Abstract
Ribosomal proteins are crucial for the proper growth and development of any organism, including plants. The ribosomal protein L24 (RPL24) is found in the large subunit of the ribosome and is responsible for the stabilization of the peptidyl transferase activity. Although RPL24 gene has been individually identified in different organisms, little is known about the genome-wide survey and expression patterns of R24 genes in Cucurbitaceae family members. We identified seven Cucurbitaceae RPL24 genes from cucumber, melon and watermelon. They were phylogenetically clustered into seven major groups. Gene structure and motif composition are relatively conserved in each group. Three-dimensional homology modelling of RPL24 proteins was performed with higher confidence level. CmRPL24-01 was isolated from melon and characterized at a molecular level. The regulation of ribosomal proteins in melon under drought stress conditions was also studied. The expression of CmRPL24-01 gene increased in melon leaf tissue at 3 h upon polyethylene glycol treatment and showed a gradual induction after 12 h. Our study provided a very useful reference for identification and functional analysis of RPL24 protein members in different plants. In addition, this research indicated a potential usage of ribosomal proteins in response to drought stress.
Introduction
Melon, which belongs to Cucurbitaceae family, is an important fruit because of its huge production of 26 million tons on 12 billion m2 area around the world.[Citation1] Cucurbitaceae family includes food plants, which are nutritionally and economically essential. This family provides a model system for gender determining, fruit ripening and vascular biology studies, which comprise of investigations of long-distance signalling pathways using xylem and phloem sap.[Citation2–4] Melon's whole genome sequence (2n = 2x = 24) has been published in 2012, thereby cucumber and melon genome sequences became very useful tools for understanding the genomic structure and evolution of two important species, which belong to the same genus, but have different chromosome numbers.[Citation5]
Ribosomes are composed of protein and RNAs. RNAs form approximately 65% portion of the ribosome, which differs in different species. The peptide bond formation reaction is catalyzed by the large subunit of the ribosome. The catalytic centre of the ribosome is named peptidyl transferase. The ribosomal protein L24 (RPL24) is a protein in the peptidyl transferase centre, which is responsible for the stabilization of the peptidyl transferase activity.[Citation6] Kyprides, Ouzounis, Woese (KOW) motif, known as RNA binding motif, is observed in many different ribosomal proteins, including in the RPL24 protein.[Citation7–12] This motif includes stabile glycine amino acids and changes hydrophobic and hydrophilic amino acid residues. KOW motif ensures the binding of the transcription factors together with the ribosomal proteins [Citation12] in bacteria. In addition, this motif may facilitate protein–protein interactions, despite of the different nucleic acid interaction regions.[Citation13] Ribosomal proteins have extra functions, besides their role in protein synthesis.[Citation14] Ribosomal protein coding genes, including rpS3A and rpA1, were knocked down in Drosophila and abnormal ovule generation and oogenesis was observed.[Citation15,Citation16] Moreover, RPL24 gene recruits translation reinitation during pistil development in Arabidopsis.[Citation17]
RPL24 gene has been individually identified in different organisms.[Citation18–20] RPL24 gene homologs have been cloned in barley,[Citation21] chickpea,[Citation22] three different yeast species, including Saccharomyces cerevisiae,[Citation23] Schizosaccharomyces pombe and Kluyveromyces lactis,[Citation24] and three different Archaebacteria, including Methanococcus jannaschii,[Citation25] Pyrococcus horikoshii [Citation26] and Archaeoglobus fulgidus.[Citation27] Although there has been a significant increase in the studies of recent developments for gene discovery, little is known about the genome-wide survey and expression patterns of RPL24 gene family in Cucurbitaceae family members, including cucumber, melon and watermelon. This is the first study for the identification, functional characterization and expression analysis of RPL24 genes in melon, cucumber and watermelon. In this study, gene, encoding one of the RPL24 (CmRPL24), was firstly isolated from melon (Cucumis melo L.) by using the rapid amplification of cDNA ends (RACE) method. However, molecular characterization studies for this gene have not been performed for Cucurbitaceae family members yet. Also, a draft of the watermelon genome sequence, which is in the same family as melon and cucumber, was reported. However, there is a little information about the RPL24 gene in watermelon. The objective of this study was the identification of RPL24 genes in Cucurbitaceae family members, including melon, watermelon and cucumber. For this purpose, bioinformatical approaches were used to detect and analyze these genes in the genomes of selected species. Conserved motifs and three-dimensional (3D) protein modelling of all RPL24 proteins were also determined. To our knowledge, there have not been any other studies for the examination of CmRPL24 gene regulation in response to drought stress with quantitative polymerase chain reaction (qPCR). Therefore, this study makes a major contribution to the research on the function of the ribosomal protein family members. In addition, the identified RPL24 gene members from Cucurbitaceae family can be used for cloning studies in agricultural applications.
