?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Rice blast caused by Magnaporthe grisea is considered to be one of the most devastating diseases in most rice-growing regions. In order to search for an effective agent to protect against rice infection, the elicitor activity of algino-oligosaccharides was evaluated in germinating rice, in which accumulation of oryzalexin C could be detected sensitively. The maximal elicitor activity was detected in germinating rice seedlings when the fractions of alginate degradation products with a degree of polymerization of 7.9 (S3) and 6.5 (S4) were used as elicitors. Algino-oligosaccharides could induce the enzyme activities of phenylalanine ammonia lyase, peroxidase and catalase in rice plant cells for protection of the plant against the invading pathogen. With utilization of algino-oligosaccharides, the disease index caused by Magnaporthe grisea could be reduced from 17.74% to 10.81% and the protection efficacy was 39.06%. These findings suggest that algino-oligosaccharides could be applied as a biological agent to control rice blast disease.
Introduction
Defence responses against plant infection by phytopathogens are well known in plant cells. If such a defence response is initiated in a timely manner, the plant infection is hampered.[Citation1] In fact, the defence response can be initiated by both endogenous and exogenous elicitors.[Citation1] Therefore, it is thoughtful to apply the plants' own defence reactions to help the plants resist phytopathogen infections.[Citation2] It has been demonstrated that some oligosaccharides can act as elicitors to stimulate the plant defence response.[Citation3] Most of these oligosaccharides are produced by enzymatic degradation of polysaccharides sourced from the structural constituents of plant or fungal cell wall or pathogen virulence factors.[Citation4] It is worthy to develop elicitor-active oligosaccharides and establish a series of alterations for resistance against various plant diseases.
Alginate is composed of β-D-mannuronate (M) and its C-5 epimer, α-L-guluronate (G), which are arranged as homopolymeric (polyM or polyG) or heteropolymeric (poly-MG) block structures, depending on the seaweed sources alginate is derived from.[Citation5] Over the last decade, increasing attention has been paid to alginate oligomers as a new functional material. They have been shown to enhance seed germination, shoot elongation and root growth.[Citation6,Citation7] For example, oligoguluronate has been reported to effectively elicit oxidative burst in the sporophyte of kelp Laminaria digitata [Citation8] and oligomannuronate and oligoguluronate, to enhance bacitracin A production in liquid culture of Bacillus licheniformis.[Citation9] The oligomers of alginate rich in polymannuronic acid could elicit a significant increase in the level of phenylalanine ammonia lyase (PAL) and peroxidase (POD) activity.[Citation10] Alginate oligomers have also been demonstrated to stimulate the production of 5′-phosphodiesterase in suspension-culture plant cells, in synergy with the effect that the active oxygen species has on the chitinase activity.[Citation11] However, all these identified oligosaccharides were produced from an acid-hydrolysed or heat-degraded alginate, in which the activities are only at trace levels and are structure-dependent.[Citation12] Therefore, the production of various alginate oligosaccharides using lyase is sought for the development of a more functional alginate for future industrial application.[Citation13]
An alginate-degrading bacterium, Flavobacterium sp. LXA, was isolated from rotting algae in our laboratory and was found to produce alginate lyase in the culture supernatant.[Citation14,Citation15] The alginate degradation products with an unsaturated urinate terminal showed elicitor activity by stimulating the accumulation of phytoalexin and inducing PAL activity in soybean cotyledons,[Citation13] suggesting a potential protection of plants against phytopathogens. In this study, the algino-oligosaccharides produced by alginate lyase were applied on rice plants. The elicitor activity of these algino-oligosaccharides on germinating rice seedlings was evaluated and their potential to protect rice plants against rice blast was also investigated. To the best of our knowledge, this is the first report that algino-oligosaccharides could elicit rice plant cells to produce phytoalexin in a defence response against the invading phytopathogen.
Materials and methods
Microorganism and culture conditions
Alginate-degrading Flavobacterium sp. LXA was isolated from rotted algae and stored in our laboratory.[Citation14] Strain LXA was cultured and maintained on an alginate medium containing 1 g sodium alginate, 0.1 g yeast extract, 0.1 g peptone and 2 g agar in 100 mL of mineral solution. The composition of the mineral solution was as follows: 0.6 g/L NaCl, 0.5 g/L K2HPO4, 0.025 g/L MgSO4 and 0.7 g/L KNO3 at pH 7.0.
