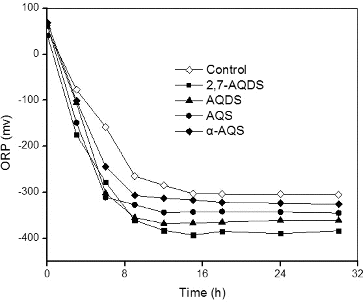
In general, all denitrification steps are catalysed by complex multisite metalloenzymes with characteristic spectroscopic and structural features,[Citation30] and the proteins required for denitrification are only produced under (nearly) anaerobic conditions.[Citation29] Thus, ORP is an important parameter during the denitrification. Redox mediators accelerate the electron transfer from a primary electron donor to a terminal electron acceptor and affect the ORP change during the denitrification process, and such mediators might increase the reaction rate by one to several orders of magnitude.[Citation31] The stabilized ORP values after 30 h of denitrification were approximately −326, −345, −361, −384 and −413 mV for the control, α-AQS, AQS, AQDS and 2,7-AQDS experiments, respectively (). These results are in agreement with similar data reported previously.[Citation3,Citation4]
Quantitative cyclic voltammetry of redox mediators
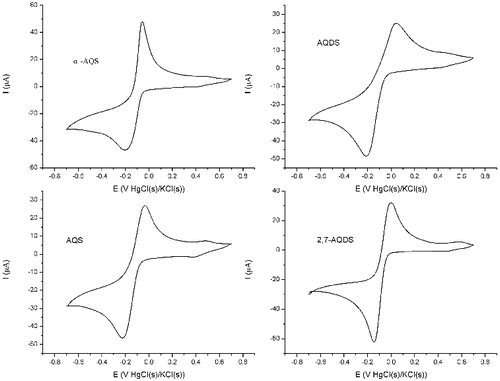
The four selected redox mediators exhibited similar electrochemical characteristics in the CVs at a scan rate of 10 mV s−1 and a concentration of 5 μmol L−1. The four selected redox mediators exhibited well-defined reduction peaks () and oxidation peaks (EpC). The tested redox mediators displayed an
potential range of 0.0 to −0.058 V vs. Hg–Hg2Cl2(s) and an
range of −0.145 to −0.215 V vs. Hg–Hg2Cl2(s) (). The values of
and
, which were from the effect of the sulphonic group, are shown in . The position of the sulphonic group relative to that of the aromatic group significantly affects the reduction potential. The reduction potential of 2,7-AQDS was the most positive, and this mediator has two sulphonic groups. Compared to the reduction potentials of redox mediators, the electron-withdrawing properties of the sulphonic group played an important role in the electroreduction of the four selected redox mediators.
Table 1. Characteristics of the cyclic voltammetry of the redox mediator.
Electrochemical reversibility is an operational concept that is dependent on the properties of the compound and on the experimental parameters used. The cyclic voltammetry of reversible (Nernstian) electrochemical reactions typically exhibits pairs of cathodic and anodic peaks in which the difference between peak potentials () is 0.059/n V (where n is the number of electrons transferred), and the ratio of the anodic and cathodic peak currents (
) is near unity.[Citation32] Under these conditions, the half-wave potential (E1/2), the potential midway between
and
, is an accurate measure of the formal redox potential (
). The observed ΔEp values for the various redox mediators () exceeded the theoretical value of 0.059 V for reversible one-electron transfer. Instead, they were within the range that is typical of quasi-reversible reactions.[Citation32] Quasi-reversible reactions, which are common in organic electrochemistry, usually exhibit kinetics of electron transfer that are slower than those required for a reversible reaction but rapid enough to yield a well-defined pair of anodic and cathodic peaks.[Citation33] The denitrification efficiency was related to the ΔEp of the four redox mediators in this study.
The structure–activity relationship of redox mediators during the denitrification process
To catalyse a reaction by an electron shuttling mechanism, the reduction potential of the catalyst must lie between that of the electron donor and the terminal electron acceptor. In earlier studies,[Citation2,Citation7] the role of the redox mediator during denitrification was believed to be similar to that of the coenzyme NADH (nicotinamide adenine dinucleotide) because a redox mediator with the quinone group had special chemical and electrochemical reduction–oxidation characteristics. Biological denitrification in nature is an oxidation–reduction reaction in which the transfer of electrons matched with protons need the help of coenzymes, such as nicotinamide adenine dinucleotide phosphate (NADPH/NADP+) and NADH/NAD+, or other redox mediators (such as quinones). The ORPs of the couples of NADPH/NADP+, NADH/NAD+ or redox mediators (like quinones) are the important factors controlling the denitrification rate, which yields characteristic CVs and is affected by the molecular structure. However, little reports present the structure–activity relationships of redox mediators and their acceleration effects in denitrification.[Citation14]
The structure–activity relationship of the four selected redox mediators was attributed to the following effects.
