 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study aimed to evaluate the relationship between physical function, disease activity, spinal mobility and bone parameters in ankylosing spondylitis (AS) patients. Fifty patients (27 men and 23 women) were examined. The clinical assessment included Bath AS Disease Activity Index, AS Disease Activity Score, Bath AS Functional Index, Bath AS Metrology Index (BASMI). Lumbar spine and femoral neck bone mineral density (BMD), spinal trabecular bone score (TBS) and the TBS T-score were calculated by dual-energy X-ray absorptiometry. Prevalence rates for osteoporosis and osteopaenia were 14% and 36%, respectively and for partially and fully degraded microarchitecture – 34% and 16%, respectively. A similar inverse correlation was observed between BASMI and TBS, TBS T-score and femoral BMD bone parameters, which were significantly lower in patients ≥45 years of age. No significant correlations were detected between any bone parameter and indicators of disease activity and physical function. Patients with a disease duration of ≥10 years tended to exhibit either normal or elevated spine BMD. Femoral BMD was lower in men. The higher BASMI was associated with an increased likelihood of TBS < 1.350 (odds ratio (OR) = 1.44, 1.05–1.97, p = 0.024) and TBS T-score < –1.00 (OR = 1.55, 1.11–2.16, p = 0.01). In summary, lumbar spine BMD can be erroneously influenced by osteoproliferation, unlike the TBS and TBS T-score. The limitations in spinal mobility predicted abnormal results for these two TBS parameters. TBS may be a better indicator of bone health than BMD in AS.
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory rheumatological disease of unknown origin that primarily affects the axial skeleton, including the sacroiliac joints and spine, and also the peripheral joints and entheses.[Citation1] New bone formation, which includes syndesmophytes and ankylosis of the spine, is almost pathognomonic for AS.[Citation2] Osteoproliferation causes abnormal spinal rigidity, which results in irreversible stiffness and loss of spinal mobility. Bony overgrowth in AS has been considered a structural damage arising from chronic immune activation and inflammation,[Citation3] although the link between inflammation and ankylosis is not completely understood in this disease.[Citation3,Citation4]
A study by Ralston et al. [Citation5] emphasized the difference between the processes taking place in cortical versus trabecular bone in AS. While trabecular bone mass decreases, leading to vertebral osteoporosis, specific sites of the cortical bone start to proliferate and expand.[Citation5,Citation3] In general, AS is associated with bone loss in the vertebrae and an increased prevalence of vertebral fractures.[Citation6,Citation7] Cooper et al. [Citation6] identified markedly increased relative risk of vertebral morphometric deformities in patients with AS compared with the control population. The review of recent literature on osteoporosis and vertebral fractures in AS published by Davey–Ranasinghe and Deodhar [Citation8] suggests that the prevalence of osteoporosis in AS patients is 25% and that of vertebral fractures is 10%. Both new bone formation and vertebral osteoporosis with its consequences might contribute to spinal pain, stiffness, loss of mobility and functional impairment in these patients. Osteopaenia is already seen in the early stages of AS.
Bone mineral density (BMD) evaluated by dual-energy X-ray absorptiometry (DXA) is commonly used to diagnose osteoporosis. However, any proper assessment of bone density is difficult in the presence of syndesmophytes, especially in later stages of AS, because they may give rise to falsely high values on DXA of the lumbar spine. Moreover, it has recently been shown that bone mass is not the only factor that contributes to bone strength. Probably because it only measures the quantity and not the quality of bone, BMD suffers limitations in fracture prediction.[Citation9,Citation10] Evaluation of bone microarchitecture might, therefore, improve the assessments of bone strength and quality.[Citation9–11] The trabecular bone score (TBS) is a new and relevant bone texture index that can be calculated by using pre-existing DXA images. It is based on the rate of grey-level amplitude variations in trabecular bone. Although it is not a direct measurement, the TBS has been shown to be an indicator of both bone microarchitecture and mechanical parameters.[Citation12]
The aim of this study was to evaluate the relationship between physical function, disease activity, spinal mobility and bone parameters – TBS and BMD in patients with ankylosing spondylitis.