For alginate lyase production, 2 mL of overnight culture of Flavobacterium sp. LXA was inoculated in 200 mL of an alginate culture medium (0.7 g sodium alginate and 0.3 g (NH4)2SO4 in 100 mL of mineral solution) and incubated at 30 °C and 150 r/min for 36 h. The culture fluid was centrifuged at 4 °C and 10,000 g for 10 min and the supernatant was collected as the fraction containing the crude enzyme.
Algino-oligosaccharides preparation
The crude enzyme was mixed with 2.5% sodium alginate at a ratio of 1:50 in 20 mmol/L phosphate-citrate buffer (pH 7.0) and incubated at 40 °C. The degradation of alginate was monitored as a function of time from 15 to 360 min. Samples were removed at intervals and boiled for 15 min to stop the enzymatic reaction. After centrifugation at 15,000 g and 4 °C for 15 min, the supernatant was mixed with absolute ethanol (analytical grade) at a 1:3 ratio and stirred at room temperature for 4 h. The pellet was collected by centrifugation at 10,000 g and 4 °C for 15 min and washed three times with ethanol. The alginate-fragmentation products were redissolved in distilled water for bioassay after drying at 30 °C. Reducing sugar [Citation16] and total sugar [Citation17] were also determined.
Germinating rice bioassay
The bioactivity of algino-oligosaccharides, i.e. their potential to induce phytoalexin accumulation in rice tissue, was determined using germinating rice. After washing and soaking in sterile distilled water for 24 h, rice seeds (Orza saliva) were placed in trays containing wet filter paper and covered with moist gauze. The seedlings were watered daily with sterile tap water. A photoperiod regime of 8 h for daylight and 16 h for darkness was applied in the germination and the temperatures in the light and dark cycles were 25 °C and 20 °C, respectively. Germinating rice seeds were detached from 5- to 7-day-old seedlings at the time when the sprouts were 1–2 cm in length. The detached germinating seeds were surface sterilized with 0.5% sodium hypochlorite for 5 min and then extensively rinsed with sterile distilled water. After immersing in solution of 0.1 mg/mL algino-oligosaccharides for 15 h, the germinating rice seeds were transferred to the moist filter papers in sterile Petri dishes (10 seeds per dish) and incubated in darkness at 25 °C for 24 h. Each group of 10 seeds was transferred in a beaker containing 10 mL of distilled water and shaken for 1 min. The absorbance of the solution at 245, 250, 268 and 274 nm was read, respectively. For a negative control, algino-oligosaccharides were replaced with sterile distilled water.
Analytical methods
All experiments were carried out in triplicate except otherwise stated.
Reducing sugar was determined by the dinitrosalicylic acid method and calculated as glucose.[Citation16] Total sugar was measured by the phenol-sulphuric acid method as described by Doubois et al.[Citation17] The average degree of polymerization (DP) of the algino-oligosaccharides was calculated by dividing the reducing sugar content into the total sugar content.
Growth of seedlings
Rice seeds (O. saliva) were surface sterilized by immersion in H2O2 for 10 min and washed five times with sterile distilled water. After soaking in sterile distilled water at 30 °C for two days and placing in trays containing wet filter paper and covered with moist gauze at 30 °C for one day, the seedlings were transferred to soil and incubated at 25 °C at a cycle of 14 h light and 10 h dark. Rice plants with 3–5 leaves were harvested for the subsequent experiments.
Treatment of rice leaves with algino-oligosaccharides
The third and fourth leaf of each rice plant were collected and washed three times with sterile distilled water. Then, they were transferred to a sterile Petri dish containing wet filter paper. The leaves were sprayed by filter-sterilized algino-oligosaccharides (1 mg/mL, DP 6.5) on the surface and incubated at 25 °C. The enzyme activities relevant for protection against the studied phytopathogen were measured at different intervals of time.
