Abstract
Uncontrolled and unplanned urbanization and industrialization due to increase of population and rapid industrial development have created severe environmental problems in Kyrgyzstan during the last few decades. In this study, Juniperus virginiana, a dioecious species, was employed in order to make assessment of the heavy metal pollution rate in the area and of the heavy metal pollution impact on the mineral nutrient status of the plant. For this study, leaf (washed and unwashed) and bark samples of J. virginiana, and its co-located soil samples were collected from eight different stations, all in the capital of Kyrgyzstan, Bishkek, in 2012 vegetation period. The standard procedures were used and the determinations of heavy metal and nutrient element contents (Ca, Cd, Cr, Cu, Fe, K, Mg, Mn, Na, Pb and Zn) in all samples were done using inductively coupled plasma-optical emission spectroscopy. According to our measurements, J. virginiana was found to be capable of accumulating a considerable amount of metals and the mineral nutrient uptake pattern was altered because of metal deposition in the plant, which showed a contamination risk in the area.
Introduction
Heavy metals, including cadmium (Cd), chromium (Cr), mercury (Hg) and lead (Pb), are chemicals, which are toxic to organisms, when they are exposed to even small doses of them. However, some of the metals, such as copper (Cu), iron (Fe), nickel (Ni) and zinc (Zn), are required by organisms as essential micronutrients in small amounts.[Citation1,Citation2] Under certain environmental conditions, an uptake of heavy metals into biological systems may occur. This may lead to bioaccumulation of these metals to toxic concentrations and may cause toxicity, resulting in illnesses or even death of organisms.[Citation3,Citation4] The size of the human population has increased dramatically in recent centuries. The anthropogenic impact in conjunction with population increase has led to an uncontrolled and unplanned urbanization and industrialization, causing health and environmental problems. Heavy metal pollution, caused by human activity, includes power industry, mining, transportation, dumping wastes, fertilizers and some of the anthropogenic-derived contamination sources.[Citation5–7]
In order to determine the specific characters of the biosphere, some organisms, known as bioindicators or biomonitors, are used.[Citation8] Some researchers define biomonitoring as the use of living organisms for measuring of the exposure level of the natural environment to pollutants.[Citation9,Citation10] Biomonitoring provides a valuable information regarding the quantity and quality of pollutants. It also has a main advantage over the other environmental protection methods, because of its lower application and maintenance cost.[Citation11,Citation12]
Higher plants, especially evergreens (rather than annual plants) are preferred for biomonitoring to give information about short- and long-term toxicity levels.[Citation4,Citation13] Especially the presence of some trace metals in soil or plant parts significantly changes the macro- and micronutrient uptake, transport and storage in plants by reducing the uptake of certain elements and increasing the uptake of others.[Citation14,Citation15]
Therefore, in the present work, leaves and barks of Juniperus virginiana and its co-located soil samples were employed in order to make assessment of the rate of the heavy metal pollution in the capital of Kyrgyzstan, Bishkek City, and to make estimation of the impact of heavy metal pollution on the mineral nutrient status of the plant.
Materials and methods
Study area
Kyrgyzstan is a country located in Central Asia, bordering Kazakhstan, Uzbekistan, Tajikistan and China. It lies between latitudes 39° and 44° N and longitudes 69° and 81° E. The population is about 5.5 million as of 2012. Kyrgyzstan is divided into seven regions: Batken, Chuy (Bishkek), Jalal-Abad, Naryn, Osh province (Osh), Talas and Issyk-Kul. The climate varies regionally. The south-western Fergana Valley is subtropical and extremely hot in the summer, with temperatures reaching 40 °C. The northern foothills are temperate and the Tian Shan varies from dry continental to polar climate, depending on the elevation.[Citation16]