Materials and methods
Plant materials, growth conditions and stress treatment
Galia type of Turkish melon seeds were obtained from Bati Akdeniz Agricultural Research Institute (Antalya, Turkey). After removing the seed coats, they were washed with distilled water three times. Then, they were transferred to plastic containers and grown in hydroponic culture containing half-strength Hoagland's solution [Citation28] for 14 d in a plant growth chamber at 24°C ± 2°C with a 16 h light and 8 h dark photoperiod at a light intensity of 400 μmol m–2 s–1. For drought stress, 10% (w/v) polyethylene glycol 6000 (PEG-6000) was added to the half-strength Hoagland's solution. Stress treatment was initiated on the 14th day of normal growth. Both treated (stress) and non-treated (control) plants were kept in the growth chamber with the same growth conditions. Samples from the treated and control plants were harvested after 0, 3, 12 and 24 h of stress application. Time point zero (0 h) was used as a control. The leaves of mature plants from three biological replicates were collected and immediately frozen in liquid nitrogen.
Total RNA extraction and cloning of CmRPL24-01 gene
Total RNA extraction was performed with TRIzol reagent (Life Technologies Corporation, Grand Island, NY, USA) according to the method of Baloglu et al.[Citation29] DNA contamination in samples was removed with DNase I (Fermentas, Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's instructions. The quality and integrity of the total RNA was checked with agarose gel electrophoresis and the Multiskan™ GO Microplate Spectrophotometer with µDrop™ Plate (Thermo Fisher Scientific, Waltham, MA, USA).
Cloning of full-length CmRPL24-01 gene was achieved with the RACE method. 5′-GAATCTACGCTCGAAGATCGACATACC-3′ and 5′-TTTTCCCCACCCGAAGTCCACATTT-3′ oligonucleotide primers were used for 5′ and 3′ RACE methods, respectively. They were designed according to the highly conserved sequences in previously identified RPL24 proteins from various organisms. The GeneRacer 5′ (5′-CGACTGGAGCACGAGGACACTGA-3′) and 3′ (5′-GCTGTCAACGATACGCTACGTAACG-3′) primers were also used for the 3′ RACE and 5′ RACE reactions, respectively. A total of 10 µg RNA was utilized for the synthesis of first-strand cDNA. PCR with specific CmRPL24 primers (Forward: 5′-ATGGGTTGGAAGGCTGCTGAA-3′, Reverse: 5′-TTAAAGTTCAGGCATGCCCT-3′) was performed using one microlitre of cDNA in a high-fidelity PCR system (Life Technologies Corp., Paisley, UK). PCR parameters were adjusted as follows: 2 min of template denaturation at 95 °C for 1 cycle, followed by 5 cycles at 95 °C (30 s), 64 °C (90 s) and 72 °C (90 s), and then 20 cycles at 94 °C (30 s), 60 °C (30 s) and 68 °C (90 s), with a final 10-min extension step at 68 °C. Then, the size of the PCR product was checked and purified from an agarose gel (prepared with tris-borate-ethylenediaminetetraacetic acid (EDTA) (TBE), 2%, w/v). The amplified CmRPL24 gene fragment was cloned into a pCR8/GW/TOPO vector (Life Technologies Corp.) and validated by colony PCR. PCR master mix was prepared and dispensed into 20 µL of the mix into the PCR tubes on ice. Individual colony was picked and resuspended in 20 µL of the PCR master mix. The PCR was performed in the following conditions: 95 °C for 3 min, 94 °C for 30 s, 55 °C for 30 s, 72 °C (1 min/kb); 25 cycles. The inserted cDNA was sequenced with an ABI 310 DNA sequencing system. All oligonucleotide primers used in this study were purchased from Sentegen Biyotek (Ankara, Turkey).