Enzyme assays
PAL was determined by the method of Carver et al. [Citation18] with some modification. The treated leaves of rice with algino-oligosaccharides were ground in a mortar in the presence of 6 mL of ice-cold Tris-HCl buffer (0.035 mol/L, pH 8.5) containing 0.0014 mol/L L-mercaptoethylamine and 10% (v/v) glycerol. The homogenate was centrifuged at 12,000 g and 4 °C for 15 min, and the supernatant was used for the assay. The reaction was started by adding 0.1 mL of enzyme extract into 0.2 mL of 0.1 mol/L L-phenylalanine in Tris-HCl buffer (0.025 mol/L, pH 8.5) and stopped with 0.4 mL of 0.1 mol/L trichloroacetic acid after incubation at 37 °C for 30 min. A control reaction mixture replaced with 0.1 mL boiled enzyme extract was also incubated in the same manner. After dilution of the reaction mixture with 3 mL of distilled water and centrifugation, the formation of cinnamate was determined by measuring the absorbance increase at 290 nm with a SpectraMax Plus 384 Microplate Reader (Molecular Devices, USA) against a corresponding reference. One unit of PAL was defined as the enzyme amount that catalyses the formation of cinnamate resulting in an increase in OD290 (optical density at 290 nm) of 0.01 per minute under the above conditions.
Catalase (CAT) was measured at 25 °C by determining the initial rate of H2O2 reduction. The treated leaves with algino-oligosaccharides were ground in a mortar in the presence of 4 mL of phosphate buffer (0.1 mol/L, pH 7.8). The homogenate was centrifuged at 12,000 g and 4 °C for 15 min and the supernatant was used for enzyme assay. The enzymatic reaction was started by injecting 0.3 mL of 0.1 mol/L H2O2 in phosphate buffer (0.1 mol/L, pH 7.8) into a sealed chamber filled with 2.7 mL of enzyme extract. The concentration of H2O2 was determined spectroscopically at 240 nm, using the SpectraMax Plus 384 Microplate Reader (Molecular Devices, USA). One unit of activity was defined as the amount of enzyme that catalyses the degradation of H2O2 leading to a decrease of 0.1 in the absorbance at 240 nm per minute under the above experimental conditions. Non-enzymatic H2O2 decomposition was subtracted from each determination.
POD was evaluated spectroscopically by the oxidation of guaiacol at pH 5.5. The enzyme extraction from treated leaves of rice was carried out as described above for the catalase assay. After 4.9 mL of 0.4% H2O2 and 0.01 mol/L guaiacol in 0.1 mol/L phosphate buffer (pH 5.5) was mixed with 0.1 mL of enzyme extract and incubated at 37 °C for 15 min, 2 mL of 20% trichloroacetic acid was added to stop the enzymatic reaction. The reaction mixture was centrifuged at 12,000 g for 15 min and the optical density of the supernatant was determined at 470 nm using the SpectraMax Plus 384 Microplate Reader (Molecular Devices, USA). The control experiment was performed with boiled enzyme. One unit of enzyme activity was defined as the amount of enzyme that catalyses the oxidation of guaiacol leading to a 0.01 decrease in optical density (OD) at 470 nm per minute under the above experimental conditions.
Growth and inoculation of rice plants
The phytopathogen Magnaporthe grisea, which causes rice blast disease, was grown on potato dextrose agar (PDA) medium for 10 days and spores were harvested with 10% sterile PDA medium. PDA medium contained 2 g dextrose and 2 g agar in 100 mL potato extraction, in which the potato extraction was prepared by boiling 20 g peeled potato in 100 mL tap water for 25 min and filtering with the seven layers of gauze. The spore suspension was adjusted with 10% sterile PDA to the appropriate concentration (105 spores/mL). Immediately after algino-oligosaccharides (1 mg/mL, DP 6.5) were sprayed, the leaves of the rice plants were inoculated with M. grisea by spraying and the plants were placed in a growth chamber (25 °C, 14 h light and 10 h dark cycle) until the leaves were harvested. The enzyme activities of PAL, POD and CAT were measured at different intervals of time. The negative controls were healthy rice plants sprayed with distilled water. The positive controls were rice plants inoculated with M. grisea and sprayed with distilled water.
Disease index of rice plants infected by M. grisea
Spore suspension of M. grisea was inoculated on the leaves of rice plants with 3–5 leaves by spraying after algino-oligosaccharides (1 mg/mL, DP 6.5) were sprayed. The rice plants were incubated in a growth chamber in dark for one day and then in light. The disease index was investigated after incubation for seven days.
