Abstract
Two invasive mussels from the genus Dreissena occur in Bulgarian inland waters – Dreissena polymorpha and Dreissena rostriformis bugensis. They are well known invasive species, but there is lack of information about the presence, quantitative parameters and distribution of their larvae. We present the first data that concern the veliger larvae dynamics in Bulgarian inland waters, with several infested reservoirs being studied within the period 2009–2012. The present study was designed to determine their distribution, density and seasonal dynamics. Veliger larvae were found in all of the studied reservoirs. Their abundance was in the range from 166 individuals per cubic meter (ind m−3) to 28,400 ind m−3 in different seasons and reservoirs. The presence of veliger larvae was detected in the plankton also during the winter: February 2011 (3.7 °C) and March 2012 at temperature 7.5 °C. The maximum abundance of larvae was found at 26 °C. The variation among annual data, which was observed in our study implied that further regular and long-term studies of the early life stages of the Dreissena mussels is required in order to monitor the dynamics of these species in the inland waters of Bulgaria.
Introduction
The mussels of genus Dreissena are of particular interest to the invasive biology because of their rapid spread and negative economic and ecological impact.[Citation1,Citation2] Two Dreissena species: Dreissena polymorpha Pallas, 1771 and Dreissena rostriformis bugensis Andrusov, 1897, inhabit the freshwater ecosystems in Bulgaria.[Citation3–8] The Dreissena mussels have several features that make them excellent invaders. One of those features is the planktonic larval stage, which facilitates their rapid dispersal among water bodies. The planktonic larvae are more evenly distributed and thus, when present, they can provide better quantitative estimates than sampling of the benthic forms. In addition, a thorough understanding of the dynamics of benthic populations requires knowledge of the abundance and distribution of the larval stages.
The study on the occurrence of Dreissena veliger larvae in the plankton samples provides an early warning of a future invasion.[Citation9]
Kozuharov et al. [Citation10,Citation11] and Stanachkova et al. [Citation12,Citation13] published some quantitative data about the veliger larvae in the zooplankton community in several water bodies. The present paper considered directly the distribution and dynamics of the veliger larvae in some Bulgarian reservoirs and their relation to certain environmental factors.
The main goal of the paper was a comparison of the quantitative parameters of the veliger larvae in different water bodies and in different seasons.
Materials and methods
The study was carried out in six reservoirs of different water volumes and depths – Ogosta, Rabisha, Poletkovtsi and Kovachitsa (the Danube river basin), Zhrebchevo and Ovcharitsa (the Aegean river basin) (). The map of distribution was done with the ESRI software ArcGIS 10. Most of the reservoirs were reported as heavily infested by Dreissena spp. – D. polymorpha was found in the reservoirs Ogosta, Rabisha, Zhrebchevo and Ovcharitsa; and D. r. bugensis in the Ogosta Reservoir.[Citation7,Citation8,Citation14] In the Kovachitsa Reservoir empty shells were recorded, while in the Poletkovtsi Reservoir no adult mussels or shells were found.[Citation8,Citation14] The plankton samples were collected in different seasons, from February to October, within the years 2009–2013 (). The samples were obtained by using quantitative plankton net with 55 μm mesh size. The veliger larvae were counted from 187 zooplankton samples by the standard methods used in limnology study.
Table 1. Samples description according the season, place and number.
Results and discussion
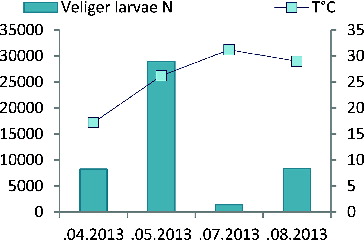
The Dreissena veliger larvae in the infested Zhrebchevo Reservoir were present in the plankton in all seasons during the study. The larvae of zebra mussel were detected in February 2011 at 3.7 °C (). Their abundance was 866 individuals in cubic meter (ind m−3) (the standard deviation was less than 1% from two iterations). Those were probably overwintering larvae. Similar observations were reported by Lucy [Citation15] about lake Lough Key, where zebra mussel larvae were detected in the plankton as early as February 2000 (7.2 °C) and March, 1999 (11 °C). In the same lake, the first definite seasonal appearance of the larvae occurred in May at temperatures higher than 12.5 °C. Previous studies have already reported that this temperature was the lowest at which the Dreissena veliger larvae were found, even in low numbers.[Citation1]
Figure 2. Seasonal dynamics of the veliger larvae N (ind m−3) in relation to water temperature in Zhrebchevo Reservoir (a) and Ogosta Reservoir (b).
