Abstract
The aim of this work was to study the status of the human epidermal growth factor receptor 2 (HER2), the oestrogen receptor (ER) and the progesterone receptor (PR) in the south-eastern region of Romania, in the population that was of a reproductive age at the time of the Chernobyl disaster. Two hundred and fifteen female patients diagnosed with invasive ductal carcinoma in the period January 2007–December 2012 were included in a retrospective follow-up study until December 2014. The urban/rural area ratio was 1.8:1. The results showed that most of the female patients had ER+/PR+, HER2+ (45.58%), the least aggressive of all the combinations studied (a two-year survival rate of 63.27%). The ER−/PR−, HER2– combination – the most aggressive one – was identified in the group with the lowest age average at the time of diagnosis (56.22 years). Of these cases, 10% presented relapses and the two-year survival rate was as low as 25%. The most frequent metastases at the time of diagnosis were seen in the ER–/PR−, HER2+ group (73.68%), followed by the ER−/PR−, HER2– group (70%). There was a low-to-medium correlation between the tumour marker combinations and the presence of metastases at the time of diagnosis and the two-year survival rate (p < 0.001). The obtained results about the specific association between the studied markers and the response to and outcome from treatment could be useful in determining the therapeutic conduct and prognosis in invasive ductal carcinoma patients from south-east Romania.
Introduction
The prognosis and evolution of breast invasive ductal carcinoma are in association with the type of receptors found in the tumour cells.[Citation1] The markers that seem to most influence this type of malignant pathology are the human epidermal growth factor receptor 2 (HER2), the oestrogen receptor (ER) and the progesterone receptor (PR).[Citation2] That is why the American Society of Clinical Oncology and the College of American Pathologists (ASCO/CAP) recommend testing the HER2, ER and PR status in all female patients with invasive breast cancer.[Citation3–5]
As highlighted in the ASCO/CAP therapeutic guide, which is based on the HER2 value, it is essential to determine the receptors of the tumour cells.[Citation6] The ER represent the main indicator for the efficiency of endocrine therapy. It is known that approximately 70% of female patients diagnosed with breast cancer are ER+.[Citation7]
In view of the therapy response, positive HER2 status is associated with relative resistance to endocrine therapy, especially to tamoxifen, but not to aromatase inhibitors.[Citation8,Citation9] Studies conducted on large patient groups identify HER2 in approximately 20% of the cases.[Citation10]
A study commissioned by the International Agency for Research on Cancer tracked breast cancer mortality in 30 European countries between 1989 and 2006 and found a trend towards increased mortality in Romania by 17%.[Citation11] Furthermore, another study on the incidence and cancer mortality in the last 20–40 years in eight European countries, including Romania, which was requested by the South Eastern European Research Oncology Group and was published in 2011, found that the most common cancer in the female population was breast cancer.[Citation12]
Romania, like other countries in the region, was affected by radiation from the Chernobyl disaster (26 April 1986). Studies conducted in the Republic of Belarus and Ukraine during 1997 and 2001 show an increase in the incidence of breast cancer after the Chernobyl disaster, especially among women who, at the time of the accident, were at a young age.[Citation13]
In Romania, several recent studies on the tumour markers in patients with invasive ductal carcinoma in the south of the country (Craiova) have been conducted. Thus, HER2 positivity was found in 50% of cases of invasive ductal carcinoma and in 17% of cases of ductal carcinoma in situ.[Citation14] Another study in the same south area of Romania followed 40 ER-negative patients diagnosed with invasive breast carcinoma and showed that the combination of ER–/PR–/HER2+ was more frequently found (81.81%) in people with a higher cancer stage (II/III).[Citation15]
In light of these reports, the aim of this work was to study the HER2, ER and PR status in a cohort of invasive ductal carcinoma patients, who were of a reproductive age at the time of the Chernobyl disaster, in south-east Romania, one of the areas most severely affected by radiation.
Subjects and methods
Subjects
The study included 215 female patients diagnosed with breast invasive ductal carcinoma between 1 January 2007 and 31 December 2012, at the ‘Sf. Andrei’ County Emergency Hospital in Galaţi and the General Railways Hospital in Galaţi (Romania). All the patients were of a reproductive age at the time of the Chernobyl disaster (26 April 1986). Detailed characteristics of the cohort of patients are presented in .
Table 1. Descriptive statistics of the studied group female patients.
