?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Biosorption of Pb(II) ions from a model solution was investigated using Streptomyces fradiae biomass as biosorbent pretreated with sodium hydroxide. The mycelium is a waste product from the biotechnological production of the macrolide antibiotic tylosin in the pharmaceutical industry. The biosorption study was conducted in a batch system with respect to initial pH, initial metal concentration and contact time. For a description of the biosorption equilibrium, Langmuir and Freundlich adsorption models were used. Equilibrium data fitted better to the Langmuir model and the calculated maximum biosorption capacity was 138.88 mg·g−1 at initial pH 5.0, contact time of 120 min, biosorbent dose of 1 g·dm−3 and concentration range for the Pb(II) ions from 10 to 200 mg·dm−3. Pseudo-first and pseudo-second order kinetic models were applied to the experimental data. The results indicated that the Pb(II) uptake process followed the Ho equation. The interference of co-present ions Cu(II) and Zn(II) on the Pb(II) biosorption was also studied. It was determined that at the highest Pb(II) concentration (200 mg·dm−3) Cu(II) and Zn(II) caused 27.22% and 24.88% decreasing in Pb(II) uptake, respectively. The obtained results could be useful in prospective applications of chemically modified waste mycelium of S. fradiae as an alternative biosorbent for Pb(II) removal from aqueous solutions.
Introduction
Environmental pollution caused by heavy metals present in different effluents is a serious public health problem, due to their toxicity even in very low concentrations. The problem concerns mainly aquatic ecosystems, where toxic metals could concentrate in food chains even at very low levels of water contamination.
Various methods have been proposed for removal of metal ions from aqueous solutions, such as hydroxide or sulfide precipitation, ion exchange, reverse osmosis, evaporation, electrodeposition.[Citation1] These methods are not completely satisfactory because of their relatively high costs and/or production of large volumes of sludge that must be treated or eliminated, resulting in surplus costs.
Among the biological methods, bioaccumulation and biosorption demonstrate promising potential for replacing conventional methods for metal concentration and removal from aqueous solutions. Bioaccumulation is a process that occurs only in living organisms through transport of contaminants and their accumulation into the cell.[Citation2] In the biosorption process, metal ions are adsorbed on the surface of dead biomass as a sorbent. Thus, biosorption is a metabolically passive process. The use of dead biomass offers the following advantages over living cells: no requirement for growth media and nutrients; low cost; high efficiency; minimization of chemical sludge; regeneration of biosorbent and possibility for metal recovery.[Citation3]
Various microorganisms, such as bacteria, yeast and fungi, have been studied for Pb(II) removal from aqueous solutions.[Citation3–10] Waste microbial mycelium from antibiotic-producing Streptomyces species is a major by-product of the biopharmaceutical industry and its disposal causes serious ecological problems. Such biomasses were studied for Cd(II),[Citation11,Citation12] Cr(III),[Citation13] Pb(II) [Citation14] and Ni(II) [Citation15] biosorption.
To the best of our knowledge, only Simeonova et al. [Citation16] and Kirova et al. [Citation17] have studied the possibilities for application of dead mycelium of tylosin-producing Streptomyces fradiae for Cu(II), Zn(II) and Ni(II) removal from aqueous solutions.
The goal of the present work was to investigate the biosorption potential of S. fradiae waste biomass pretreated with sodium hydroxide for removal of Pb(II) ions from aqueous solution. The effects of pH, contact time and initial metal concentration on the biosorption capacity of the biomass were studied. The biosorption of Pb(II) was characterized by the Langmuir and Freundlich models. The kinetics of biosorption of Pb(II) ions was described using pseudo-first order and pseudo-second order models. The interference of co-present ions, copper and zinc, on the Pb(II) biosorption capacity was also studied.
Materials and methods
Biosorbent preparation
Waste Streptomyces fradiae biomass was provided by ‘Biovet’ AD, Peshtera, Bulgaria. The raw biomass was powdered by mortar and pestle, filtered under vacuum, washed several times with distilled water until pH 6 was reached in the filtrate and oven-dried at 80 °C for 12 h. The dried biomass was stored at 4 °C until further use. Five grams of the heat inactivated biomass were boiled at 110 °C for 15 min in 100 cm3 of 1 mol·dm−3 NaOH. The alkali-treated biomass was filtered under vacuum, washed with distilled water and oven-dried at 80 °C for 12 h. The dried biomass was stored at 4 °C until further use.