The disease index was defined as follows: Grade 0, when there was no rice blast observed; Grade 1, when there were less than five blast spots and their diameter was less than 1 cm; Grade 3, when there were 6–10 blast spots and their diameter was more than 1 cm; Grade 5, when there were 11–25 blast spots and 10%–25% of the leaf area was affected; Grade 7, when there were more than 26 blast spots affecting 26%–50% of the leaf area; Grade 9, when more than 50% of the leaf area was affected. The following formula was used:(1)
(1) where NL is the number of rotten leaves, G is grade, T is total number of tested leaves; and 9 is the highest value of grade.
(2)
(2) where CK means disease index in control experiment, PT means disease index in the inoculated leaves.
Results and discussion
There is growing experimental evidence about the bioactivity of alginate hydrolysate and its derivatives in terms of growth stimulation of plant roots or Bifidobacterium,[Citation19] regulation of physiological process in plants [Citation6] and induction of cytotoxic cytokine production in human mononuclear cells [Citation20] (for review see [Citation4]). Naturally, this has resulted in increasing attention on the alginate oligomer. In our previous studies about alginate lyase produced by Flavobacterium sp. LXA, it was demonstrated that alginate could be decomposed controllably by alginase to form algino-oligosaccharides and that algino-oligosaccharides with an average DP of 6.8 showed maximal elicitor activity in the soybean cotyledon bioassay.[Citation13] The DP of algino-oligosaccharides was related to the phytoalexin accumulation in soybean cotyledon, which is an important attribute for biological activities of oligosaccharides.[Citation21] The phytoalexin production also depends on the concentration of added algino-oligosaccharides. When the concentration was lower than 0.003% (w/v), almost no elicitor activity could be detected.[Citation13] Since rice blast caused by M. grisea is regarded as one of the most important diseases of rice due to its widespread distribution, as a further step in our research, we evaluated the potential elicitor activity of these algino-oligosaccharides on germinating rice seedlings, as well as their possible protective effect against rice blast disease.
Elicitor activities of algino-oligosaccharides determined by rice germination bioassay
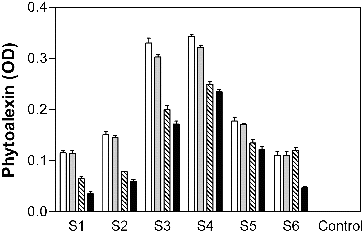
Alginate is a single-chain structure without branches, in which β-D-mannuronate (M) and α-L-guluronate (G) occur as random sequences. When it was degraded by alginase, the DP decreased with time, resulting in an unsaturated uronic acid-containing oligosaccharides.[Citation13,Citation15] In this study, algino-oligosaccharides were prepared by incubating sodium alginate with alginase for a different length of time, and the reducing sugar content and the total sugar content were determined. The obtained alginate degradation products were with different DP, including 11.4, 9.2, 7.9, 6.5, 5.3 and 4.2, and the respective algino-oligosaccharides were designated as S1, S2, S3, S4, S5 and S6. Germinating rice seeds were selected as a model plant tissue to detect the elicitor activity of algino-oligosaccharides. The elicitor activities on germinating rice were assayed by determining the accumulation of phytoalexin in rice seed tissues as a marker for plant disease resistance.
In accordance with spectroscopic analysis indicating that some phytoalexins show a distinct peak in absorption in the ultraviolet spectrum, four kinds of phytoalexins have been detected by measuring the absorbance at a wavelength of 245, 250, 268 and 274 nm, which corresponds to oryzalexin C, oryzalexin A, phytocassane A/D and phytocassane B/C, respectively.[Citation22,Citation23] Thus, the accumulation of phytoalexin in the germinating rice seeds was measured spectroscopically after treatment with different alginate degradation products. As shown in , the four kinds of phytoalexins were elicited in the rice-seed tissues by algino-oligosaccharides. Although there were differences in the accumulation of phytoalexins elicited in the rice seeds by each of the studied alginate degradation products, there was similarity in the overall trends. The absorbance was highest at 245 nm when algino-oligosaccharides with different DP were applied on the germinating rice seeds (), suggesting that the accumulation of the oryzalexin C could be considered more sensitive as a marker for assaying the elicitor activity. The accumulation of phytoalexins in the germinating rice elicited by algino-oligosaccharides S3 and S4, whose DP is 7.9 and 6.5, respectively, was higher than that by all other alginate degradation products. These results differed from the ones obtained previously in the soybean cotyledon assay [Citation13], presumably suggesting that oligosaccharides of a wider DP could be recognized by rice seedlings as compared with soybean cotyledon.