Bishkek (42°52′29″ N, 74°36′44″ E) is the capital and the largest city of Kyrgyzstan and also the country's cultural, economic and financial centre. Bishkek is the most populated city in Kyrgyzstan. Its population is about 1,000,000.[Citation17] It has a continental Mediterranean climate and the average precipitation is around 440 millimetres per year. The average daily high temperatures range from 3 °C during January to about 31 °C during July.[Citation18]
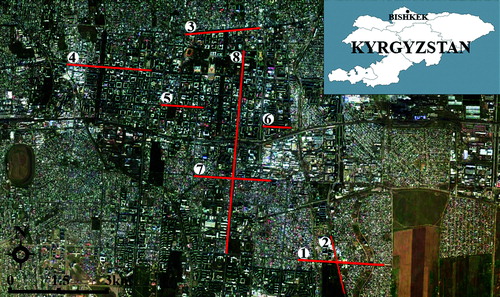
In this research, the study materials (parts of J. virginiana and co-located soil samples) were collected from eight locations in Bishkek. The location names are Akhunbaev Street, Shabdan Baatyr Street, Jibek Jolu Street, Chuy Street, Moskovskaya Street, Bokonbaeva Street, Gorky Street and Yusup Abdrahmanov & Baitik Baatyr Streets. The locations of the eight sampling sites are shown in
Measurement of element concentrations
The plant (bark and leaves) and soil samples were collected from different parts of Bishkek during the study period of 2012 (). Eight sampled plants per location were used and three analytical repetitions were done for the statistical analyses. Leaf samples were divided into two subsamples; half of them were thoroughly washed with running deionized distilled water to remove dust particles in a standardized procedure and the remaining leaf samples were analysed unwashed. The air-borne removal rates (%) on the leaves were calculated by comparisons of the unwashed and washed leaves' data. Plant parts were isolated and oven-dried at 80 °C for 48 h and then 0.2 g was taken and transferred into Teflon vessels. After this, 8 mL of 65% (v/v) HNO3 (Merck) was added. Soil samples (about 500 g) were collected from a depth of about 10 cm with a stainless steel shovel. They were also oven-dried at 80 °C for 48 h and passed through a 2-mm sieve. After that, 0.3 g was weighed and 9 mL 65% (v/v) HNO3, 3 mL 37% (v/v) HCl and 2 mL 48% (v/v) HF (Merck) were added. Samples were mineralized in a microwave oven (Berghof-MWS2) as follows: 5 min at 145 °C, 5 min at 165 °C and 20 min at 175 °C. After cooling, the samples were filtered by using Whatman filters and the volume was made up to 50 mL with ultrapure water (Human–Zeneer Power II) in volumetric flasks. These were stored in falcon tubes. After this, 10, 50, 100, 250 and 500 µg/L of standard solutions, prepared by using multi-element stock solutions 1000 mg/L (Merck), were used for drawing calibration curve and element (Ca, Cd, Cr, Cu, Fe, K, Mg, Mn, Na, Pb and Zn) measurements in mg/kg dry weight (DW). The measurements were done by inductively coupled plasma optical emission spectroscopy (PerkinElmer-Optima 7000 DV).
Statistical analyses
All calculations were based on DW of soil, bark and leaf. Statistical analyses, such as one-way analyses of variance with Tukey's post hoc honest significant difference (HSD) and Pearson correlation, were performed using IBM SPSS Statistics 20 software. The levels of statistical significance were expressed as **P < 0.01 level (2-tailed). IBM SPSS Statistics (Version 20) and the paired samples t-test module were employed for the comparison of the means of the heavy metals in the unwashed and washed leaf samples. The Bonferroni correction criterion was used for the decision of the significance when carrying out comparisons between metals at the same time. The Bonferroni correction is a correction factor used for performing several statistical tests simultaneously. The correction provides avoiding spurious positives.
Results and discussion
The results of analyses of the plant parts (unwashed and washed leaves and barks) and soil samples collected from eight stations in Bishkek are presented in . According to our results, the average highest and lowest heavy metal accumulations (Cd, Cr and Pb) in the plant parts were found to be ∼2.33 mg/kg DW in bark samples collected from location 4 and ∼1.28 mg/kg DW in washed leaf samples collected from location 2 for Cd, respectively. The average highest and lowest Cr accumulations in the plant parts were ∼7.60 mg/kg DW in unwashed leaf samples collected from location 4 and ∼2.63 mg/kg DW in bark samples collected from location 2, respectively. The average highest and lowest Pb accumulations in the plant parts were ∼26.58 mg/kg DW in unwashed leaf samples collected from location 4 and ∼11.93 mg/kg DW in bark samples collected from location 2, respectively. The average highest and lowest metal accumulations in co-located soils were found to be ∼7.53 mg/kg DW, collected from location 4 and ∼5.03 mg/kg DW, collected from location 2 for Cd, respectively. The average highest and lowest Cr accumulations in co-located soils were found to be ∼49.42 mg/kg