Model solutions
Pb(II) stock solution (1000 mg·dm−3) was prepared from analytical grade Pb(NO3)2 (Merck, Darmstadt, Germany) in deionized water containing 10 cm3 concentrated nitric acid. Working solutions were prepared immediately before use by adequate dilution.
Fourier transform infrared spectroscopy (FTIR) analysis
The functional groups present on the surface of the pretreated S. fradiae biomass before and after Pb(II) sorption were determined by FTIR analysis, using a Thermo Nicolet Avatar 330 FT-IR spectrometer (Thermo Electronic Corporation). The samples were prepared as KBr discs and the FTIR spectra were recorded in the region of 4000–400 cm−1.
Biosorption of Pb(II) from single solutions
In order to evaluate the effect of pH, initial Pb(II) concentration and contact time, a set of biosorbtion experiments were carried out in a batch system. The pH of metal solutions was adjusted to values between 2.0 and 5.0, using 0.1 mol·dm−3 HNO3 or 0.1 mol·dm−3 NaOH. Biosorption of Pb(II) was carried out in 250 cm3 Erlenmeyer flasks by adding 0.1 g of biosorbent to 100 cm3 metal solution of a desired concentration and pH value at 25 ± 1 °C, on a magnetic stirrer with a speed of 300 r·min−1 for 120 min. Samples were taken at definite time intervals and centrifuged at 3000 r·min−1 for 10 min. The concentration of Pb(II) ions in the liquid supernatant was determined.
Biosorption of Pb(II) ions from binary model solutions
To determine the biosorption characteristics of Pb(II) ions in binary metal solutions, the initial concentration of Pb(II) ions was varied between 10 and 200 mg·dm−3, whereas the concentrations of Cu(II) or Zn(II) ions were 25 mg·dm−3. The experiments were carried out at optimal pH value for Pb(II) biosorption, as determined for the single metal-ion solution, and using the same procedures as in the single biosorption experiments.
All glassware used in the biosorption experiments was washed with 10% HNO3 and subsequently rinsed with deionized water to avoid binding of the metal ions to it.
Analysis of metal ions
The residual metal concentrations in the supernatants were determined using a Perkin Elmer atomic absorption spectrometer PinAAcle 900 T (THGA/FLAME) at 283.31 nm.
Calculation of metal uptake and removal efficiency
The metal uptake was calculated by the simple difference method [Citation18]:
(1)
(1)
The removal of metal ions was determined using EquationEquation (2)(2)
(2) :
(2)
(2) where Ci and Cf are the initial and final concentration of Pb(II) in solution (mg·dm−3); V is the volume of the solution in the flask (dm3); and W is the mass of the biosorbent (g).
Equilibrium modelling
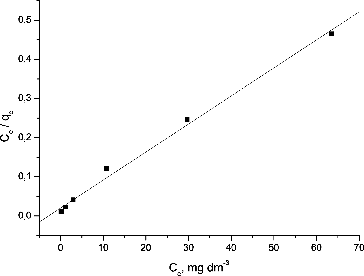
The Langmuir and Freundlich models were used to describe the biosorption equilibrium [Citation19]. The linearized Langmuir isotherm allows the calculation of the maximum adsorption capacity and Langmuir constant and was presented by the following equation:
(3)
(3) where qe and qm are the metal uptake at equilibrium and the maximum metal uptake, respectively, (mg·g−1); Ce is the metal concentration at equilibrium (mg·dm−3) and b is the constant related to the affinity of the binding sites (dm3·mg−1).
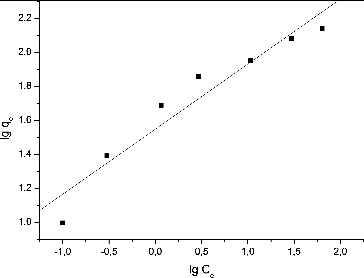
The Freundlich isotherm was linearized as follows and Freundlich constants were determined:
(4)
(4) where KF (dm3·g−1) and n (dimensionless) are the Freundlich adsorbent constant and the exponent characterizing the system.
Kinetic models
In order to evaluate the biosorption processes of Pb(II) ions on NaOH-pretreated S.fradiae biomass, pseudo-first and pseudo-second order kinetic models were used.