Figure 1. Elicitor activity of algino-oligosaccharides on germinating rice.
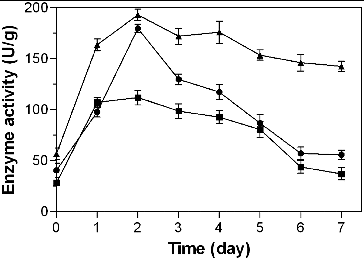
Eliciting activity on rice leaves by algino-oligosaccharides
The potential of algino-oligosaccharides to elicit the enzyme activity of PAL, POD and CAT in rice leaves was studied (). An increase in PAL activity in rice leaves was detected after treatment with algino-oligosaccharides, reaching a maximum on the second day. Increased PAL activity is considered a direct response of host plant to attempted penetration by pathogens, which is associated with the resistance of plants to fungal diseases.[Citation24,Citation25] It has been proved that PAL activity could be induced in an exogenous elicitor treatment.[Citation26] After the maximum in PAL activity was observed on the second day following treatment with algino-oligosaccharides, PAL activity was decreased sharply in the following days. These results are in agreement with the suggestion that algino-oligosaccharides have an ability to induce the expression of PAL in plant cells like other oligosaccharides described as elicitors.[Citation19] The POD activity in rice leaves was also highest on the second day after treatment with algino-oligosaccharides, after which it gradually decreased. The CAT activity was also maximally elicited one and two days after the treatment with algino-oligosaccharides and then slowly decreased. This similarity in the observed trends in POD, CAT and PAL following elicitation by algino-oligosaccharides reflect the synergistic roles of these enzymes as protectants.
Effect of algino-oligosaccharides on infection of rice plant by M. grisea
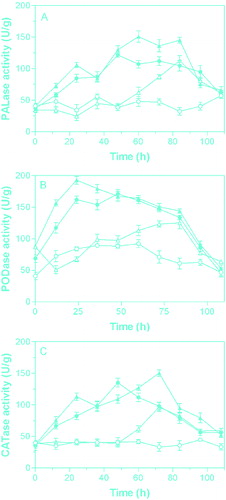
To assay the eliciting activity of algino-oligosaccharides against the phytopathogen M. grisea, the algino-oligosaccharides were sprayed on the leaves of rice plants with or without inoculation of M. grisea (). The highest PAL activity in the leaves was obtained at 84 h after the plant leaves were inoculated with M. grisea in the positive control (plants developing rice blast but not treated with algino-oligosaccharides). After algino-oligosaccharides were sprayed on the rice plant leaves, an increase in PAL activity was observed both with and without inoculation of phytopathogen. However, the time needed to reach maximum PAL activity in the presence of algino-oligosaccharides was much less than that without treatment with algino-oligosaccharides, which could be considered an indication of the ability of the studied algino-oligosaccharides to protect rice plants against M. grisea. Moreover, the PAL activity elicited by algino-oligosaccharides in the plant leaves inoculated with phytopathogen was higher than that in the non-inoculated plants. Similar results were also obtained for the elicitation of POD and CAT activity by algino-oligosaccharides. To the best of our knowledge, these are the first results to demonstrate the elicitor activity of algino-oligosaccharide in rice plants.
Figure 3. Changes in (A) phenylalanine ammonia lyase (PAL) activity, (B) peroxidase (POD) activity and (C) catalase (CAT) activity in inoculated leaves (▴) and uninoculated leaves (•) of rice plants after spraying with algino-oligosaccharides. Positive control (△): inoculated leaves on the rice plant sprayed with distilled water. Negative control (○): uninoculated leaves on the rice plant sprayed with distilled water.