DW collected from location 4 and ∼32.91 mg/kg DW collected from location 2, respectively. The average highest and lowest Pb accumulations in co-located soils were ∼129.48 mg/kg DW collected from location 4 and ∼86.46 mg/kg DW collected from location 2, respectively. Meanwhile, the contents of the mineral nutrients (Ca, Cu, Fe, K, Mg, Mn, Na and Zn) in the plant parts and co-located soils were determined in order to make assessment of the excessive metal accumulation effects on the mineral nutrient uptake. In our study, the average highest and lowest macronutrient values in the plant parts were found to be ∼4161.04 mg/kg DW in unwashed leaf samples collected from location 2 and ∼1657.34 mg/kg DW in bark samples collected from location 4 for Ca, respectively. The average highest and lowest Mg values in the plant parts were ∼4102.30 mg/kg DW in unwashed leaf samples collected from location 2 and ∼653.21 mg/kg DW in bark samples collected from location 4, respectively. The average highest and lowest K values in the plant parts were ∼6399.99 mg/kg DW in unwashed leaf samples collected from location 4 and ∼1926.90 mg/kg DW in bark samples collected from location 2, respectively. The average highest and lowest micronutrient values in the plant parts were found to be ∼18.61 mg/kg DW in unwashed leaf samples collected from location 4 and ∼10.50 mg/kg DW in washed leaf samples collected from location 2 for Cu, respectively. The average highest and lowest Fe values in the plant parts were ∼227.94 mg/kg DW in unwashed leaf samples collected from location 4 and ∼136.97 mg/kg DW in bark samples collected from location 2, respectively. The average highest and lowest Mn values in the plant parts were found to be ∼19.28 mg/kg DW in unwashed leaf samples collected from location 4 and ∼11.29 mg/kg DW in washed leaf samples collected from location 2, respectively. The average highest and lowest Na values in the plant parts were ∼304.50 mg/kg DW in unwashed leaf samples collected from location 4 and ∼104.66 mg/kg DW in bark samples collected from location 2, respectively. The average highest and lowest Zn values in the plant parts were ∼84.32 mg/kg DW in unwashed leaf samples collected from location 4 and ∼17.53 mg/kg DW in bark samples collected from location 2, respectively. The average highest and lowest macronutrient values in co-located soils were found to be ∼16,283.68 mg/kg DW collected from location 2 and ∼9296.61 mg/kg DW collected from location 4 for Ca, respectively, ∼5140.64 mg/kg DW collected from location 2 and ∼2995.99 mg/kg DW collected from location 4 for Mg, respectively, and ∼4876.44 mg/kg DW collected from location 4 and ∼3272.38 mg/kg DW collected from location 2 for K, respectively. The average highest and lowest micronutrient values in co-located soils were found to be ∼66.38 mg/kg DW collected from location 4 and ∼44.83 mg/kg DW collected from location 2 for Cu, respectively, ∼7082.57 mg/kg DW collected from location 4 and ∼4709.26 mg/kg DW collected from location 2 for Fe, respectively. The average highest and lowest Mn values in co-located soils were ∼314.40 mg/kg DW collected from location 4 and ∼209.41 mg/kg DW collected from location 2, respectively. The average highest and lowest Na values in co-located soils were found to be ∼168.63 mg/kg DW collected from location 4 and ∼113.09 mg/kg DW collected from location 2, respectively, and for Zn – ∼381.13 mg/kg DW collected from location 4 and ∼255.48 mg/kg DW collected from location 2, respectively. According to the literature, the normal values of Cd, Cr, Cu, Fe, Mn, Pb and Zn in plants are in the ranges of 0.2–0.8, 0.006–18, 5–30, 2–250, 30–300, 0.1–10 and 25–150 mg/kg DW, respectively, and values between or over 5–30, >100, 20–100, 400–1000, 300–500, 30–300 and 100–400 mg/kg DW, respectively, are accepted as toxic.[Citation19–23] According to these values, the concentrations of Cr, Cu (except location 4) and Zn in this study were within the normal limits in the plants collected from all locations. The normal limits were only exceeded for Cd, Fe and Pb in the plants from all locations. Cu at location 4, Fe at location 4, Pb at locations 3–8 and Zn at location 4 were in the range of toxic level in this work (the total concentrations of metals in plants were measured by taking both washed leaf and bark sample values together for each station).
Table 1. Concentrations of Ca, Cd, Cr, Cu, Fe, K, Mg, Mn, Na, Pb and Zn (mg/kg DW) in the parts of J. virginiana and co-located soil samples.