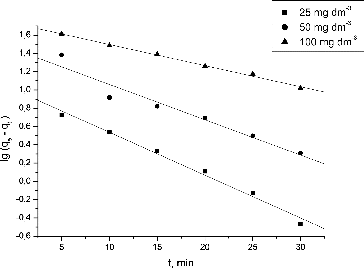
After integration and applying boundary conditions t = 0 to t = t and qt = 0 to qt = qt, the pseudo-first order equation was given as [Citation20]:
(5)
(5) where qt and qe are the biosorption uptake of Pb(II) ions at time t and at equilibrium (mg·g−1); k1 is the pseudo-first order rate constant (min−1) and t is the contact time (min).
By plotting lg(qe − qt) against t, the values of qe and k1 can be calculated by the intercept and the slope of the plot, respectively.
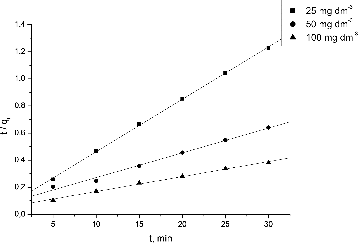
The pseudo-second order rate equation [Citation21] was expressed in a linear form as:
(6)
(6) where k2 is the pseudo-second order rate constant (g·mg−1·min−1).
The plot of (t/qt) and t of this equation give a linear relationship from which qe and k2 were determined.
Results and discussion
FTIR analysis
Biosorption is defined as the property of microorganisms to accumulate metal ions by adsorption on the cell surface. The major constituent of the Streptomyces cell wall is peptidoglycan linked with teichoic acid and polysaccharides. The functional groups of these molecules can play an important role in the biosorption of metal ions.[Citation22–24] For this reason, to study the mechanism of Pb(II) removal by waste NaOH-treated S. fradiae mycelium from tylosin production, the active chemical groups on the cell surface before and after Pb(II) removal were evaluated by FTIR spectroscopy. The obtained results are shown in .
Figure 1 . FTIR spectra of the waste S. fradiae biomass before (a) and after (b) Pb(II) biosorption.
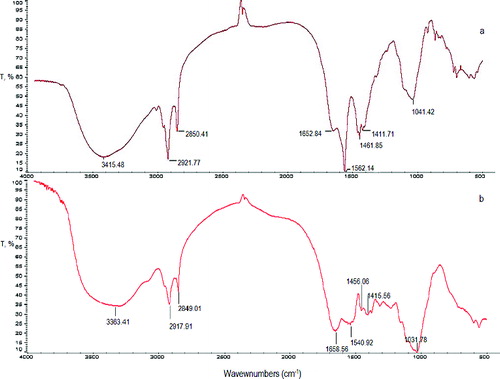
The spectrum of the waste S. fradiae biomass treated with NaOH showed several bands. The broad and strong band at 3415.48 cm−1 could be attributed to the overlapping of –OH and –NH stretching. The presence of –CH stretching vibrations was confirmed by the adsorption peaks at 2921.48 and 2850.41 cm−1. The stretching of –OH groups could occur from carboxylic acids and the peak is extremely broad in the range from 3400 to 2400 cm−1 and often interferes with the adsorption of –CH groups.[Citation23] The bands at 1652.84 and 1562.14 cm−1 could be assigned to amide I (–C–O stretching coupled with –N–H deformation mode) and amide II (–N–H deformation coupled to –C = N– deformation) and/or asymmetric stretching vibration of COO− groups, respectively. The peak at 1461.85 cm−1 corresponds to bending deformations of –CH2 or –OH bending in carboxylic groups. The peaks situated at 1411.70 сm−1 and 1041.42 сm−1 could be due to symmetric stretching of COO− vibrational stretching and/or stretching of О–Н of alcohols and Р–О–С groups. Thus, the FTIR analysis of the pretreated waste S. fradiae biomass showed presence of hydroxyl, carboxyl and amine groups on the biosorbent surface.
The FTIR spectra of the metal loaded biomass, which is presented in (b), showed that the peaks expected at 3415.48, 1652.84, 1562.14, 1461.85, 1411.71 and 1041.42 cm−1 were changed and shifted to 3363.41, 1658.56, 1540.92, 1456.06, 1415.56 and 1031.78 cm−1. The cause of these shifts is difficult to be determined, but the results revealed interactions between the Pb(II) ions, hydroxyl, amino and carboxyl groups on the biomass surface similar to those reported by other authors [Citation8,Citation13,Citation23].