Subjects and methods
Patients
Fifty patients [27 men (54%) and 23 women (46%)] diagnosed with AS, according to the Modified New York criteria,[Citation13] were included in this cross-sectional study. All participants were Caucasian. The exclusion criteria were specific endocrine disorders (hyperparathyroidism, hyperthyroidism), systemic high-dose corticosteroid therapy, alcohol abuse and chronic renal or liver diseases. All patients gave their consent to participate in the study.
Clinical and laboratory assessments
The clinical assessment included a collection of demographic data like age and sex, recording basic clinical parameters like height, weight and body mass index (BMI, kg/m2) and obtaining details regarding any therapy administered for AS.
The disease activity state was assessed at the time point of measuring the bone parameters by using the Bath Ankylosing Spondylitis Disease Index (BASDAI),[Citation14] the Ankylosing Spondylitis Disease Activity Score (ASDAS),[Citation15] the inflammatory biomarkers erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). The ASDAS score was calculated by using the most widely adopted formula
To measure the functional disability, the participants were asked to complete the Bath ankylosing spondylitis functional index (BASFI).[Citation16] Disease status and changes in spinal movement were assessed by using the Bath ankylosing spondylitis metrology index (BASMI).[Citation17]
Bone parameters measurement
All DXA scans were performed by using a GE Lunar (GE Healthcare Lunar, Madison, WI, USA) scanner, adopting standard procedures supplied by the manufacturer for scanning and analysis. The DXA device was cross-calibrated by using anthropomorphic phantoms, as part of a standardized daily quality control protocol and exhibited a stable long-term performance [coefficient of variation (CV) < 0.5%]. Anthropometric measurements (height and weight) and calculation of BMI were done at the time of DXA scanning. BMD was measured for the lumbar spine (posterior–anterior projection at L1-L4) and for the femoral neck. A T-score ≤ –2.5 was defined as osteoporosis and between –2.5 and –1.0 - as osteopaenia, as per the World Health Organization (WHO) classification scheme.[Citation18] Male patients were categorized by using the same criteria used for the categorization of the females.
The TBS is an index that characterizes the bone texture obtained by macroscopic representation (DXA image). It was evaluated based on the grey-level analysis of the DXA images as the slope at the origin of the log–log representation of the experimental variogram.[Citation19,Citation20] TBS measurement was performed by using a specific software that had been installed onto the DXA device (TBS iNsight v1.9, Medimaps group, Geneva, Switzerland). The software uses posteroanterior raw spine images from the densitometer, including the BMD region of interest and edge detection, so that TBS calculation is performed over the exact same region of interest as the BMD measurement. We calculated an adjusted TBS for men, as this software version had been designed for women. The cut-off points for TBS values in postmenopausal woman were used, established by a working group of TBS users from different countries.[Citation21] With this grading scheme, a TBS ≥ 1.350 is considered normal, a TBS between 1.200 and 1.350 is considered consistent with partially degraded microarchitecture and a TBS ≤ 1.200 signifies fully degraded microarchitecture. These ranges were created to be analogous to the three BMD categories: normal bone mass, osteopaenia and osteoporosis and could be used to detect women at high risk of osteoporotic fracture independently and in addition to the BMD classification.[Citation22] A normal range for TBS in men has not yet been proposed,[Citation12] so we used the same category thresholds as for women. From the TBS raw score, a TBS T-score was calculated.
Statistical analysеs
Statistical analyses were performed by using the Statistics Package for the Social Sciences (SPSS) version 16.0 (SPSS Inc., Chicago, IL). Spearman's correlation coefficients were calculated to assess univariate associations between clinical, laboratory and bone parameters. Intergroup comparisons were performed by using the Mann–Whitney test for continuous variables and χ2 analyses, Fisher's exact test for categorical variables. Binary logistic regression analysis was undertaken to investigate the independent contributions of BASDAI, ASDAS-CRP, BASFI and BASMI as predictors of dichotomous dependent variables for the TBS, TBS T-score and femoral neck BMD. Other covariates entered into the model were patient's age and sex, disease duration, ESR, CRP and therapy for AS. The main explanatory variables were used as continuous variables. TBS scores were grouped into two categories: ≥1.350 and <1.350. Femoral neck BMD scores and the TBS T-score were also subdivided into two categories: ≥–1.00 and <−1.00, when treated as dependent variables. Odds ratios (ORs) with a 95% confidentiality interval (CI) were calculated to identify any association between the BASMI score and both the TBS category score and the T-score. In addition, OR were calculated to assess the associations between patient's age and femoral neck BMD, as a categorical variable. Results were expressed as regression coefficients. A two-tailed p-value of <0.05 was considered significant.