Quantitative real-time PCR analysis
For the identification of CmRPL24 gene expression level changes under drought stress, qPCR with specific primers (5′-GGTTGGAAGGCTGCTCAAAA-3′ and 5′-CATGGAGGGGAGCTTCAACT-3′) was performed by using DNA from stress treated and non-treated leaf tissues. All primers used throughout this study were designed according to the CmRPL24 gene sequence by CLC Genomics Workbench, version 7.5. A cucumber 18S rRNA gene (GenBank ID: X51542.1), amplified with primers 5′-GTGACGGGTGACGGAGAATT-3′ and 5′-GACACTAATGCGCCCGGTAT-3′, was used as an internal control. Control and gene-specific primers were optimized according to the melting temperatures. Three biological replicates were carried out and triplicate quantitative assays for each replicate were performed by using SYBR Green PCR Master mix kit (QIAGEN, Hilden, Germany). Relative gene expression was calculated by dividing RPL24 gene threshold cycle (CT) value to 18S rRNA gene CT value. The ΔCT and ΔΔCT were calculated by the equations ΔCT = CT target – CT reference and ΔΔCT = ΔCT treated sample – ΔCT untreated sample (0 h treatment).[Citation30] For all chart preparations, selected RNA relative amount was evaluated for gene expression level using the 2−ΔΔCT. At the same time, the standard errors of mean among replicates were calculated. Student's t-test was used to obtain the statistical significance of the difference between treated samples and untreated samples (0 h treatment under abiotic stress). If P-values were <0.01, we considered the CmRPL24 gene as differentially expressed gene.
Sequence retrieval and identification of other RPL24 genes from Cucumis sativus, C. melo and Citrullus lanatus
CmRPL24-01 gene was firstly isolated from melon (C. melo L.) by using the RACE method.[Citation31] Other RPL24 proteins from melon (CmRPL24), cucumber (CsRPL24) and watermelon (ClaRPL24) were identified by performing a basic local alignment search tool for proteins (BLASTP) at Melonomics database (https://melonomics.net/),[Citation5] Phytozome v9.1 database (www.phytozome.net/) [Citation32] and Cucurbit Genomics database (http://www.icugi.org/cgi-bin/ICuGI/index.cgi),[Citation33] respectively. Moreover, the Hidden Markov model (HMM) profiles of the KOW domains (PFAM ID: PF00467) in the Pfam database (http://pfam.sanger.ac.uk/) were searched against the Phytozome, Melonomics and Cucurbit Genomics databases for three species. All hits with expected values less than 1.0 were retrieved. Each sequence was checked for the presence of the conserved KOW domains by SMART (http://smart.emblheidelberg.de/) [Citation34] and Pfam (http://pfam.sanger.ac.uk/) [Citation35] proteomics servers to verify the conserved domains of RPL24 proteins.
Chromosomal location and gene structure prediction
Physical positions of the RPL24 genes on the cucumber and watermelon chromosomes were determined by searching their corresponding locus numbers in the Phytozome and the Cucurbit Genomics database, respectively. An exon and intron numbers and their locations of all RPL24 genes were identified using gene structure display server (gsds.cbi.pku.edu.cn/) [Citation36] through comparison of their predicted coding sequence with their corresponding genomic sequence. Some physicochemical characteristics including the number of amino acids, molecular weight and theoretical isoelectric point (pI) were computed using ProtParam tool (http://www.expasy.org/tools/protparam.html).
Sequence alignment, phylogenetic analysis and identification of conserved motifs
The amino acid sequences were imported into MEGA5 [Citation37] and multiple sequence alignments were performed using ClustalW with gap open and gap extension penalties of 10 and 0.1, respectively.[Citation38] The alignment file was then used to construct an unrooted phylogenetic tree based on the neighbour-joining (NJ) method [Citation39] and after bootstrap analysis for 1000 replicates, the tree was displayed using interactive tree of life (iTOL) (http://itol.embl.de/index.shtml).[Citation40] Protein sequence motifs were identified using the multiple expectation maximization (EM) for motif elicitation (MEME) (http://meme-suite.org/).[Citation41] The analysis was performed by keeping number of repetitions, any; maximum number of motifs, 20; and optimum width of the motif, ≤50. Discovered MEME motifs (≤1e-30) were searched in the InterPro database with InterProScan.[Citation42]
Homology analysis of RPL24 proteins between melon, cucumber and watermelon
For determination of homology ratio among the melon and the two other species' amino acid sequences of RPL24, BLASTP search (http://blast.ncbi.nlm.nih.gov/Blast.cgi) was performed against peptide sequences of cucumber and watermelon. Hits with e-value ≤1e-10 and at least 80% identify were considered significant.