Bishkek suffers many of the problems of developing cities, including pollution and rapid increase of population. In this study, J. virginiana was used as a biomonitor organism to provide information on pollution rate of Bishkek. The data for pollution rate were deduced from the determined metal concentrations of Ca, Cd, Cr, Cu, Fe, K, Mg, Mn, Na, Pb and Zn in the plant's parts and co-located soils. Also, the effects of metal deposition on the mineral uptake were analysed in J. virginiana. The data for heavy metal accumulation showed that the highest concentrations of heavy metals were consistently found in location 4, which was relatively close to heavily populated areas (). The growing population has increased the urbanization of Bishkek and with it more motor vehicles, traffic jams and smog have started to pollute the air. Therefore, it can be said that the anthropogenic effects, especially heavy traffic and urbanization, are the main sources of heavy metal pollution in Bishkek.
For organisms, the main abiotic stress agent is heavy metals. Elevated amounts of heavy metals can lead to toxicity, resulting in inhibition and/ or alteration of physiological processes in plants.[Citation24,Citation25] For example, excessive accumulation of Pb in plants causes decrease in the mitotic index, seed germination, root elongation and biomass, and inhibition of chlorophyll biosynthesis.[Citation26–31] Cd [Citation32] or Cu [Citation33] inhibits leaf elongation as a result of induced preferential decrease of cell wall elasticity. Inhibition of K uptake and photosynthesis in leaves of cucumber plants could be the result of Cu accumulation, preventing cell expansion.[Citation34] In general, toxicity caused by heavy metals results in growth reduction in plants, due to probable indirect effects of the heavy metals (impeding of uptakes, transport and the use of several elements such as Ca, Fe, Mg, Mn, P and Zn) on the metabolism of macro- and micronutrients.[Citation35,Citation36] Excessive accumulation of some heavy metals (Cd, Fe and Pb) was observed in the plant and soil samples in this study. Experimental data obtained for estimation of metal deposition impact on mineral nutrient uptake and composition of J. virginiana indicated that mineral nutrient metabolism of J. virginiana was not adversely affected by metal accumulation. However, as a consequence of metal depositions, the presence of heavy metals strongly influenced the uptake of mineral elements and, in particular, that of K, Mg and Na, whose concentrations were high in J. virginiana. Also, high concentrations of K, Mg and Na were observed in J. virginiana, in comparison with the soil samples in the present study. K is the major solute contributing to osmotic pressure and ionic strength.[Citation37] Also, the uptake of heavy metals was competitively inhibited by K, Ca and Mg.[Citation35] Our data suggested that K was actively transported by the plant for restoring the osmotic pressure and ionic strength disturbed by heavy metals. The plant may also take action to protect itself by increasing the Mg and Ca levels, as a response to heavy metal stress, but after a threshold point, the plant does not confer a resistance to heavy metal stress, leading to decrease in the uptake of Ca and Mg. In general, the uptake of heavy metals (Cd, Cr, Cu, Pb and Zn) was high, whereas the uptake of Ca and Mg was low in location 4, showing the effects of heavy metal deposition on the uptakes of mineral elements.
The Cd concentrations in the barks and leaves of J. virginiana represent the total Cd accumulations for long and short terms, respectively. When leaf and bark materials were analysed for heavy metal accumulation, the concentrations of Cd in bark samples were found to be high, in comparison with unwashed and washed leaf samples from all locations. Also, the amounts of Cu were found to be similar in bark and washed samples from all locations. This proves progressive accumulations of Cd and Cu in J. virginiana over long-term period in Bishkek.
When statistical evaluation was done in terms of using linear correlation relationship based on the obtained data from the elements' concentrations in leaves of J. virginiana and co-located soils, relatively high positive correlations (>0.94, >0.99) were found between the values of heavy metals (Cd, Cr, Cu, Fe, Mn, Pb and Zn) and mineral nutrients (Ca, K and Na) in leaves of J. virginiana and the values of heavy metals (Cd, Cr, Cu, Fe, Mn, Pb and Zn) in the co-located soil samples (). Negative high correlation (−>0.93, −>0.96) existed between the soil contents of other elements measured in this study and the content of Mg present in leaves of J. virginiana (). It appeared that there was a high positive correlation between the soil values of K and Na and the values of heavy metals (Cd, Cr, Cu, Fe, Mn, Pb and Zn) and Ca measured in leaves of J. virginiana (>0.95, >0.99), and high negative correlation between the soil values of K and Na and the value of Mg, measured in leaves of J. virginiana (−>0.94) (). There was a high negative correlation (−>0.93, −>0.97) between the soil concentrations of Ca and Mg and heavy metals (Cd, Cr, Cu, Fe, Mn, Pb and Zn), and mineral nutrients (K and Na) concentrations examined in leaves of J. virginiana. However, a high positive correlation (>0.98, >0.99) was detected between soil Mg–leaf Mg contents and soil Ca–leaf Mg contents ().