Results and discussion
Cohort of patients
In total, 50 AS patients were studied. With 27 males and 23 females, the male to female ratio was roughly 1: 1. The mean (±SD) age of the patients was 41.6 ± 10.1 years (range 25.9–61.3 years). Only two of the women, included, in the study, were postmenopausal.
The mean (± SD) disease duration was 12.6 ± 8.5 years (range 1–37). In terms of drug usage, 11 patients (22%) were only on first-line therapy, a non-steroidal anti-inflammatory drug at the time of the study, 5 (10%) were being treated with a synthetic disease-modifying antirheumatic drug (sulfasalazine or methotrexate) and 34 (68%) were receiving a tumour necrosis factor (TNF)-blocking agents (etanercept, adalimumab or golimumab). None of the patients exhibited evidence of a fracture in any of the visualized vertebrae.
presents key characteristics of the 50 patients. The means (± SD) for bone parameters were: lumbar spine TBS (1.319 ± 0.145), lumbar spine TBS T-score (–0.807±1.514), lumbar spine BMD T-score (–0.862 ± 1.739) and femoral neck BMD T-score (–1.436 ± 1.048). The wide ranges of these scores reflect the broad spectrum of our population with AS. By using the WHO categorization scheme, 50% of the patients were deemed to have a normal lumbar BMD, 36% were osteopaenic and 14% were osteoporotic. This compares with normal TBS in 50% of the patients, with partially degraded and fully degraded microarchitecture seen in 34% and 16%, respectively.
Table 1. Clinical characteristics of the 50 patients.
Associations between bone parameters and clinical characteristics
Spearman's correlation coefficients were calculated to investigate univariate associations between bone parameters and cross-sectionally assessed BASDAI, ASDAS-CRP, BASFI and BASMI for the entire group. We found moderately strong inverse correlations between TBS and BASMI (r = –0.34; p = 0.02), between the TBS T-score and BASMI (r = –0.35; p = 0.01) and between femoral BMD and BASMI (r = –0.395; p = 0.004). The correlation coefficient for the relationship between the TBS T-score and age was –0.29 (p = 0.045), suggesting a weak inverse association, while TBS only exhibited a borderline inverse correlation with age (r = –0.27; p = 0.06). Femoral BMD was moderately inversely correlated with age (r = –0.44; p = 0.001). In addition, the lumbar spine TBS and TBS T-score were moderately to strongly correlated with BMI (both r = –0.65; p < 0.001). No significant correlations were observed between bone parameters and other markers of disease activity, physical function or applied therapy administered for AS.
When comparing patients, who were 45 years old or older with those, who were under this age, the former had a significantly lower TBS (1.266 ± 0.14 vs. 1.355 ± 0.14; p = 0.03) () and TBS T-score (–1.429 ± 1.49 vs. –0.392 ± 1.41; p = 0.03) (). In addition, older patients had a lower femoral neck BMD (–1.95 ± 0.88 vs. –1.09 ± 1.03; p = 0.003) (). No significant differences were observed in TBS, TBS T-score or femoral neck BMD between patients, whose disease had been present for ≥10 years versus <10 years (–). Interestingly, patients, who have had the disease for over 10 years tended to have higher lumbar spine BMD than those with a shorter duration of the disease (–0.423 ± 1.80 vs. –1.302 ± 1.59, respectively; p = 0.07) ().
Figure 1. Comparing TBS by sex, age (under 45 years vs. ≥45 years) and disease duration (under 10 years vs. ≥10 years).
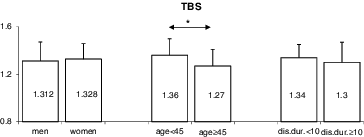
Figure 2. Comparing TBS T-scores by sex, age (under 45 years vs. ≥45 years) and disease duration (under 10 years vs. ≥10 years).