Inductive and resonance effects
In solution, the reduction reaction mechanism of quinone is as follows: H+ attacks the carbon atom of carbonyl, which links to the quinone ring. The π bond is destroyed, and the charge of the benzene ring becomes positive and that of the O atom becomes negative. As the reduction reaction proceeds, the system receives an electron, the H+ is transferred to the O2− and OH− is formed.
Anthraquinone is a planar molecule, and all of the carbon atoms are sp2 hybridized. Except for the σ-type bond, which is formed by the sp2 hybrid orbitals, there are π14 bonds throughout. The carbonyl carbon and the carbonyl oxygen of anthraquinone form a σ bond and a π bond. The carbonyl π bond and the anthraquinone ring form a delocalized π bond. Therefore, anthraquinone exhibits good reductive activity. Because the H atom of the anthraquinone was replaced by various substituents, there are two effects between the substituents and anthraquinone: one is the electron withdrawal effect and the other is the conjugated effect. The substituents can be divided into two groups based on their effect on the redox potential of quinones.[Citation21,Citation22] One group consists of electron-donating groups with a (+) I effect, which lower the redox potential of quinone/hydroquinone (E0(Q/QH2)) relative to the unsubstituted quinone (X = H). These include X = –CH3, –O−, –OH and –NH2. The second group consists of electron-withdrawing groups with a (−) I effect, which increase the E0 (Q/QH2). These include X = –SO3, –Cl and –Br.
As the H atom of anthraquinone was replaced by a sulphonic group, the oxidation and reduction of anthraquinone became difficult due to the strong electron-withdrawing effect of the sulphonic group. The m-sulphonic group of anthraquinone has a higher electron-withdrawing effect than the o-sulphonic group, and thus the anthraquinone with the m-sulphonic group was easy to reduce and difficult to oxidize. of the anthraquinone with the m-sulphonic group was more positive than that of the anthraquinone with the o-sulphonic group, and
was the reverse for them.
of the anthraquinone with the o-sulphonic group was approximately 1, and this molecule exhibited good redox reversibility, however,
of the anthraquinone with the m-sulphonic group was approximately 0.51, and this molecule exhibited the lowest redox reversibility. Although two H atoms of anthraquinone were replaced by two sulphonic groups, the stronger withdrawing effects of the two sulphonic groups led to a positive transfer of the
and
in the cyclic voltammetry and led to low redox reversibility. The positive transfer of the
and
was clearer in the 2,7-AQDS experiment than in the AQDS experiment. The ranking of the studied mediators based on the
in the cyclic voltammetry is 2,7-AQDS > AQDS > AQS > α-AQS, which related and affected their acceleration capability.
Quantum chemical calculations
Orbital analysis: according to Fukui's frontier orbital theory,[Citation24] during chemical reactions, the nature of the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) in reacting species is very important. The energy difference between the HOMO and LUMO is referred to as the HOMO–LUMO energy gap, ΔE = ELUMO − EHOMO, which is an important indicator of the molecular stability. Larger values of ΔE indicate greater stability of a molecule.
The energies of the HOMOs and LUMOs of the four redox mediators and their energy gaps (ΔE) are listed in . As shown in , the ranking of the mediators based on their ΔE values is α-AQS > AQS > AQDS > 2,7-AQDS, which indicates that the reaction activity of the four redox mediators follows this order. The results of the orbital analysis might explain the acceleration activity during the denitrification (A)).
Table 2. Energy of HOMOs (EHOMO) and LUMOs (ELUMO) of the studied molecules and their energy gaps ΔE.
AIM analysis: according to the AIM theory,[Citation26] the Laplacian of the electron density is the second derivative of the electron density ρ(rc), and
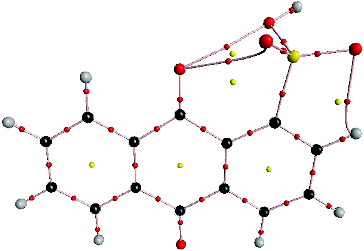
, where λi is one of the eigenvalues of the Hessian matrix of the electron density. If a critical point has two negative and one positive eigenvalue, it is called a (3, −1) saddle point or the bond critical point. If a critical point has two positive and one negative eigenvalues, it is called a (3, +1) critical point or the ring critical point (RCP). The (3, +1) critical points are characteristic of a ring structural region. presents the molecular structure of α-AQS.