Effect of pH
The pH of the aqueous solution is considered as one of the most critical parameters influencing the biosorption processes. It affects the behaviour and speciation of metal ions; the dissociation of functional groups on the active sites of the biosorbent and the metal ion–sorbent interactions.[Citation25] To avoid precipitation of metal hydroxides, the initial pH of the solution was chosen according to the speciation diagram for Pb(II) ions. The dominant species in the pH range from 2.0 to 5.0 were Pb2+ and Pb(OH)+ and at pH values higher than 5, several low-soluble hydroxide species are observed.[Citation26]
The effect of the initial pH on the Pb(II) removal by waste NaOH-pretreated S. fradiae biomass was evaluated in the range from 2.0 to 5.0 to avoid Pb(OH)2 precipitation. The results are shown in .
Figure 2. Effect of pH on biosorption of Pb(II) by waste S. fradiae biomass. Note: Ci = 50 mg·dm−3, V = 100 cm3, W = 1 g·dm−3, t = 120 min.
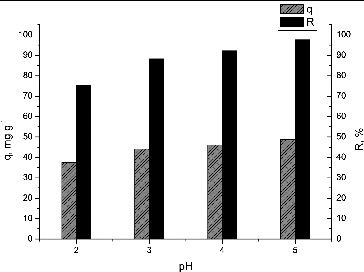
The biosorption efficiency increased from 75.2% to 97.68% with the increase in pH from 2.0 to 5.0. The highest metal uptake (48.84 mg·g−1) was obtained at pH 5.0. Therefore, pH 5.0 was selected for all further experiments.
These results could be explained by the fact that as pH increases, this causes deprotonation of functional groups on the cell wall of the biosorbent. Thus, negatively charged sites are formed and they electrostatically attract positively charged metal ions. The low removal efficiency at low pH was apparently due to the presence of higher concentrations of hydrogen ions in the solution which competed with Pb(II) ions for the adsorption sites on the biosorbent surface. These results were in good agreement with data for the effect of pH on Pb(II) biosorption reported by other authors.[Citation7,Citation8,Citation13,Citation25]
Effect of contact time and initial concentration of Pb(II) on biosorption uptake
The effect of contact time on the biosorption uptake of Pb(II) ions using NaOH-pretreated S. fradiae biomass at different initial concentrations is illustrated in .
Figure 3. Effect of contact time and initial Pb(II) concentration on the NaOH-pretreated S. fradiae biomass biosorption capacity. Note: pH = 5.0, V = 100 cm3, W = 1 g·dm−3, t = 120 min, Ci = 25, 50, 100 mg·dm−3.
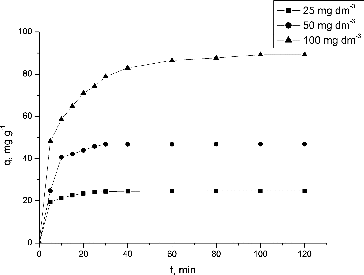
As shown, at an initial Pb(II) concentration of 25 mg·dm−3, the Pb(II) uptake was rapid and equilibrium was reached within the first 20 min. For 50 and 100 mg·dm−3, equilibrium was reached after 30 and 80 min, respectively. After 120 min, the Pb(II) uptake was 24.70 48.84 and 89.23 mg·g−1 when 25, 50 and 100 mg·dm−3 initial Pb(II) concentrations were used. Thus, 120 min were selected as an optimum contact time for further experiments. These results are in agreement with the reports of Selatnia et al. [Citation14] and Simeonova et al. [Citation16].
Adsorption isotherm analysis
Proper quantification of the sorption equilibrium is required for commercial-scale application of the biosorption technique. The Langmuir and Freundlich isotherms are more frequently used. The Langmuir adsorption isotherm is based on the assumption that the surface is homogeneous, with all the adsorption sites having equal adsorbate affinity and adsorption at one site not affecting the adsorption at an adjacent site. The Freundlich adsorption isotherm describes heterogeneous surface adsorption and does not assume monolayer biosorption.[Citation22] The linearized forms of the Langmuir and Freundlich isotherms are presented in and , respectively.
The calculated model constants and correlation coefficients for Pb(II) biosorption by NaOH-treated S. fradiae biomass are given in .
Table 1. Isotherm model constants and correlation coefficients for biosorption of Pb(II) ions.
The experimental data fitted better to the Langmuir model compared to the Freundlich model. The maximum Pb(II) uptake per unit of dry S. fradiae biomass treated with NaOH was found to be 138.88 mg·g−1. These results are comparable with the maximum biosoprtion capacity of NaOH-treated dead biomass of Streptomyces rimosus (135 mg·g−1) obtained by Selatnia et al. [Citation14]. The authors carried out the Pb(II) removal from aqueous solutions at contact time of 3 h, biomass concentration of 3 g·dm−3 and a stirring speed of 250 r·min−1. However, it is noteworthy that the maximum biosorption capacity is a function of the experimental conditions. That is why it is generally very difficult to compare the results from a particular experiment with those in other reports where biosorption experiments have been performed at different process parameters.