3D protein modelling of RPL24 proteins
All the RPL24 proteins were searched against the protein data bank (PDB) [Citation43] by BLASTP (with the default parameters) to identify the best template having similar sequence and known 3D structure. The data was fed in Phyre2 (Protein Homology/AnalogY Recognition Engine; http://www.sbg.bio.ic.ac.uk/phyre2) for predicting the protein structure by homology modelling under ‘intensive’ mode.[Citation44]
Results and discussion
Cloning and characterization of CmRPL24-01 gene in melon
CmRPL24-01 gene was amplified with RACE techniques from melon fruit total RNA. Using degenerate and RACE primers, an 848 bp cDNA clone (GenBank accesion no: EU853460) was obtained. Nucleotide sequence analysis of this cDNA showed that it corresponded to a RPL24 full-length cDNA, termed C. melo RPL24 (CmRPL24-01). The cloned cDNA had an open reading frame extending from position 220 in the frame to position 699, coding for a mature 159 amino acid protein with a predicted molecular mass of 17.6 kDa (). The predicted polypeptide sequence revealed that the CmRPL24-01 gene encodes a basic protein with an pI of 10.04. No canonical polyadenylation-processing signals (AATAAA or AATGAA) were found, which is common in most plant genes.[Citation45]
Identification of the RPL24 genes in the cucumber, melon and watermelon genomes
RPL24 genes in three cucurbit species (cucumber, melon and watermelon) were identified using basic local alignment search tool (BLAST) and HMM searches. Then, all predicted RPL24 proteins were subjected to Pfam domain searches to verify the presence of KOW motifs with Pfam Id number (PF00467). A total of seven putative RPL24 genes, belonging to these species, were identified after the removal of different transcripts of the same gene (). CsRPL24, CmRPL24 and ClaRPL24 were entitled based on their scientific names - C. sativus (cucumber), C. melo (melon) and C. lanatus (watermelon), respectively. The particularization of Cucurbitaceae RPL24 proteins is listed in , which shows the number of amino acids (length), molecular weight, pI and homology. According to the detailed information, the molecular weight of the RPL24 protein sequences ranged from 11.27 kDa (CsRPL24-03) to 21.5 kDa (ClaRPL24-01), whereas the pI ranged from 9.74 (CsRPL24-02) to 10.24 (CsRPL24-01). Although individual RPL24 genes have been identified in different plant species, such as rice,[Citation20] barley [Citation21] and chickpea,[Citation21] identification of this family genes on a genomic level was firstly performed in Cucurbitaceae family members in our study. The more surprising correlation is with the protein length of plants' RPL24 genes. For example, rice RPL24 gene encodes 190 amino acids,[Citation20] which is very similar to CsRPL24-02, CmRPL24-02 and ClaRPL24-01 genes from cucumber, melon and watermelon, respectively. In addition, barley and chickpea RPL24 genes encode proteins with 162 and 164 amino acids, respectively.[Citation22,Citation21] Their protein lengths resembled to CmRPL24-01 and ClaRPL24-02 genes. This interprets that evolution of those genes might have arisen from a similar origin.
Table 1. Catalogue of seven RPL24 genes in cucumber, melon and watermelon.
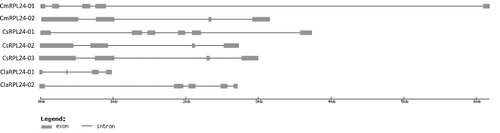
Although RPL24 genes, belonging to cucumber and watermelon, were physically mapped to their corresponding chromosomes, we did not achieve mapping of CmRPL24 genes on melon chromosomes due to the absence of genome browser function in Melonomics database. The exact position (in bp) of each RPL24 genes of the three species is shown in
It is commonly known that exon–intron organization plays a crucial role in understanding the evolutionary and functional relationships.[Citation46] In order to understand the gene structure of RPL24 genes, exon and intron structure analysis was also performed for the Cucurbitaceae members' RPL24 genes (). We detected that all RPL24 genes contained introns, whose numbers ranged from three to five (). Examination of the intron–exon organization indicated that family members of RPL24s with higher homology rate shared similar gene structures in respect to intron number or exon length. Especially, CmRPL24-02 gene structure was identical with CsRPL24-02 and CsRPL24-03. It could be suggested that RPL24 genes may be regarded as having a similar history with higher homology rate among the Cucurbitaceae family members.