illustrates that there are three ring structures in the anthraquinone molecule. The values of ρ(rc) of the middle ring's RCP of α-AQS, AQS, AQDS and 2,7-AQDS are 0.01634, 0.01630, 0.01628, and 0.01628, respectively. The ρ(rc) of the RCP is related to the aromaticity of the molecule: smaller values of ρ(rc) of the RCP indicate lower aromaticity.[Citation26] The lower aromaticity also indicates a lower effect on the denitrification electron. In other words, a small ρ(rc) of the RCP means that the negative charges are concentrated in oxygen atoms rather than the ring structure of the molecule. The larger negative charge concentrated in the oxygen atoms causes electrophilic substitution reactions to occur more easily. The ranking of the mediators based on the ρ(rc) of the RCPs is α-AQS > AQS > AQDS > 2,7-AQDS, which also indicates that the reaction activity of these four compounds follows this order. The calculated results are consistent with the former experimental results.
Inductive and resonance effects influence the overall electron characteristics of a given quinone molecule. In addition, the molecular structures of quinones are the primary factors controlling their reversible electron transfer reactions and redox (E0) properties. The molecular structure and stability of a redox mediator could be used to assess and predict the ability of a quinone molecule to catalyse a biogeochemical process (e.g., denitrification).
Conclusions
A simple and practical method for calculating thermodynamic parameters was employed based on the semi-quinone stability constants and the redox potentials of natural quinone products. Our results indicate that a combination of various computationally inexpensive quantum chemical methods provides reasonable agreement with the experimental data. The primary advantage of the present approach is that it can be readily applied to high-throughput processes in screening of potential redox cycling agents.
Supplemental data
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/13102818.2015.1027504.
J_Guo1_Supplementary_appenix.pdf
Download PDF (9 KB)Acknowledgements
The calculation methods benefited from the kind help of Dr Xiaoyan Li (Hebei Normal University) and Dr Guanghui Ding (Dalian Maritime University).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Van der Zee FP, Cervantes FJ. Impact and application of electron shuttles on the redox (bio)transformation of contaminants: a review. Biotechnol Adv. 2009;27:256–277.
- Hong Y, Gu J. Bacterial anaerobic respiration and electron transfer relevant to the biotransformation of pollutants. Int Biodeterioration Biodegradation. 2009;63:973–980.
- Guo J, Kang L, Yang J, Wang X, Lian J, Li H, Liu C, Li Z, Yue L. Study on a novel non-dissolved redox mediator catalyzing biological denitrification (RMBDN) technology. Bioresour Technol. 2010;101:4238–4241.
- Guo J, Zhou J, Wang D, Tian C, Wang P, Salah Uddina M, Yu H. Biocalalyst effects of immobilized anthraquinone on the anaerobic reduction of azo dyes by the salt-tolerant bacteria. Water Res. 2007:41:426–432.
- Guo J, Kang L, Wang X, Yang J. Decolorization and degradation of azo dyes by redox mediator system with bacteria. In: Atacag Erkurt H, editor. Biodegradation of Azo Dyes. Heidelberg: Springer; 2009. p. 85–100.
- Field JA, Cervantes FJ. Microbial redox reactions mediated by humus and structurally related quinones. In: Perminova IV, Hatfield K, Hertkorn N, editors. Use of humic substances to remediate polluted environments: from theory to practice. Vol. 52, NATO Science Series. Dordrecht: Springer; 2005. p. 343–352.
- Cervantes FJ, Garcia-Espinosa A, Moreno-Reynosa MA, Rangel-Mendez JR. Immobilized redox mediators on anion exchange resins and their role on the reductive decolorization of azo dyes. Environ Sci Technol. 2010;44:1747–1753.
- Rau J, Knackmuss HJ, Stolz A. Effects of different quinoid redox mediators on the anaerobic reduction of azo dyes by bacteria. Environ Sci Technol. 2002;36:1497–1504.
- O'Loughlin EJ, Burris DR, Delcomyn CA. Reductive dechlorination of trichloroethene mediated by humic-metal complexes. Environ Sci Technol. 1999;33:1145–1147.
- Collins R, Picardal F. Enhanced anaerobic transformations of carbon tetrachloride by soil organic matter. Environ Toxicol Chem. 1999;18:2703–2710.
- Kappler A, Haderlein SB. Natural organic matter as reductant for chlorinated aliphatic pollutants. Environ Sci Technol. 2003;37:2714–2719.