Kinetic studies
Various kinetic models could be used for analysis of the biosorption process. In the present study, pseudo-first and pseudo-second order models were applied to the kinetic experimental data ( and ). The calculated Lagergren and Ho kinetic constants for biosorption of Pb(II) by waste S. fradiae biomass and their corresponding regression correlation coefficients are presented in .
Table 2. Kinetic constants for Pb(II) biosorption by waste S. fradiae biomass.
The correlation coefficients for the pseudo-second order model were very high for all the Pb(II) concentrations studied by us. The calculated equilibrium uptakes for the Pb(II) ions indicated that this model fitted better to the experimental values.
Effect of interfering ions on Pb(II) biosorption
Available studies report mostly biosorption experiments carried out in single-ion model solutions; however, industrial wastewaters are usually polluted with more than one heavy metal. For this reason, from a practical point of view it is very important to clarify the influence of co-present ions, such as Cu(II) and Zn(II), onto Pb(II) removal from aqueous solutions by NaOH pretreated waste mycelium of S. fradiae. The obtained results are shown in .
Figure 8. Biosorption of Pb(II) onto S. fradiae biomass. Notes: 1 – Pb(II) ions only; 2 – Pb(II) and Cu(II) ions; 3 – Pb(II) and Zn(II) ions.
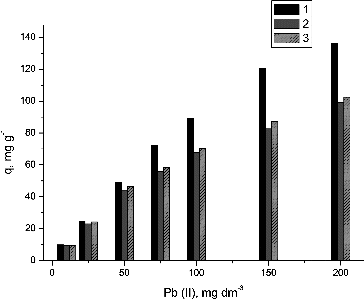
It was obtained that the co-present ions decreased the effectiveness of waste biomass of S. fradiae as a Pb(II) biosorbent. When the initial Pb(II) concentration was 200 mg·dm−3, the presence of Cu(II) ions caused a 27.22% decrease in the biosorption uptake of Pb(II). The decrease was similar in the presence of Zn(II) ions (24.88%). This similarity in the effect of Zn(II) and Cu(II) on the Pb(II) biosorption could be explained by the similarity between copper and zinc in terms of molecular mass (63.57 and 65.38), ionic radii (73 and 74 pm) and electronegativity (1.90 and 1.65 Pauling) and their covalent indices (χm2r), which are 6.41 and 4.54, respectively [Citation9].
Conclusions
The biosorption capacity of NaOH pretreated S. fradiae biomass, a waste product from the industrial biotechnological production of the macrolide antibiotic tylosin, was studied for Pb(II) ions removal from dilute aqueous solutions. The optimal process parameters were initial pH 5.0 and contact time 120 min. The experimental data at the studied conditions fitted well to the Langmuir adsorption isotherm and maximum Pb(II) uptake of 138.88 mg g−1 was calculated. The kinetic of Pb(II) biosorption onto chemically modified waste biomass of S. fradiae followed a pseudo-second order model. The obtained results suggest that chemically modified waste mycelium of S. fradiae could be considered a prospective and cheap alternative biosorbent for Pb(II) removal from aqueous solutions but can show decreased effectiveness caused by co-present ions. Further experiments will be focused on the optimization of process parameters for Pb(II) desorption and recovery of the biosorbent for reusage. The obtained results are a basis for development of environmentally friendly biotechnology for purification of wastewaters contaminated with heavy metals through utilization of waste mycelium from antibiotic production.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Zouboulis AI, Loukidou, MX, Matis KA. Biosorption of toxic metals from aqueous solutions by bacterial strain isolated from metal-polluted soils. Process Biochem. 2004;8:909–916.
- Zabochnicka-Świątek M, Krzywonos, M. Potentials of biosorption and bioaccumulation, processes for heavy metal removal. Pol J Environ Stud. 2014;2:551–561.
- Çabuk A, Ilhan S, Filik C, Çalişkan F. Pb2+ biosorption by pretreated fungal biomass. Turk J Biol. 2005;29:23–28.
- Gupta R, Mohapatra H. Microbial biomass: an economical alternative for removal of heavy metals from waste water. Indian J Exp Biol. 2003;41:945–966.