Phylogenetic relationship of Cucurbitaceae RPL24 proteins and identification of their conserved domain
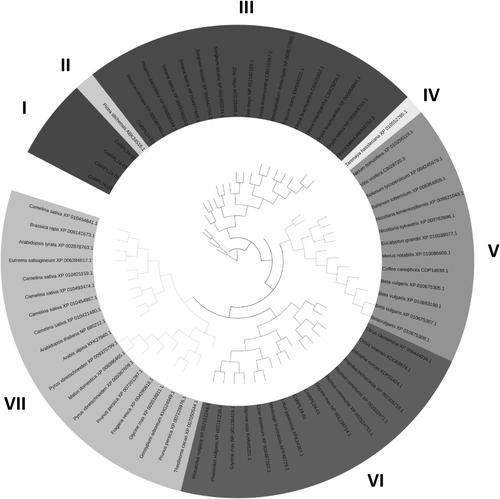
The amino acid sequences of all plant RPL24 proteins were submitted to the phylogenetic analysis for clustering the Cucurbitaceae family members (). The phylogenetic tree was constructed using 71 plant RPL24 proteins through neighbour-joining method. Based on the analysis, a total of seven clusters were identified for plant RPL24 proteins. Higher bootstrap values were obtained between internal branches, because bootstrap analysis was replicated 1000 times. This clearly indicated statistically reliable pairs of potential homologous derivation. In general, Cucurbitaceae RPL24 proteins, clustered together, shared similar motif composition. They are mainly found in clusters that are classified in Groups I and VI (). Except ClaRPL24-02, Cucurbitaceae RPL24 proteins generally exhibited closer relationships to RPL24 proteins from dicotyledons than to those from monocotyledons. These results suggested that although the plant RPL24 proteins are evolutionarily diverse, a number of them may have undergone further differentiation in monocotyledon and dicotyledon lineages.
The sequences were aligned by ClustalW at MEGA5 and the unrooted phylogenetic tree was deduced by NJ method. Distinct clusters were assigned with roman numerals. The accession numbers of all RPL24 proteins are shown in the phylogenetic tree.
Motif compositions of cucurbits RPL24 proteins were also examined to control the reliability of the phylogenetic analysis. To examine the diversity of RPL24 proteins in Cucurbitaceae family members, three conserved motifs were predicted by the MEME programme ( and ). RPL24s plays a crucial role in generating the first intermediate during the 50S ribosomal subunit assembly. All predicted cucurbit RPL24 proteins have a KOW motif which is known as an RNA-binding motif.[Citation47] Majority of the closely related cucurbits RPL24 proteins such as CmRPL24-02, CsRPL24-02 and CsRPL24-03 have a common Motif 1 composition. Other than the KOW domain region, Cucurbitaceae RPL24 proteins also contain one additional conserved motif (Motif 2) that may demonstrate possible function sites or take part in the ribosomal subunits assembly functions. The motif analysis provides additional evidence for proving other analysis including exon–intron structure and phylogenetic analysis. In general, motif-sequence conservation or variation between the proteins defined a functional equivalence or diversification, in respect to the different biological functions.[Citation48]
Table 2. Amino acid composition of the Cucurbitaceae RPL24 protein motifs.