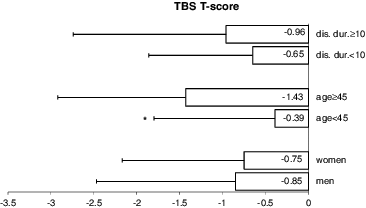
Figure 3. Comparing femoral neck BMD by sex, age (under 45 years vs. ≥45 years) and disease duration (under 10 years vs. ≥10 years).
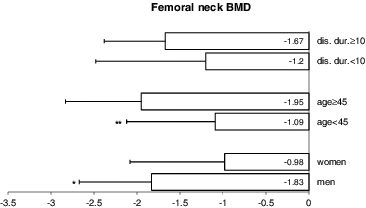
Figure 4. Comparing lumbar spine BMD by sex, age (under 45 years vs. ≥45 years) and disease duration (under 10 years vs. ≥10 years).
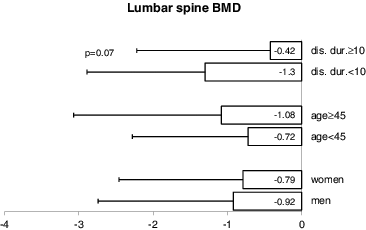
When comparing men and women, the disease duration at the time of study entry (14.4 ± 9.1 vs. 10.4 ± 7.3; p = 0.08), patient's age (42.4 ± 10.4 vs. 40.7 ± 9.8; p = 0.55) and BMI (26.5 ± 3.98 vs. 24.7 ± 4.85; p = 0.16) were not significantly different. Femoral neck BMD, when expressed as a T-score, was lower in men than in women. The mean femoral neck BMD for men was –1.826 ± 0.84 vs. –0.978 ± 1.10 for women (p = 0.01) (). No significant sex differences were apparent in lumbar spine BMD, TBS or TBS T-score (, and ).
Adjustment for age, sex, disease duration and other covariates
To further investigate the relationship between bone parameters and both clinical and laboratory assessments of the disease, while adjusting for age, sex, disease duration and other potential confounders, multivariate analysis was performed by using binary logistic regression with TBS, TBS T-score and hip BMD T-score as a dichotomous dependent variable. presents the results of this analysis. Disease duration, sex, BASDAI, ASDAS-CRP, BASFI, ESR, CRP and therapy for AS did not exhibit a significant independent contribution in the models. The parameter estimate, like a regression coefficient, describes the independent relationship between the explanatory variable (BASMI, age) and the dependent variables (TBS, TBS T-score and femoral neck BMD T-score). ORs represent the likelihood of degraded versus normal microarchitecture and the likelihood of osteopaenia/osteoporosis versus normal BMD. Only BASMI independently contributed to explaining TBS. Those with a higher BASMI were more likely to have either partially or fully degraded trabecular microarchitecture, with an OR of 1.44 (95% CI from 1.05 to 1.97, p = 0.024). Other regression models revealed that increased axial impairment also significantly increased a subject's likelihood of having an abnormal TBS T-score: on average, the increase with one unit in BASMI was associated with odds of a TBS T-score <–1.00 of 1.55 (95% CI from 1.11 to 2.16, p = 0.010). The subject's age was independently associated with a femoral neck BMD <–1.00 (OR = 1.08, 95% CI from 1.01 to 1.15, p = 0.03).
Table 2. Multivariate relationship between BASMI and TBS / TBS Т-score and between age and femoral neck T-score, established by binary logistic regression in 50 patients with AS.
When comparing subjects with lumbar spine BMD ≥−1.00 versus <–1.00, we failed to reveal any significant differences in sex distribution, in mean age, disease duration, BASMI, BASDAI, ASDAS-CRP, BASFI, ESR or CRP and therapy administered for AS. For this reason, multivariate analysis with spine BMD as a dependent variable was not performed.
Final remarks
Osteopaenia and osteoporosis are common in patients with AS. In our cohort, reduced bone mass (osteopaenia and osteoporosis) were detected in half of the patients. DXA is a practical and safe method to measure BMD.[Citation23] However, it has limitations in AS, especially since spinal measurements might be artificially elevated as a consequence of sclerosis of the vertebral margins, syndesmophytes or total bony bridging between vertebrae. Our study was consistent with this conjecture, as we identified a trend towards higher and more ‘normal’ values of lumbar spine bone density in patients with longstanding AS. The observations that osteoproliferative changes can confound BMD spine measurements gave us grounds to evaluate the TBS in patients with AS.