Study design
The study design was that of a retrospective study with a two-year follow-up, until December 2014. Informed consent was obtained from all patients. All criteria of medical and deontological ethics and the law of Romania were met and the study was approved by the Ethics Committee of the Faculty of Medicine and Pharmacy, “Lower Danube” University of Galati.
Diagnosis criteria
Diagnosis was made following histopathological examination, a procedure of haematoxylin–eosin staining and immunohistochemically following tumour biopsy. All female patients underwent a mammographic examination. HER2, ER and PR tests were performed for all the patients included in the study and the two-year survival rate was followed up.
Determination and interpretation of tumour markers
Determination and interpretation of the HER2, ER and PR values were performed by immunohistochemistry, according to the ASCO/CAP HER2 Test Guideline Recommendations and the ASCO/CAP ER and PgR Guideline Recommendations.[Citation3–5] To determine the HER2, ER and PR values, the tumour tissue obtained by tumour biopsy was processed immunohistochemically (BenchMark XT, Ventana) in the first 6 weeks following biopsy, and fixation was performed within 6–48 hours.
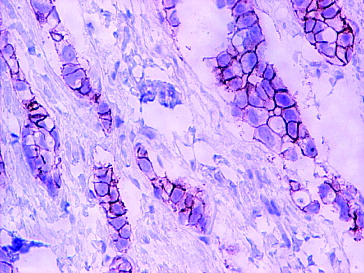
HER2 status, as a function of the cell membrane staining pattern, was expressed as follows:
3+, positive HER2 expression: intense and complete staining of the circumferential cell membrane ();
2+, equivocal for HER2 protein expression: incomplete, low or moderate staining of the membrane in more than 10% of the tumour cells; or intense staining of the circumferential membrane in less than 10% of the tumour cells;
1+, negative HER2 expression: incomplete and low membrane staining detectable in less than 10% of the tumour cells.
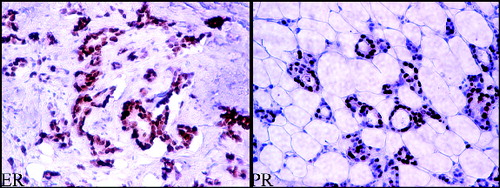
To determine the ER and PR receptors (), 4 μm paraffin-embedded tissue sections were used, stained with 6F11 monoclonal antibodies (for ER) and 1A6 (for PR). The ER and PR status was considered positive, when ≥1% of the tumour cells were immunoreactive.
Statistical analysis
Data analysis was performed using Crosstabs analysis in SPSS version 20.0. The ϕ, C and V coefficients were calculated to determine whether two nominal variables are correlated. The databases used contain mostly nominal values (the values of the variable associated to the tumour markers are their IDs; the values for the variables indicating the survival are ‘yes’/‘no’, etc.). The analysed variables were considered correlated if the p-values associated to each coefficient were less than α = 0.05. In that case, the three coefficients indicate the strength of the correlation.
Results and discussion
Studies on different population groups selected based on geographical or other criteria can give valuable information about any local specifics in the frequency of occurrence of tumour markers and their possible association with tumour progression and therapeutic outcome. By analogy with other reports in different countries, we made an attempt to identify the specific features of the female population diagnosed with invasive ductal carcinoma in the south-east of Romania. Since there are data suggesting a possible relation between the Chernobyl radiation and breast cancer in the Republic of Belarus, [Citation16] this study included a section of the Romanian population who were in their reproductive age approximately three decades ago, when the Chernobyl disaster happened. Moreover, the south-east of Romania was chosen as being one of the regions in Romania most severely affected by radiation released in the Chernobyl explosion.
Frequency of occurrence of tumour markers
It has been suggested that there are differences in the frequency of the HER2, ER and PR markers depending on the geographical region. For example, in 2013, Kuzhan et al. [Citation17] reported that in a cohort of 648 women with breast cancer (in 2006–2012) there is a relation between HER2, ER and PR, and the place of origin, Turkey, Arabia and Kurdistan. Their results showed that the ER+/PR+ combination is more frequently encountered in the Kurdish female patients, while the ER–/PR–/HER2– has the greatest frequency of occurrence in the patients from Turkey.[Citation17] Amend et al. [Citation18] underlined some differences between breast cancer in European-American and in African-American women, the latter presenting more aggressive cancer types at younger ages, a possible cause being lower social-economic status and the limited access to medical assistance. The African-American patients were observed to be more frequently ER– and PR– than the European-American ones, but there were no major differences in the expression of HER2.[Citation18]
In this study, to evaluate the frequency of occurrence of the three studied markers in a cohort of female patients from south-east Romania, the patients were divided into five groups depending on the type of markers present (ER, PR and HER2). Detailed descriptive statistics of the cohort of patients are shown in .