Table 2. Correlation matrix (R) data between soil and washed leaf (Leaf) obtained by Pearson correlation method.
Also, according to our data, location 4 was heavily contaminated, whereas location 2 was relatively the cleanest region and the washing procedure reduced all metal accumulation charges on the leaves of the plant. In these regions, the highest and lowest air-borne removal rates on the leaves were found to be ∼9.06% and ∼9.08% for Cd, ∼19.46% and ∼18.21% for Cr, and ∼15.83% and ∼15.36% for Pb, respectively (). Our results indicated that the amounts of heavy metals, other than iron, were changed considerably by washing. According to Bonferroni correction (used to cancel out the problem of multiple comparisons in statistics), the alpha level was corrected as 0.005 (alpha is divided by the number of tests performed simultaneously) instead of 0.05 ().
Table 3. The percentage changes (% removal) of heavy metal amounts found in the leaves of the plant before and after washing.
Table 4. The results of paired samples t-test for unwashed and washed leaf samples.
Conclusions
From the results of this study, it can be said that station 4 was the most affected area from the contamination in Bishkek, the largest city of Kyrgyzstan. The data indicated that the presence of heavy metals strongly influenced the uptake of mineral elements, in particular, that of Mg, K and Na, whose concentrations were high in J. virginiana as a consequence of metal depositions. It appeared that the variations of heavy metals found in the soil were directly reflected in the accumulation of these heavy metals in the leaves of J. virginiana. Based on the high correlation values that existed between the soil and plant part (leaf) samples, it can be said that the presence of Ca and Mg in the soil appeared to have a direct effect on the accumulation of heavy metals in the leaves of J. virginiana. Overall, heavy metals are transported and stored within J. virginiana and for estimations of metal accumulations, J. virginiana could be used as a biomonitor organism.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Freedman B. Environmental Ecology: the ecological effects of pollution, disturbance, and other stresses. 2nd ed. San Diego (CA): Academic Press; 1995.
- Osma E, Ozyigit II, Leblebici Z, et al. Determination of heavy metal concentrations in tomato (Lycopersicon esculentum Miller) grown in different station types. Rom Biotechnol Lett. 2012;17(1):6962–6974.
- Foy CD, Chaney RL, White MC. The physiology of metal toxicity in plants. Ann Rev Plant Physiol. 1978;29:511–566.
- Yasar U, Ozyigit II, Yalcin IE, et al. Determination of some heavy metals and mineral nutrients of bay tree (Laurus nobilis L.) in Bartin City, Turkey. Pak J Bot. 2012;44:81–89.
- Avino RB, Lopez-Moya JR, Navarro-Avino JP. Health implications: trace elements in cancer. In: Prasad MNV, editor. Trace elements as contaminants and nutrients: consequences in ecosystems and human health. Hoboken (NJ): Wiley; 2008. p. 495–521.
- Ugulu I, Dogan Y, Baslar S, et al. Biomonitoring of trace element accumulation in plants growing at Murat Mountain. Int J Environ Sci Technol. 2012;9:527–534.
- Osma E, Ozyigit II, Demir G, et al. Assessment of some heavy metals in wild type and cultivated purslane (Portulaca oleracea L.) and soils in Istanbul, Turkey. Fresenius Environ Bull. 2014;23(9):2181–2189.
- Markert B. Plants as biomonitors: indicators for heavy metals in the terrestrial environment. Weinheim: VCH Publisher; 1993.
- DeCaprio AP. Biomarkers: coming of age for environmental health and risk assessment. Environ Sci Technol. 1997;31(7):1837–1848.
- Kamrin M. Biomonitoring basics (a report from biomonitoring info.org). Manassas (VA): Environmental Health Research Foundation; 2004.
- Akguc N, Ozyigit II, Yarci C. Pyracantha coccinea Roem. (Rosaceae) as a biomonitor for Cd, Pb and Zn in Mugla province (Turkey). Pak J Bot. 2008;40(4):1767–1776.
- Dogan Y, Ugulu I, Baslar S. Turkish red pine as a biomonitor: a comparative study of the accumulation of trace elements in the needles and bark. Ekoloji. 2010;19(75):88–96.
- Dogan I, Ozyigit II, Demir G. Influence of aluminum on mineral nutrient uptake and accumulation in Urtica pilulifera L. J Plant Nutr. 2014;37:469–481.