Potential of algino-oligosaccharides to protect against rice blast disease
Although elicitor activity has been shown to reside in some alginate oligomers,[Citation10,Citation11] there has been little research on its potential application in protection against rice blast disease. So far, the use of chemical pesticides appears to offer marginal protection from the disease.[Citation27] In this study, the disease index and protection efficacy were investigated in rice plants and the results are shown in . When algino-oligosaccharides were applied to the rice plant for protection against rice blast, the disease index was decreased from 17.74% to 10.81%. Respectively, the protection effectiveness of algino-oligosaccharides against rice blast was 39.06%. This effectiveness is very similar to that achieved by other authors using different substances.
Table 1. Efficiency of algino-oligosaccharides against rice blast.
For example, the application of salicylic acid in protection of rice plants against M. grisea has been reported to show a reduction in the number of lesions by more than 50%.[Citation28] Recently, a novel secreted protein elicitor from the rice pathogen M. oryzae was shown to reduce the leaf blast score by about 40%.[Citation29] However, none of these elicitors is of oligosaccharide nature. To the best of our knowledge, this is the first study to apply algino-oligosaccharides in protection against rice blast disease. The algino-oligosaccharide fraction tested in this study shows the necessary characteristics of a potential bio-pesticide for protection against rice plant disease, but with much more advantages, such as being environmentally friendly, low cost and not inducing drug resistance.
Conclusions
The elicitor activity of bioactive algino-oligosaccharides could be detected by the germinating rice bioassay. Algino-oligosaccharides with a wide range in the DP showed elicitor activity in the rice seedlings. Algino-oligosaccharides could induce the activity of PAL, POD and CAT in rice plant cells for protection of plants against the invading pathogen. Potentially, algino-oligosaccharides could be applied as a biological agent to protect against rice blast disease.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Guo J, Du G, Chen J, Chen X, Li X. Oligosaccharides act as elicitors to protect plant against crop disease based on knowledge of plant defence response mechanism. In: Hertsburg CT, editor. Sugar beet crops: growth, fertilization and yield. New York, NY: Taylor & Francis; 2009. p. 1–42.
- Welbaum G, Sturz AV, Dong Z, Nowak J. Fertilizing soil microorganisms to improve productivity of agroecosystems. Crit Rev Plant Sci. 2004;23:175–193.
- Shibuya N, Minami E. Oligosaccharide signalling for defence responses in plant. Physiol Mol Plant Pathol. 2001;59:223–233.
- Li X, Chen X. Biodegradation of polysaccharide sourced from virulence factor or plant and pathogenic cell wall constituent and its application in management of phytopathogenic disease. In: Wang BY, editor. Environmental biodegradation research focus. New York, NY: Taylor & Francis; 2007. p. 1–47.
- Gacesa P. Alginates. Carbohydrate Polym. 1988;8:161–182.
- Hu X, Jiang X, Hwang H, Liu S, Guan H. Promotive effects of alginate-derived oligosaccharide on maize seed germination. J Appl Phycol. 2004;16:73–76.
- Xu X, Iwamoto Y, Kitamura Y, Oda T, Muramatsu T. Root growth-promoting activity of unsaturated oligomeric uronates from alginate on carrot and rice plants. Biosci Biotechnol Biochem. 2003;67:2022–2025.
- Küpper FC, Müller DG, Peters AF, Kloareg B, Potin P. Oligoalginate recognition and oxidative burst play a key role in natural and induced resistance of sporophytes of Laminariales. J Chem Ecol. 2002;28:2057–2081.
- Murphy T, Parra R, Radman R, Roy I, Harrop A, Dixon K, Keshavarz T. Novel application of oligosaccharides as elicitors for the enhancement of bacitracin A production in cultures of Bacillus licheniformis. Enzyme Microbial Technol. 2007;40:1518–1523.
- Chandía NP, Matsuhiro B, Mejías E, Moenne A. Alginic acids in Lessonia vadosa: partial hydrolysis and elicitor properties of the polymannuronic acid fraction. J Appl Phycol. 2004;16:127–133.
- Akimoto C, Aoyagi H, Dicosmo F, Tanaka H. Synergistic effect of active oxygen species and alginate on chitinase production by Wasabia japonica cells and its application. J Biosci Bioeng. 2000;89:131–137.