Most female patients presented with ER+/PR+ and HER2+, and the least with ER–/PR– and HER2+ or ER–/PR– and HER2–. Of the studied patients, only 15.82% presented one of the following variants: ER+/PR– or ER–/PR+ with HER2+ and HER2–, respectively.
A study in western India reported a relation among the type of receptors, the tumour degree and the patients’ age in young female patients already menopausal: HER2/neu 0 or 1+ was in association with ER+/PR+ and histopathological grade II.[Citation1] However, another report shows that young menopausal female patients are more frequently ER+/PR– or ER–/PR+; whereas the association among ER+/PR+, HER2 value 0 or 1+ and histopathological grade II is more frequently observed in elderly women with an age average of 68.9 years.[Citation19]
In our study, the average age of onset of breast cancer was generally the fifth decade of life. The ER–/PR–/HER2– variant was present in the group with the lowest age average and ER–/PR–/HER2+ in the group with the highest one.
Among all the groups studied, most women were residents of urban areas. The patients living in an urban environment were almost twice as numerous as those from a rural environment (urban/rural = 1.8/1). It could be speculated that this could, at least in part, be due to differences in eating habits; more specifically, due to higher consumption of organic food in rural parts, with a possible effect of reducing the risk of cancer.[Citation20] This hypothesis is supported by works demonstrating that diets poor in saturated fats and alcohol, but rich in fruits, vegetables and cereals, constitute a protection factor against breast cancer.[Citation21] Other factors might also include the quality of healthcare [Citation18] or the level of pollution.[Citation22]
Furthermore, of all the female patients with a family history of breast cancer, the urban/rural ratio was 16:5. Family history of breast cancer was more frequently encountered in the female patients presenting the ER–/PR–, HER2+ marker combination, but the greatest number of relapses appeared in those with the ER–/PR–, HER2– markers.
Correlation between tumour markers and tumour aggressiveness
Accumulating evidence suggests that there is association between the ER, PR and HER2 status and the tumour aggressiveness. For review, see [Citation23]. Recent studies support the conclusion that triple-negative breast cancer is associated with a high risk of relapse, distal metastases and a low survival rate. For example, Yildiz et al. [Citation24] conducted a study on 204 breast cancer female patients in north-east Turkey and noted that ER–/PR–/HER2– is the most aggressive type, being associated with tumour grades from 2 to 3 (92.5%), with an increased risk of relapse (27.9%) and increased mortality (13.2%). A study conducted at the University of Rochester Medical Center between 1997 and 2008 also showed that, among 447 cases of breast invasive ductal carcinoma, the triple-negative breast cancers (ER–/PR–/HER2–) had the most severe prognosis of all.[Citation25] Similar reports are available for other regions as well, e.g. Morocco.[Citation26] Rais et al. [Citation26] conducted, for a period of 2 years (2007–2008), a study on 152 female patients and noted that the triple-negative breast cancer encountered in 16.5% of the cases studied was also highly aggressive, being associated in 2/3 of the cases with tumour formations over 2 cm and in 48% of the cases with axillary adenopathy and with a 5-year survival rate of 76.5%.
In view of these data, we assessed the possible correlation between the tumour marker combination and the presence of sentinel nodes, respectively metastases, at the time of diagnosis, as well as the two-year survival rate (). The descriptive statistics showed that adenopathies were more frequent in the ER+/PR+ and HER2– group (59%), and, at the level of the entire group studied, 53.49%. However, there was no statistical relation (p = 0.629 > α = 0.05) between these two parameters.
Table 2. Presence of adenopathies as a function of the combination of markers present.
At the time of diagnosis, the greatest number of metastases was discovered in the ER–/PR– and HER2+ female patients (73.68% of the cases) and the ER–/PR– and HER2– ones (70% of the cases). The lowest number of metastases was observed in the ER+/PR+ and HER2– group (22.7%) (). The statistical analysis showed a significant association of tumour markers and the presence of metastases at the time of diagnosis (p < 0.001 < α = 0.05), the ER–/PR– combination being the most aggressive one. The most common metastases were those in the bone tissue, and the least frequent ones, in the thoracic wall ().