- Akguc N, Ozyigit II, Yasar U, et al. Use of Pyracantha coccinea Roem. as a possible biomonitor for the selected heavy metals. Int J Environ Sci Technol. 2010;7(3):427–434.
- Ozyigit II, Vardar F, Yasar U, et al. Long-term effects of aluminum and cadmium on growth, leaf anatomy, and photosynthetic pigments of cotton. Commun Soil Sci Plant Anal. 2013;44:3076–3091.
- The official website of Nations Online, All Countries of the World. [Internet] [cited 2015 Feb]. Available from: http://www.nationsonline.org/oneworld/kyrgyzstan.htm
- The official website of Geohive-Population Statistics. [Internet] [cited 2015 Feb]. Available from: http://www.geohive.com/cntry/kyrgyzstan.aspx
- The official website of World Meteorological Organization. [Internet] [cited 2015 Feb]. Available from: http://worldweather.wmo.int/en/city.html?cityId=210
- Ross SM, editor. Sources and forms of potentially toxic metals in soil-plant systems. In: Toxic metals in soil-plant systems. Chichester: Wiley; 1994. p. 3–25.
- Kabata-Pendias A, Pendias H. Trace elements in soils and plants. 3rd ed. Boca Raton (FL): CRC Press; 2001.
- Davies FT, Puryear JD, Newton RJ, et al. Mycorrhizal fungi increase chromium uptake by sunflower plants: influence on tissue mineral concentration, growth, and gas exchange. J Plant Nutr. 2002;25:2389–2407.
- Zayed AM, Terry N. Chromium in the environment: factors affecting biological remediation. Plant Soil. 2003;249:139–156.
- Kabata-Pendias A, Mukherjee AB. Trace elements from soil to human. New York (NY): Springer; 2007.
- Zenk MH. Heavy metal detoxification in higher plants – a review. Gene. 1996;179:21–30.
- Zornoza P, Vazquez S, Esteban E, et al. Cadmium-stress in nodulated white lupin: strategies to avoid toxicity. Plant Physiol Biochem. 2002;40:1003–1009.
- Balsberg-Pahlsson AM. Toxicity of heavy metals (Zn, Cu, Cd, Pb) to vascular plants. A literature review. Water Air Soil Pollut. 1989;47:287–319.
- Kumar G, Singh RP. Nitrate assimilation and biomass production in Sesamum indicum L. seedlings in a lead enriched environment. Water Air Soil Pollut. 1993;66:163–171.
- Fargasova A. Effect of Pb, Cd, Hg, As and Cr on germination and root growth of Sinapis alba seeds. Bull Environ Contam Toxicol. 1994;52(3):452–456.
- Xiong Z-T. Bioaccumulation and physiological effects of excess lead in a roadside pioneer species Sonchus oleraceus L. Environ Pollut. 1997;97:275–279.
- Wierzbicka M. The effect of lead on the cell cycle in the root meristem of Allium cepa L. Protoplasma. 1999;207:186–194.
- Patra M, Bhowmik N, Bandopadhyay B, et al. Comparison of mercury, lead, and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environ Exp Bot. 2004;52:199–223.
- Poschenrieder C, Gunse B, Barcelo J. Influence of cadmium on water relations, stomotal response and abscissic acid content in expanding bean leaves. Plant Physiol. 1989;90:1365–1371.
- Maksymiec W, Bednara J, Baszynski T. Responses of runner plants to excess copper as a function of plant growth stages: effects on morphology and structure of primary leaves and their chloroplast ultrastructure. Photosynthetica. 1995;31:427–435.
- Alaoui-Sosse B, Genet P, Vinit-Dunand F, et al. Effect of copper on growth in cucumber plants (Cucumis sativus) and its relationships with carbohydrate accumulation and changes in ion contents. Plant Sci. 2004;166(5):1213–1218.
- Clarkson DT, Luttge U III. Mineral nutrition: divalent cations, transport and compartmentation. Progr Bot. 1989;51:93–112.
- Harrison RB, Henry CL, Xue D. Magnesium deficiency in Douglas-fir and grand fir growing on a sandy outwash soil amended with sewage sludge. Water Air Soil Pollut. 1994;75:37–50.
- Schroeder JI, Ward JM, Gassmann W. Perspectives on the physiology and structure of inward-rectifying K-channels in higher plants. Biophysical implications for K-uptake. Annu Rev Biophys Biomol Struct. 1994;23:441–471.