Homology modelling of Cucurbit RPL24 proteins
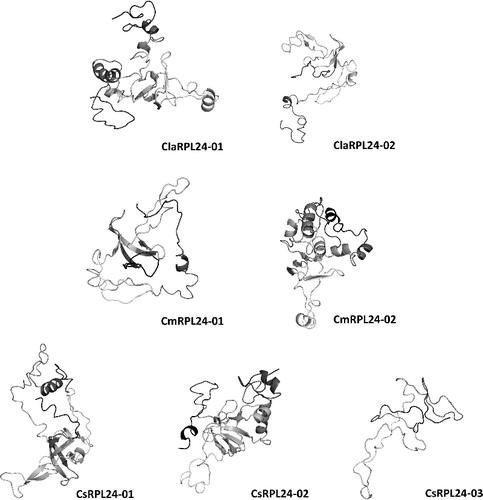
BLASTP search was performed against the PDB to construct the homology pattern of seven Cucurbitaceae RPL24 proteins. In order to improve the accuracy of alignment, prediction of homology modelling and the intensive mode in Phyre2 were used.[Citation49] In addition, Phyre2 has a new ab initio folding simulation termed as Poing,[Citation50] to model areas of proteins without any significant homology for known structures. All seven RPL24 proteins were modelled at >90% reliability and the residue percentage varied from 80 to 100 (). The secondary structures were predominantly constituted of β sheets and rare incurrence of α helices. Thus, all suggested protein structures were assessed to be highly reliable, which offered a preliminary basis for understanding the molecular function of cucurbit RPL24 proteins. In addition, a homology modelling analysis may be informative for the functional characterization of proteins.[Citation31]
Drought stress responses of CmRPL24-01 gene in melon
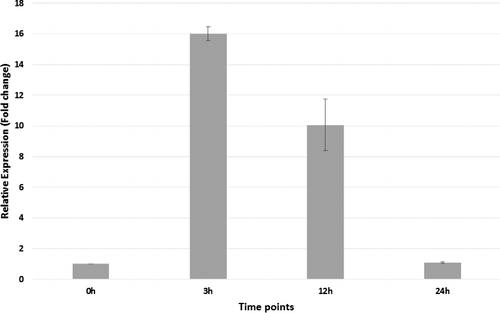
In order to reveal the response of CmRPL24-01 gene to drought stress, we measured the expression levels of this gene in melon leaves exposed to drought-stress at different time-points (0, 3, 12 and 24 h). Although CmRPL24-01 gene was up-regulated in melon leaves upon 3 h and 12 h of PEG treatment, the expression level of this gene peaked at 3 h (). The CmRPL24-01 gene expression levels were sharply decreased after 12 h of drought stress treatment. These results indicated that CmRPL24-01 gene expression has increased as an early response to water shortage and then it has gone back to its natural level. It is well known that plant growth is severely affected by stress conditions. Ribosomal proteins play an important role for proper growth and development of any organism.[Citation51] There has been a limited number of studies related with the regulation of genes expression of ribosomal proteins in plants, until recently. Ribosomal proteins have been reported to be induced or repressed under different stress conditions.[Citation52–56] Rogalski et al. [Citation57] indicated that chloroplast rpL33 gene was required for plant survival under cold stress in Arabidopsis. In a recent study, it has been shown that rpL32 gene from rice was transcriptionally down-regulated under various treatments (sucrose, cold, drought and abscisic acid (ABA) in rice.[Citation58] The study have also reported that this transcriptional repression was associated with the removal of transcription factors from specific promoter elements.[Citation51] These results agree with the findings of our study, in which CmRPL24-01 gene was strongly induced by drought stress treatment. It is possible to hypothesize that CmRPL24-01 gene might likely play a role in drought stress response for melon. Although altered expression of ribosomal proteins under stress conditions have been shown to involve the translational regulatory [Citation59–64] and post-transcriptional mechanisms,[Citation65–67] little is known about the stress-specific transcriptional regulation of ribosomal proteins. Therefore, this study makes a major contribution to research on molecular and functional characterization of Cucurbitaceae RPL24 family members.
Conclusions
In this study, the identification and bioinformatics analysis of cucumber, melon and watermelon RPL24 genes at the whole genome level were conducted. A total of seven RPL24 genes were identified. Gene structure, phylogenetic relationship and sequence characteristics were investigated. Besides the bioinformatics analysis, CmRPL24-01 gene was also cloned from melon and its expression level increased after drought stress treatment. The obtained results will help to gain deeper understanding of RPL24 genes and could contribute to the discovery of new RPL24 members in other Cucurbitaceae family members and to the selection of these candidate genes for functional and cloning studies.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Winfield IJ. FAO statistical yearbook 2012: world food and agriculture. J Fish Biol. 2012;81:2095–2096.