Table 3. Presence of metastases as a function of the combination of markers present.
Table 4. Types of metastases at the time of the diagnosis as a function of the type of markers.
Between January 2000 and December 2004, Wang et al. [Citation27] conducted a study on 835 breast cancer female patients undergoing mastectomy and found that ER–/PR– was associated with the highest rate of distal metastases and mortality. During the same period, also in China, a retrospective study on 111 breast cancer female patients showed that the (ER–/PR–) and HER2 overexpression (HER+++) type was associated with a poor prognosis (at 5 years: disease-free survival rate of 70.7%; overall survival rates of 73.1%).[Citation28] Using data from the European Organization for Research and Treatment of Cancer from 9938 breast cancer female patients, van der Hage et al. [Citation29] found that the ER+ female patients had a higher 7-year survival rate than the ER- ones (95%, p = 0.02). The same was true for the PR+ ones over the PR– ones (95% survival chance for the PR+ ones, p = 0.01).[Citation29]
In our study, as the cohort consisted of female patients diagnosed with invasive ductal carcinoma until 2012, only the two-year survival rate could be traced (). The data analysis showed that there was a statistically significant positive correlation (p < 0.001 for the ϕ, V, and C coefficients) between the tumour marker combination and the two-year rate survival.
Table 5. Two-year survival as a function of the combination of markers present.
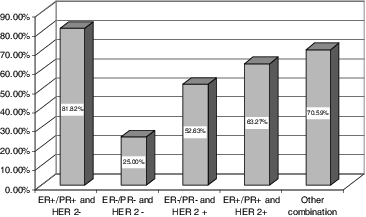
When the two-year survival rate was studied as a function of the presence and type of the tumour markers, the best survival rate was observed in the female patients with ER+/PR+ and HER2+ (). This was, in fact, the group that contained most of the studied patients. The most aggressive combination was shown to be ER–/PR– and HER2–, followed by ER–/PR– and HER2+. The greatest number of tumour relapses (10%) was seen in the group with the youngest female patients, who presented with ER–/PR– and HER2–, where the greatest number of deaths at 2 years since the diagnosis was also observed (75%). These results are in consistence with the above-mentioned studies and indicate similar trends in the studied cohort of patients from south-east Romania as compared to other parts of the world.
Figure 3. Two-year survival as a function of the type of tumour markers present expressed as percentage.
Taken together, the results from this study throw more light on the local specifics of the occurrence of different ER/PR/HER2 types among breast invasive ductal carcinoma patients in south-east Romania and their association with disease progression. The obtained data illustrate the importance of tumour-marker screening tests, especially in populations susceptible to a certain pathology, in view of the underlying therapeutic decisions and prognosis evaluation.
Conclusions
The results from this study showed that female patients in the industrialized urban areas of south-east Romania accounted for almost twice the number of those in the rural areas. The statistical analysis showed a correlation between the type of markers and the presence of metastases. Most metastases were bone ones and were especially associated with the ER–/PR– and HER2+ type. There was also correlation between the two-year survival and the markers present, with the best survival in the ER+/PR+/HER2 + group. The highest aggressiveness belonged to the ER–/PR– and HER2– group, where the greatest number of relapses was seen, and the two-year mortality was also the highest. For this group, the average age at the time of the diagnosis was the lowest, these women being younger when the Chernobyl disaster happened. Our future research will focus on the possible correlation between diet and survival in invasive ductal carcinoma in south-eastern Romania.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Singhai R, Patil V, Patil A. Status of HER-2/neu receptors and Ki-67 in breast cancer of Indian women. Int J Appl Basic Med Res. 2011;1(1):15–19.
- Bozhok AA, Semiglazov VF, Semiglazov VV, et al. Prognostic and predictive factors in breast cancer. Vopr Onkol. 2005;51(4):434–443. Russian.
- Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline update. J Clin Oncol. 2013; 31(31):3997–4013.
- Hammond MEH, Hayes DF, Dowsett M, et al. ASCO-CAP guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28(16):2784–2795.
- Hammond MEH, Hayes DF, Dowsett M, et al. American society of clinical oncology/college of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 2010;134(7):e48–e72.
- Partridge AH, Rumble RB, Carey LA, et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2-negative (or unknown) advanced breast cancer: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2014;32(29):3307–3329.
- Lumachi F, Brunello A, Maruzzo M, et al. Treatment of estrogen receptor-positive breast cancer. Curr Med Chem. 2013;20(5):596–604.