- Parvathi K. Lead biosorption onto waste beer yeast by-product, a means to decontaminate effluent generated from battery manufacturing industry. Electron J Biotechnol. 2007;1:92–105.
- Gőksungur Y, Üren S, Gűvenç, U. Biosorption of copper ions by caustic treated waste baker's yeast biomass. Turk J Biol. 2003;27:23–29.
- Svekova L, Spanelova M, Kubal M, Guibal E. Cadmium, lead and mercury biosorption on waste fungal biomass issued from fermentation industry. I. Equilibrium studies. Sep Purif Technol. 2006;1:142–153.
- Pan J, Liu R, Tang H. Surface reaction of Bacillus cereus biomass and its biosorption for lead and copper ions. J Environ Sci. 2007;19:403–408.
- Puranik PR, Paknikar KM. Biosorption of lead, cadmium, and zinc by Citrobacter strain MCM B-181: characterization studies. Biotechnol Prog. 1999;15:228–237.
- Marandi R, Doulati Ardejani F, Amir Afshar H. Biosorption of Lead (II) and Zinc (II) ions by pre-treated biomass of Phanerochaete chrysosporium. Int J Mining Environ Issues. 2010;1:9–16.
- Puranik PR, Chabukswar NS, Paknikar KM. Cadmium biosorption by Streptomyces pimprina waste biomass. Appl Microbiol Biotechnol. 1995;6:1118–1121.
- Selatnia A, Bakhti MZ, Madani A, Kertous L, Mansouri Y. Biosorption of Cd2+ from aqueous solution by a NaOH-treated bacterial dead Streptomyces rimosus biomass. Hydrometallurgy. 2004;1–4:11–24.
- Sharma I, Goya D. Kinetic modeling: chromium (III) removal from aqueous solution by microbial waste biomass. J Sci Ind Res. 2009;68:640–646.
- Selatnia A, Boukazoula A, Kechid N, Bakhti MZ, Chergui A, Kerchich Y. Biosorption of lead (II) from aqueous solution by a bacterial dead Streptomyces rimosus biomass. Biochem Eng J. 2004;2:127–135.
- Selatnia A, Madani A, Bakhti MZ, Kertous L, Mansouri Y, Yous R. Biosorption of Ni2+ from aqueous solution by a NaOH-treated bacterial dead Streptomyces rimosus biomass. Miner Eng. 2004;7–8:903–911.
- Simeonova A, Godjevargova T, Ivanova D. Biosorption of heavy metals by dead Streptomyces fradiae. Environ Eng Sci. 2008;5:627–634.
- Kirova G, Velkova Z, Gochev V. Copper (II) removal by heat inactivated Streptomyces fradiae biomass: surface chemistry characterization of the biosorbent. J BioSci Biotech. 2012;SE/ONLINE:77–82.
- Ullah I, Nadeem R, Iqbal M, Manzoor Q. Biosorption of chromium onto native and immobilized sugarcane bagasse waste biomass. Ecol Eng. 2013;60:99–107.
- Li XN, Xu QY, Han GM, Zhu WQ, Chen ZH, He XB, Tian XJ. Equilibrium and kinetic studies of copper (II) removal by three species of dead fungal biomasses. J Hazard Mater. 2009;1–3:469–474.
- Ho Y-S. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics. 2004;1:171–177.
- Ho Y-S. Review of second-order models for adsorption systems. J Hazard Materi B. 2006;2:681–689.
- Mosbah R, Sahmoune, MN. Biosorption of heavy metals by Streptomyces species – an overview. Cent Eur J Chem. 2013;9:1412–1422.
- Veneu DM, Torem ML, Pino GAH. Fundamental aspects of copper and zinc removal from aqueous solutions usin a Streptomyces lunalinharesii strain. Miner Eng 2013;48:44–50.
- Selatnia A, Boukazoula A, Kechid N, Bakhti MZ, Chergui A. Biosorption of Fe3+ from aqueous solution by a bacterial dead Streptomyces rimosus biomass. Process Biochem. 2004;39:1643–1651.
- Mahendra A, Liakopoulou-Kyriakides M. Binding mechanism and biosorption characteristics of Fe(III) by Pseudomonas sp. cells. J Water Sustain. 2013;3:117–131.
- Gomez-Sarrano V, Macias-Garcia A, Esplinosa-Mansilla A, Valenzuela-Calahorro C. Adsorption of mercury, cadmium and lead from aqueous solution on heat-treated and sulphurized activated carbon. Water Res. 1998;32:1–4.