- Huang S, Li R, Zhang Z, et al. The genome of the cucumber, Cucumis sativus L. Natl Genet. 2009;41:1275–1281.
- Lough TJ, Lucas WJ. Integrative plant biology: role of phloem long-distance macromolecular trafficking. Annu Rev Plant Biol. 2006;57:203–232.
- Xoconostle-Cázares B, Xiang Y, Ruiz-Medrano R, et al. Plant paralog to viral movement protein that potentiates transport of mRNA into the phloem. Science. 1999;283:94–98.
- Garcia-Mas J, Benjak A, Sanseverino W, et al. The genome of melon (Cucumis melo L.). Proc Natl Acad Sci USA. 2012;109(29):11872–11877.
- Reyes R, Vazquez D, Ballesta JP. Peptidyl transferase center of rat-liver ribosome cores. Eur J Biochem. 1977;73:25–31.
- Hartzog GA, Fu J. The Spt4-Spt5 complex: a multi-faceted regulator of transcription elongation. Biochim Biophys Acta. 2013;1829(1):105–115.
- Werner F. A nexus for gene expression-molecular mechanisms of Spt5 and NusG in the three domains of life. J Mol Biol. 2012;417(1–2):13–27.
- Ben-Shem A, Garreau de Loubresse N, Melnikov S, et al. The structure of the eukaryotic ribosome at 3.0 Å resolution. Science. 2011;334(6062):1524–1529.
- Klinge S, Voigts-Hoffmann F, Leibundgut M, et al. Crystal structure of the eukaryotic 60S ribosomal subunit in complex with initiation factor 6. Science. 2011;334(6058):941–948.
- Steiner T, Kaiser JT, Marinkovic S, et al. Crystal structures of transcription factor NusG in light of its nucleic acid- and protein-binding activities. EMBO J. 2002;21:4641–4653.
- Kyrpides NC, Woese CR, Ouzounis CA. KOW: a novel motif linking a bacterial transcription factor with ribosomal proteins. Trends Biochem Sci. 1996;21(11):425–426.
- Zhang Y, Cheng YT, Bi D, et al. MOS2, a Protein containing G-patch and KOW motifs, is essential for innate immunity in arabidopsis thaliana. Curr Biology. 2005;15(21):1936–1942.
- Wool IG. Extraribosomal functions of ribosomal proteins. Trends Biochem. 1996;21:164–165.
- Reynaud E, Bolshakov VN, Barajas V, et al. Antisense suppression of the putative ribosomal protein S3A gene disrupts ovarian development in Drosophila melanogaster. Mol Gen Genet. 1997;256:462–467.
- Qian S, Hongo S, Jacobs-Lorena M. Antisense ribosomal protein gene expression specifically disrupts oogenesis in Drosophila melanogaster. Proc Natl Acad Sci USA. 1988;85:9601–9605.
- Nishimura T, Wada T, Okada K. A key factor of translation reinitiation, ribosomal protein L24, is involved in gynoecium development in Arabidopsis. Biochem Soc Trans. 2004;32:611–613.
- Kousteni S, Tura-Kockar FD, Ramji P. Sequence and expression analysis of a novel Xenopus laevis cDNA that encodes a protein similar to bacterial and chloroplast ribosomal protein L24. Gene. 1999;235:13–18.
- Read LK, Militello KT, Nerantzakis GE. Cloning and characterisation of a cDNA encoding the Trypanosoma brucei ribosomal protein L24p. Int J Parasitol. 1999;29:601–605.
- Tsutsumi N, Takusagawa S, Suzuki H. Molecular cloning and nucleotide sequencing of nuclear genes coding for the chloroplast ribosomal proteins L13, L24, L28 of rice (Oryza sativa L.) Plant Sci. 1996;121:167–174.
- Rasmussen SK, Klausen J. Ribosomal protein L24E homologue (accession No. X94296) is expressed in barley endosperms (PGR96-011). Plant Physiol. 1996;110:1047–1048.
- Estebsan R, Labrador E, Dopico B. Ribosomal protein L24 homologue is expressed in Cicer arietinum L. epicotyls. Plant Physiol. 1998;117:717–718.
- Baronas-Lowell DM, Warner JR. Ribosomal protein L30 is dispensible in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:5235–5243.