- Iwamoto M, Kurachi M, Nakashima T, Kim D, Yamaguchi K, Oda T, Iwamoto Y, Muramatsu T. Structure-activity relationship of alginate oligosaccharides in the induction of cytokine production from RAW264.7 cells. FEBS Lett. 2005;579:4423–4429.
- An QD, Zhang GL, Wu HT, Zhang ZC, Zheng GS, Luan L, Murata Y, Li X. Alginate-deriving oligosaccharide production by alginase from newly isolated Flavobacterium sp. LXA and its potential application in protection against pathogens. J Appl Microbiol. 2008;106:161–170.
- An QD, Zhang GL, Wu HT, Zhang ZC, Zheng GS, Zhang YL, Li X, Murata Y. Properties of an alginate-degrading Flavobacterium sp. LXA isolated from rotting algae from coastal China. Can J Microbiol. 2008;54:314–320.
- An QD, Zhang GL, Wu HT, Zhang ZC, Gong W, Liu Y, Li X, Murata Y. Production and partial properties of alginase from newly isolated Flavobacterium sp. LXA. Proc Biochem. 2008;43:842–847.
- Miller GL. Use of dinitrosalicylic acid for determination of reducing sugar. Anal Chem. 1959;31:426–428.
- Doubois M, Gilles K, Hamilton JK, Rebirs PA, Smith F. Colorimeric method for determination of sugars and substance. Anal Chem. 1956;28:350–356.
- Carver TLW, Robbins MP, Zeyen RJ, Dearne GA. Effects of PAL-specific inhibition on suppression of activated defence and quantitative susceptibility of oats to Erysiphe graminis. Physiol Mol Plant Pathol. 1992;41:149–163.
- Akiyama H, Endo T, Nakakita R, Murata K, Yonemoto Y, Okayama K. Effect of depolymerized alginates on the growth of Bifidobacteria. Biosci Biotechnol Biochem. 1992;56:355–356.
- Iwamoto Y, Xu X, Tamura T, Oda T, Muramatsu T. Enzymatically depolymerized alginate oligomers that cause cytotoxic cytokine. Biosci Biotechnol Biochem. 2003;67:258–263.
- Park PJ, Je JY, Byun HG, Moon SH, Kim SK. Antimicrobial activity of hetero-chitosans and their oligosaccharides with different molecular weights. J Microbiol Biotechnol. 2004;14:317–323.
- Akatsuka T, Kodama O, Sekido H, Kono Y, Takeuchi S. Novel phytoalexins (oryzalexins A, B and C) isolated from rice blast leaves infected with Pyricularia oryzae. Part I: isolation, characterization and biological activities of oryzalexins. Agric Biol Chem. 1985;49:1689–1694.
- Koga J, Shimura M, Oshima K, Ogawa N, Yamauchi T, Ogasawara N. Phytocassanes A, B, C and D, novel diterpene phytoalexins from rice, Oryza sativa L. Tetrahedron. 1995;51:7907–7918.
- Hahlbrock K, Scheel D. Physiology and molecular biology of phenylpropanoid metabolism. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:347–369.
- Shiraishi T, Yamada T, Nicholson RL, Kunoh H. Phenylalanine ammonia-lyase in barley: activity enhancement in response to Erysiphe graminis sp. hordei (race 1) a pathogen, and Erysiphe pisi, a non pathogen. Physiol Mol Plant Pathol. 1995;46:153–162.
- Ramanathan A, Samiyappan R, Vidhyasekaran P. Induction of defence mechanisms in greengram leaves and suspension cultured cells by Macrophomina phaseolina and its elicitors. J Plant Disease Protect. 2000;107:245–257.
- Aktar MW, Sengupta D, Chowdhury A. Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol. 2009;2:1–12.
- Daw BD, Zhang LH, Wang ZZ. Salicylic acid enhances antifungal resistance to Magnaporthe grisea in rice plants. Australas Plant Pathol. 2008;37:637–644.
- Chen M, Zhang C, Zi Q, Qiu D, Liu W, Zeng H. A novel elicitor identified from Magnaporthe oryzae triggers defense responses in tobacco and rice. Plant Cell Rep. 2014;33:1865–1879.