TBS is a new texture measurement that reflects the trabecular microstructure.[Citation19,Citation20,Citation24]. In this way, the TBS provides an indirect index of trabecular microarchitecture quality: high TBS value is associated with better bone structure, whereas low TBS value indicates worse bone structure.[Citation12] Changes in TBS are less studied in AS. One small study demonstrated lower TBS in AS patients relative to healthy controls, suggesting alterations in bone microarchitecture.[Citation25] Our findings support these results. By using the TBS, we found microarchitectural deterioration of bone tissue in half of our AS patients. As expected, adverse alterations in bone microarchitecture were more prominent in older patients. Sex did not appreciably modify the odds of having an altered trabecular structure; the TBS seems to be equally decreased in men and women.
We did find that men in our study had a significantly lower femoral neck BMD than women of the same age and disease duration. This might have been due to the milder overall course of AS in women. The hip BMD T-score was also less in older patients, decreasing progressively with age. On multivariate analysis, age independently, albeit marginally, predicted a femoral BMD in the osteopaenia or osteoporosis range.
Furthermore, we assessed the relationship between bone parameters (TBS and BMD) and physical function, disease activity and spinal mobility in patients with AS. Some earlier studies have shown that a reduced lumbar spine BMD is related to disease severity.[Citation26–28] In this study, we failed to identify any association between bone parameters (TBS and BMD) and disease activity measures or functional outcomes, or with the level of administered therapy. However, we observed an inverse correlation between spinal mobility and the three bone parameters (TBS, TBS T-score and femoral BMD), indicating an association between worsened bone health and impaired axial status. Multivariate analysis demonstrated a nearly 1.5-fold higher risk of degraded bone microarchitecture with each unit elevation in the BASMI. Lost spinal mobility, as indicated by the BASMI, also appears to affect the TBS T-score and to increase the risk of low bone mass among AS patients. This suggests that bone assessment panels that incorporate the TBS, either as a normatively adjusted score or as a T-score, may be a better choice for evaluating bone health in AS than relying on BMD alone.
One of the major strengths of our study was that we assessed bone health in AS patients by utilizing the full panel of bone parameters obtained by DXA imaging.
A methodical shortcoming was the cross-sectional design. It would have been preferable to prospectively follow patients, starting at an early stage of the disease and observing the progression of bone microstructure deterioration over time and incidence of osteoporotic fracture. Another study limitation was the small number of patients, which limits not only the power of statistical analysis but the generalizability of results; clearly, the results that we observed in our study must be confirmed in larger populations. Also, we did not collect information on the presence or absence of vertebral syndesmophytes or other bone proliferation by X-ray, due to incomplete data across the entire group of patients. This may have caused an underestimation of factors associated with worsened bone health, especially since the degree of spinal mobility appears to influence TBS and spinal mobility is influenced by the degree of osteoproliferation. This being said, ours is one of the few studies investigating TBS in AS patients and a good first step to understanding this relationship.
Conclusions
In summary, our results provided further evidence that lumbar spine BMD may be falsely influenced by new bone formation in ankylosing spondylitis. However, measures of TBS in the spine are not impacted/affected by bone proliferation, which likely renders them more accurate parameters to evaluate bone than spine BMD in these patients. TBS also appears to reflect limitations in spinal mobility. Based upon these findings, we conclude that, in addition to measuring BMD by DXA, measuring TBS might improve the assessment of bone health in patients with AS.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Sieper J, Braun J, Rudwaleit M, et al. Ankylosing spondylitis: an overview. Ann Rheum Dis. 2002;61(3):8–18.
- Baraliakos X, Listing J, Rudwaleit M, et al. Progression of radiographic damage in patients with ankylosing spondylitis: defining the central role of syndesmophytes. Ann Rheum Dis. 2007;66:910–915.
- Schett G, Rudwaleit M. Can we stop progression of ankylosing spondylitis? Best Pract Res Clin Rheum. 2010;24:363–371.
- Van der Heijde, Maksymowych W. Spondyloarthritis: state of the art and future perspectives. Ann Rheum Dis. 2010;69:949–954.