- Dunnivant FM, Schwarzenbach RP, Macalady DL. Reduction of substituted nitrobenzenes in aqueous solutions containing natural organic matter. Environ Sci Technol. 1992;26:2133–2141.
- Li L, Wang J, Zhou J, Yang F, Jin C, Qu Y, Li A, Zhang L. Enhancement of nitroaromatic compounds anaerobic biotransformation using a novel immobilized redox mediator prepared by electropolymerization. Bioresour Technol. 2008;99:6908–6916.
- Guo J, Liu H, Qu J, Lian J, Zhao, L, Jefferson W, Yang J. The structure activity relationship of non-dissolved redox mediators during azo dye bio-decolorization processes. Bioresour Technol. 2012;112:350–354.
- Aranda-Tamaura C, Estrada-Alvarado MI, Texier AC, Cuervo F, Gomez J, Cervantes FJ. Effects of different quinoid redox mediators on the removal of sulphide and nitrate via denitrification. Chemosphere. 2007;69:1722–1727.
- O'Loughlin EJ. Effects of electron transfer mediators on the bioreduction of lepidocrocite (γ-FeOOH) by Shewanella putrefaciens CN32. Environ Sci Technol. 2008;42:6876–6882.
- Field JA, Sierra-Alvarez R, Cortinas I, Feijoo G, Moreira MT, Kopplin M, Gandolfi AJ. Facile reduction of arsenate in methanogenic sludge. Biodegradation. 2004;15:185–196.
- Fredrickson JK, Kostandarithes HM, Li SW, Plymale AE, Daly MJ. Reduction of Fe(III), Cr(VI), U(VI), and Tc(VII) by Deinococcus radiodurans R1. Appl Envrion Microbiol. 2000;66:2006–2011.
- Zhang YQ, Zahir ZA, Amrhein C, Chang A, Frankenberger WT. Application of redox mediator to accelerate selenate reduction to elemental selenium by Enterobacter taylorae. J Agric Food Chem. 2007;55:5714–5717.
- Liu G, Yang H, Wang J, Jin R, Zhou J, Lv H. Enhanced chromate reduction by resting Escherichia coli cells in the presence of quinone redox mediators. Bioresour Technol. 2010;101:8127–8131.
- Uchimiya M, Stone AT. Reversible redox chemistry of quinones: impact on biogeochemical cycles. Chemosphere. 2009;77:451–458.
- Cape JL, Bowman MK, Kramer DM. Computation of the redox and protonation properties of quinones: towards the prediction of redox cycling natural products. Phytochemistry. 2006;67:1781–1788.
- Aeschbacher M, Sander M, Schwarzenbach R. Novel electrochemical approach to assess the redox properties of humic substances. Environ Sci Technol. 2010;44:87–93.
- Fukui K, Yonezawa T, Shingu H. A molecular orbital theory of reactivity in aromatic hydrocarbons. J Chem Phys. 1952;20:722–725.
- Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels A, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill, PMW, Johnson BG, Chen W, Wong MW, Andres JL, Head-Gordon M, Replogle ES, Pople JA. Gaussian 03, revision D.01. Pittsburgh, PA: Gaussian, Inc.; 2004.
- Bader RFW. Atoms in molecules – a quantum theory. Oxford: University of Oxford; 1990.
- Biegler-Könìng FJ, Derdau R, Bayles D, Bader RFW. AIM2000 progam version 1. Bielefeld, Germany; 2000.
- Xi Z, Guo J, Lian J, Li H, Zhao L, Liu X, Zhang C, Yang J. Study the catalyzing mechanism of dissolved redox mediators on bio-denitrification by metabolic inhibitors. Bioresour Technol. 2013;140:22–27.
- Bothe H, Ferguson S, Newton WE. Biology of the nitrogen cycle. Amsterdam: Elsevier Science Ltd.; 2006.
- Berks BC, Ferguson SJ, Moir JW, Richardson DJ. Enzymes and associated electron transport systems that catalyse the respiratory reduction of nitrogen oxides and oxyanions. Biochimica Biophysica Acta. 1995;1232:97–173.
- Cervantes FJ. Quinones as electron acceptors and redox mediators for the anaerobic biotransformation of priority pollutants. Wageningen: Wageningen University; 2002.
- Tsujimura S, Kano K, Ikeda T. Bilirubin oxidase in multiple layers catalyzes four-electron reduction of dioxygen to water without redox mediators. J Electroanal Chem. 2005;576:113–120.
- Nurmi JT. Physical environmental electrochemistry electrochemical properties of natural organic matter and iron powders. Portland: Oregon Health and Science University; 2005.