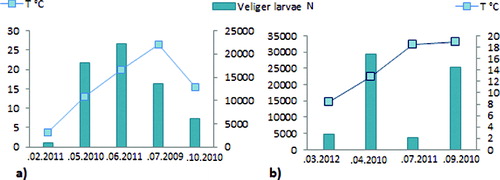
In the Zhrebchevo Reservoir, the quantitative parameters of the veliger larvae increased with the increase of water temperature. The highest larval density (22,300 ind m−3) was detected at temperature of 20 °C (). The larval number decreased during the autumn period, at the end of the spawning period and in October, they were detected only in low numbers – 6159 ind m−3. The optimal water temperature for spawning and development of early life stages of zebra mussel was reported to be 20 °C.[Citation16] Water temperatures above 26 °C seemed to be unfavourable for the development of veliger larvae and their number decreases.[Citation17]
Significant changes in the quantitative parameters of the veligers were found in the ecotone zone of the system Tundzha river – Zhrebchevo Reservoir during the investigated period. The seasonal variations were between 166 ind m−3 and 1875 ind m−3. In July 2009, the larval abundance increased from the ecotone station to the dam, but the trend was not repeated for the rest of samplings.
In the Ogosta Reservoir, the veliger larvae were found in the plankton in March, 2012, at temperature of 7.5 °C (). Most probably those were also overwintering larvae.
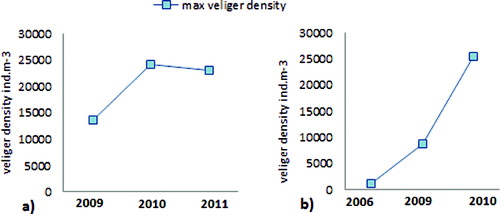
The larval density in the plankton varied with a tendency to increase over the years ().
Figure 3. Maximum values of the veliger larvae density (ind m−3) in Zhrebchevo Reservoir in 2009–2011 (a) and in Ogosta Reservoir in September 2006, 2009 and 2010 (b).
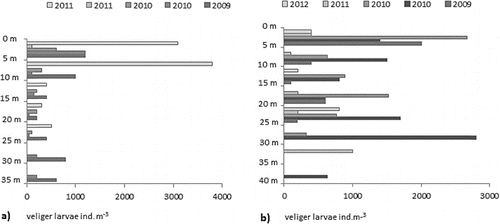
The vertical distribution of the veliger larvae in the reservoirs Ogosta and Zhrebchevo corresponded with spatial distribution of adult mussels in relation to the water depth (). D. r. bugensis has better survivability in the deepest parts of the reservoirs; on the other side, D. polymorpha prefers the shallow ecotone zone between the river and the reservoirs.[Citation13]
Figure 4. Vertical distribution of the abundance of the veliger larvae at the sites with the highest depths in Zhrebchevo Reservoir (a) and Ogosta Reservoir (b).
The Rabisha Reservoir differs from the other studied reservoirs regarding its origin, morphologic and hydrological conditions. It was also reported as heavily infested by zebra mussel.[Citation7,Citation14] Temperature is a key factor for the increasing veliger larvae abundance in Rabisha in the studied period ((a)). A decrease of the veliger larvae density was observed compared to previous studies [Citation10,Citation11] – from 7893 ind m−3 in 2006 to 3943 ind m−3 in 2010 ((b)).
Figure 5. Seasonal dynamics of the veliger larvae in the Rabisha Reservoir in 2009–2010; (a) maximum values of the veliger larvae density (ind m−3) in the Rabisha Reservoir in 2009–2010 compared to previous studies from 2006 after Kozuharov et al.[Citation11] (b).
![Figure 5. Seasonal dynamics of the veliger larvae in the Rabisha Reservoir in 2009–2010; (a) maximum values of the veliger larvae density (ind m−3) in the Rabisha Reservoir in 2009–2010 compared to previous studies from 2006 after Kozuharov et al.[Citation11] (b).](/cms/asset/0228f434-f365-424f-af41-551fbc22cd45/tbeq_a_1047180_f0005_b.gif)
During some of the studied seasons, the abundance of the veliger larvae was higher at site 1, near the dam, compared to the site at the water intake tower – site 2 (). Probably the strong, constant winds blow up most of the veligers to the dam. Most probably this is the reason for the high quantities of the larvae at this station. The water intake tower was colonized strongly by the mussels, because of its suitable substrate, but the constant regime of water outrunning, drifted out the veligers from this area. Our observations showed that some factors, such as the bottom substrate, which is a key element for the development of the adult mussels, as well as the fluctuations in the water level of the reservoirs, can cause a decrease in the density of the larvae in the plankton.