- Konecny G, Pauletti G, Pegram M, et al. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst. 2003;95:142–153.
- Ellis MJ, Coop A, Singh B, et al. Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: evidence from a phase III randomized trial. J Clin Oncol. 2001;19:3808–3816.
- Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6,556 breast cancer tissues. Clin Breast Cancer. 2004;5:63–69.
- Autier P, Boniol M, LaVecchia C, et al. Disparities in breast cancer mortality trends between 30 European countries: retrospective trend analysis of WHO mortality database. BMJ. 2010;341:c3620.
- Vrdoljak E, Wojtukiewicz MZ, Pienkowski T, et al. Cancer epidemiology in Central and South Eastern European countries. Croat Med J. 2011;52(4):478–487.
- Pukkala E, Kesminiene A, Poliakov S, et al. Breast cancer in Belarus and Ukraine after the Chernobyl accident. Int J Cancer. 2006;119(3):651–658.
- Fota GL, Stepan A, Ciurea RN. The evaluation of the immunoexpression of Her2/neu oncoprotein in ductal carcinoma in situ in association with invasive ductal carcinoma of the breast. Rom J Morphol Embryol. 2012;53(3 Suppl):805–881.
- Enache DE, Georgescu CV, Pătrană N. Negative estrogen-receptor invasive breast carcinoma: mammographic aspects, correlations with HER2/neu oncoprotein status. Rom J Morphol Embryol. 2012;53(3 Suppl):755–776.
- Varma G, Varma R, Huang H, et al. Array comparative genomic hybridisation (aCGH) analysis of premenopausal breast cancers from a nuclear fallout area and matched cases from Western New York. Br J Cancer. 2005;93(6):699–708.
- Kuzhan A, Adli M, Eryigit Alkis H, et al. Hormone receptor and HER2 status in patients with breast cancer by races in southeastern Turkey. J BUON. 2013;18(3):619–622.
- Amend K, Hicks D, Ambrosone C. Breast cancer in African-American women: differences in tumor biology from European-American women. Cancer Res. 2006;66:8327–8330.
- Mustać E, Zamolo G, Petković M, et al. Breast infiltrating ductal carcinoma: analysis of hormone, HER-2 receptors and Ki-67 proliferation marker. Coll Antropol. 2008;32(3):741–746.
- Yao LH, Jiang YM, Shi J, et al. Flavonoids in food and their health benefits. Plant Foods Hum Nutr. 2004;59(3):113–122.
- Holmes MD, Willett WC. Does diet affect breast cancer risk? Breast Cancer Res. 2004;6(4):170–178.
- Hystad P, Villeneuve PJ, Goldberg MS, et al. Exposure to traffic-related air pollution and the risk of developing breast cancer among women in eight Canadian provinces: a case–control study. Environ Int. 2015;74:240–248.
- Elias AD. Triple-negative breast cancer: a short review. Am J Clin Oncol. 2010;33(6):637–645.
- Yıldız B, Fidan E, Ozdemir F, et al. Clinicopathological characteristics of triple-negative breast cancers in the northeast region of Turkey. Balkan Med J. 2014;31(2):126–131.
- Zhang Z, Wang J, Tacha DE, et al. Folate receptor α associated with triple-negative breast cancer and poor prognosis. Arch Pathol Lab Med. 2014; 138(7):890–895.
- Rais G, Raissouni S, Aitelhaj M, et al. Triple negative breast cancer in Moroccan women: clinicopathological and therapeutic study at the National Institute of Oncology. BMC Womens Health. 2012;12:35. Available from: http://www.biomedcentral.com/1472-6874/12/35.
- Wang SL, Li YX, Song YW, et al. Prognostic value of estrogen receptor, progesterone receptor and human epidermal growth factor receptor-2 in node positive breast cancer patients treated by mastectomy. Zhonghua Zhong Liu Za Zhi. 2010;32(7):520–525. Chinese.
- Fan Y, Guan Y, Zhao WH, et al. Clinicopathological characteristics and prognostic factors of breast cancer with estrogen- and progesterone-receptor negative and HER-2 overexpression. Zhonghua Zhong Liu Za Zhi. 2008;30(12):917–920. Chinese.
- van der Hage JA, Mieog JS, van de Vijver MJ, et al. Efficacy of adjuvant chemotherapy according to hormone receptor status in young patients with breast cancer: a pooled analysis. Breast Cancer Res. 2007;9(5):R70.