- Ralston SH, Urquhart GD, Brzeski M, et al. Prevalence of vertebral compression fractures due to osteoporosis in ankylosing spondylitis. BMJ. 1990;300:563–565.
- Cooper C, Carbone L, Michet CJ, et al. Fracture risk in patients with ankylosing spondylitis: a population based study. J Rheumatol. 1994;21:1877–1882.
- Vosse D, Landewe R, van der Heijde D, et al. Ankylosing spondylitis and the risk of fracture: results from a large primary care-based nested case-control study. Ann Rheum Dis. 2009;68:1839–1842.
- Davey-Ranasinghe N, Deodhar A. Osteoporosis and vertebral fractures in ankylosing spondylitis. Curr Opin Rheumatol. 2013;25(4):509–516.
- Rice JC, Cowin SC, Bowman JA. On the dependence of the elasticity and strength of cancellous bone on apparent density. J Biomech. 1988;21:155–168.
- Majumdar S. A review of magnetic resonance (MR) imaging of trabecular bone micro-architecture: contribution to the prediction of biomechanical properties and fracture prevalence. Technol Health Care. 1998;6:321–327.
- Turner CH, Cowin SC, Rho JY, et al. The fabric dependence of the orthotropic elastic constants of cancellous bone. J Biomech. 1990;23:549–561.
- Silva BC, Leslie WD, Resch H, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. JBMR. 2014;29(3):518–530.
- Van der Linden SM, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–368.
- Garrett S, Jenkinson T, Kennedy LG, et al. A new approach to defining disease status in ankylosingspondylitis: the Bath ankylosing spondylitis disease activity index. J Rheumatol. 1994;21:2286–2291.
- Lukas C, Landewe R, Sieper J, et al. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis. 2009;68:18–24.
- Calin A, Garrett S, Whitelock H, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath ankylosing spondylitis functional index. J Rheumatol. 1994;21:2281–2285.
- Jenkinson TR, Mallorie PA, Whitelock HC, et al. Defining spinal mobility in ankylosing spondylitis (AS): the Bath AS metrology index. J Rheumatol. 1994;21:1694–1698.
- Kanis JA, WHO Study Group. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. Osteoporos Int. 1994;4:368–381.
- Hans D, Barthe N, Boutroy S, et al. Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J Clin Densitom. 2011;14(3):302–312.
- Winzenrieth R, Michelet F, Hans D. Three-dimensional (3D) microarchitecture correlations with 2d projection image gray-level variations assessed by trabecular bone score using high -resolution computed tomographic acquisitions: effects of resolution and noise. J Clin Densitom. 2013;16(3):287–296.
- Cormier C, Lamy O, Poriau S. TBS in routine clinical practice: proposals of use [Internet]. Plan-les-Outes, Switzerland: Medimaps Group. 2012; Available from: http://www.medimapsgroup.com/upload/MEDIMAPS-UK-WEB.pdf
- Hans D, Goertzen AL, Krieg MA, et al. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res. 2011;26(11):2762–2769.
- El Maghraoui A, Roux C. DXA scanning in clinical practice. QJM. 2008;101:605–617.
- Pothuaud L, Carceller P, Hans D. Correlations between grey‐level variations in 2D projection images (TBS) and 3D microarchitecture: applications in the study of human trabecular bone microarchitecture. Bone. 2008;42(4):775–787.
- Toussirot E, Mourot L, Wendling D, et al. Trabecular bone score in rheumatoid arthritis and ankylosing spondylitis and changes during long term treatment with TNFA blocking agents. Ann Rheum Dis. 2013;72(Suppl3):1008.
- Maillefert JF, Aho LS, El Maghraoui A, et al. Changes in bone density in patients with ankylosing spondylitis: a two-year follow-up study. Osteoporos Int. 2001;12:605–609.
- Gratacos J, Collado A, Pons F, et al. Significant loss of bone mass in patients with early, active ankylosing spondylitis: a follow up study. Arthritis Rheum. 1999;42:2319–2324.
- Ghozlani I, Ghazi M, Nouijai A, et al. Prevalence and risk factors of osteoporosis and vertebral fractures in patients with ankylosing spondylitis. Bone. 2009;44:772–776.