Figure 6. Dynamics of the veliger larvae of D. polymorpha in the Rabisha Reservoir at stations 1, 2 and 3 in 2009–2010, compared to previous studies from 2006 after Kozuharov et al.[Citation11].
![Figure 6. Dynamics of the veliger larvae of D. polymorpha in the Rabisha Reservoir at stations 1, 2 and 3 in 2009–2010, compared to previous studies from 2006 after Kozuharov et al.[Citation11].](/cms/asset/5752a1d7-30b2-4032-a0e5-5ff8f21055db/tbeq_a_1047180_f0006_b.gif)
During our study, veliger larvae were found also in the reservoirs Poletkovtsi and Kovachitsa. In the Poletkovtsi Reservoir no adult mussels or shells of Dreissena spp. were recorded in the previous studies.[Citation8,Citation14] However, Kozuharov et al. [Citation10,Citation11] reported on the occurrence of veliger larvae in the plankton of the Poletkovtsi Reservoir in 2006. Our results showed that for the next four year period the quantities of the larvae have increased up to 4780 ind m−3 for all three sampling points () and the frequency of occurrence reached 75%. The reservoir was used intensively for fishery and the presence of larvae may be explained with possible continuous introduction of larvae together with the fish stocking material.
Figure 7. Dynamics of the veliger larvae in the Poletkovtsi Reservoir at station 1, 2 and 3 in September 2009 compared to previous studies from 2006 after Kozuharov et al.[Citation11].
![Figure 7. Dynamics of the veliger larvae in the Poletkovtsi Reservoir at station 1, 2 and 3 in September 2009 compared to previous studies from 2006 after Kozuharov et al.[Citation11].](/cms/asset/04a12ccf-964e-4570-af2f-a6aff9c072da/tbeq_a_1047180_f0007_b.gif)
In the Kovachitsa Reservoir, the presence of large amounts of empty shells, single live individuals and veliger larvae was reported in previous studies.[Citation8,Citation14] During our study, veliger larvae of low density, 320 ind m−3, were found in the reservoir.
In the infested Ovcharitsa Reservoir, the maximum abundance of veliger larvae (28,400 ind m−3) was detected in May 2013 at 26 °C (). It was the highest quantity among all of the studied reservoirs. In the same year in July, the abundance of the larvae decreased to 1404 ind m−3, when the water temperature reached 31 °C.
The Ovcharitsa Reservoir is a thermal power plant cooling reservoir. Comparatively high water temperatures have been maintained during the whole year. It was reported that high temperatures, over 30 °C, are unfavourable for the mussels and can cause mortality, which can affect the veliger larvae abundance and seasonal dynamics.[Citation18] Another reason for the low densities of the larvae in July 2013 might be the settlement of the majority of the veligers. Settlement rates reflect the survival of veligers through the post-veliger stage to the settled juvenile. The spawning of D. polymorpha in the Ovcharitsa Reservoir happens earlier than other investigated reservoirs, which reflects on the larval dynamics of the veliger larvae. Similar high settlement rate was observed by Churchill [Citation19] for the larval dynamics of the zebra mussel population in a North Texas Reservoir.
Conclusions
Veliger larvae were found in all of the studied reservoirs. Their abundance was in the range from 166 ind m−3 to 28,400 ind m−3 in different seasons and reservoirs. The presence and dynamics of the veliger larvae in the reservoirs indicated potential infestation by Dreissena mussels and reflected the state of the invaded populations. The changes in the density of the veliger larvae may be due to changes of environmental factors, such as water temperature, fluctuations in the water level, type of substrate, etc., in the studied reservoirs. Therefore, regular and long-term monitoring for the presence and abundance of the veliger larvae, as well as for ecological status of the water bodies in terms of the Water Framework Directive EU 2000/60 is recommended. Such important monitoring of the Iskar reservoir, which is the biggest reservoir in Bulgaria, has already been provided. This reservoir has high importance because it is the main water source for drinking water of Sofia city and because it produces electricity by two water power plants what have been built there.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Nalepa TF, Schloesser DW, editors. Zebra mussels, biology, impacts and control. Boca Raton (FL): Taylor & Francis Taylor & Francis; 1993.
- Van Der Velde G, Rajagopal S, Bij De Vaate A, editors. The zebra mussel in Europe. Leiden: Taylor & Francis; 2010.
- Hubenov Z. Dreissena (Bivalvia: Dreissenidae) – systematics, autochthonous and anthropogenic areas. Acta Zool Bulg. 2005;57(3):259–268.
- Hubenov Z, Trichkova T. Dreissena bugensis (Mollusca: Bivalvia: Dreissenidae) – new invasive species for the Bulgarian malacofauna. Acta Zool Bulg. 2007;59(2):203–210.
- Kozuharov D, Trichkova T, Hubenov Z, et al. Distribution of Dreissena polymorpha in human-made lakes along the Lesnovska River (tributary of Iskar River, Danube drainage basin). Paper presented at: Proceedings of the Anniversary Scientific Conference of Ecology; 2008 Nov 1; Plovdiv, Bulgaria.
- Kozuharov D, Trichkova T, Botev I, et al. Invasion of Dreissena polymorpha (Pallas, 1771) to reservoirs in the Struma River basin (Aegean Sea drainage basin, South-West Bulgaria). Biotechnol Biotechnol Equip. 2009;23(Special Edition/ On-Line):192–196.
- Trichkova T, Kozuharov D, Hubenov Z, et al. Characteristics of zebra mussel (Dreissena polymorpha) populations in infested reservoirs, North-West Bulgaria. J Nat Hist. 2008;42:619–631.
- Trichkova T, Kozuharov D, Hubenov Z, et al. Current distribution of Dreissena species in the inland waters of Bulgaria. Int Sci Publications: Ecol Saf. 2009;3(1):507–516.
- Rusev B. [The mussel Dreissena polymorpha – major threat to hydrotechnical facilities]. Priroda. 1965;3:85–87. Bulgarian.
- Kozuharov D, Trichkova T, Borisova P, Stanachkova M. The zooplankton composition in two reservoirs in the North-West Bulgaria in relation to Dreissena spp. occurrence. Biotechnol Biotechnol Equip. 2009;23:271–275.
- Kozuharov D, Trichkova T, Stanachkova M, et al. Comparative analysis of zooplankton composition in reservoirs of North-West Bulgaria: relation to water physicochemical parameters and Dreissena infestation. Acta Zool Bulg. 2013;65(3):359–370.
- Stanachkova M, Kozuharov D, Trichkova T, et al. Changes in zooplankton community of Jrebchevo Reservoir before and after infestation by Dreissena polymorpha (Pallas, 1771). Paper presented at: Scientific Youth Conference “Kliment's Days”; 2010 Nov 22–23; Sofia, Bulgaria.
- Stanachkova M, Kozuharov D, Trichkova T, et al. Development of zooplankton of Ogosta Dam within 2009–2010 period in relationship with mussels from Dreissena Genus. Paper presented at: Proceedings International Conference Ecology – Interdisciplinary Science and Practice; 2012 Oct 25–26; Sofia, Bulgaria.
- Trichkova T, Kotsev A, Popov A, et al. Assessment of zebra mussel (Dreissena polymorpha) infestation risk using GIS for water basins in the North-West Bulgaria. Paper presented at: 15th International Conference on Aquatic Invasive Species (ICAIS); 2007 Sept 23–27; Nijemegen, The Netherlands.
- Lucy F. Early life stages of Dreissena polymorpha (zebra mussel): the importance of long term datasets in invasion ecology. Aquat Invasions. 2006;1–3:171–182.
- Neumann D, Jenner H. The zebra mussel Dreissena polymorpha. Ecology, biological monitoring and first applications in the water quality management. Stuttgart: Taylor & Francis; 1992.
- Wilhelm S, Adrian R. Long term response of Dreissena polymorpha Pallas (Molusca: Bivalvia). Freshw Biol. 2007;12:552–558.
- Matthews A, McMahon RF. Effects of temperature and temperature acclimation on survival of zebra mussel (Dreissena polymorpha) and Asian clams (Corbicula fluminea) under extreme hypoxia. J Molluscan Stud. 1999;65:317–325.
- Churchill JC. Spatio-temporal spawning and larval dynamics of a zebra mussel (Dreissena polymorpha) population in a North Texas Reservoir: implications for invasions in the southern United States. Aquat Invasions 2013;8(4):389–406.