- Eng FJ, Warner JR. Structural basis for the regulation of splicing of a yeast messenger RNA. Cell. 1991;65:797–804.
- Bult CJ, White O, Olsen GJ, et al. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science. 1996;273:1058–1073.
- Kawarabayasi Y, Sawada M, Horikawa H, et al. Complete sequence and gene organization of the genome of a hyper-thermophilic archaebacterium, Pyrococcus horikoshii OT3. DNA Res. 1998;5:55–76.
- Venter HP, Clayton RA, Tomb JF, et al. The complete genome sequence of the hyperthermophilic, sulphate-reducing archaeon Archaeoglobus fulgidus. Nature. 1997;390:364–370.
- Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. Calif Agric Exp Stn Circ. 1950;347:1–32
- Baloglu MC, Eldem V, Hajyzadeh M, et al. Genome-wide analysis of the bZIP transcription factors in cucumber. PLoS One. 2014;9:e96014.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression datausing real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408.
- Baloglu MC, Patir MG. Molecular characterization, 3D model analysis, and expression pattern of the CmUBC gene encoding the melon ubiquitin-conjugating enzyme under drought and salt stress conditions. Biochem Gen. 2014;52:90–105.
- Goodstein DM, Shu S, Howson R, et al. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 2012;40:D1178–D1186.
- Guo S, Zhang J, Sun H, et al. The draft genome of watermelon (Citrullus lanatus) and resequencing of 20 diverse accessions. Natl Genet. 2012;45:51–58.
- Letunic I, Doerks T, Bork P. Smart 7: recent updates to the protein domain annotation resource. Nucleic Acids Res. 2012;40:D302–D305.
- Punta M, Coggill PC, Eberhardt RY, et al. The Pfam protein families database. Nucleic Acids Res. 2012;40:D290–D301.
- Guo AY, Zhu QH, Chen X, et al. GSDS: a gene structure display server. Yi Chuan. 2007;29:1023–1026.
- Tamura K, Peterson D, Peterson N, et al. Mega5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739.
- Thompson JD, Gibson TJ, Plewniak F, et al. The Clustal_x windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882.
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425.
- Letunic I, Bork P. Interactive tree of life v2: online annotation and display of phylogenetic trees made easy. Nucleic Acids Res. 2011;39:W475–478.
- Bailey TL, Elkan C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Paper presented at: Intelligent Systems for Molecular Biology. Proceedings of the Second International Conference. August 14–17, 1994; Menlo Park, CA.
- Quevillon E, Silventoinen V, Pillai S, et al. InterProScan: protein domains identifier. Nucleic Acids Res. 2005;33:W116–W120.
- Berman HM, Westbrook J, Feng Z, et al. The protein data bank. Nucleic Acids Res. 2000;28:235–242.
- Kelley LA, Sternberg MJ. Protein structure prediction on the Web: a case study using the Phyre server. Natl Protoc. 2009;4(3):363–371.
- Pateraki I, Sanmartin M, Kalamaki MS, et al. Molecular characterization and expression studies during melon fruit development and ripening of L-galactono-1,4-lactone dehydrogenase. J Exp Bot. 2004;55(403):1623–1633.
- Hu LF, Liu SQ. Genome-wide identification and phylogenetic analysis of the ERF gene family in cucumbers. Genet Mol Biol. 2011;34:624–633.
- Marchler-Bauer A, Derbyshire MK, Gonzales NR, et al. CDD: NCBI's conserved domain database. Nucleic Acids Res. 2015;43(Database issue):D222–D226.
- Puranik S, Sahu PP, Srivastava PS, et al. NAC proteins: regulation and role in stress tolerance. Trends Plant Sci. 2012;17:1360–1385.
- Söding J. Protein homology detection by HMM–HMM comparison. Bioinform. 2005;21:951–60.
- Jefferys BR, Kelley LA, Sternberg MJ. Protein folding requires crowd control in a simulated cell. J Mol Biol. 2010;397:1329–1338.
- Mukhopadhyay P, Reddy MK, Singla-Pareek SL, et al. Transcriptional downregulation of rice rpL32 gene under abiotic stress is associated with removal of transcription factors within the promoter region. PLoS ONE. 2011;6(11):e28058.
- Kim KY, Park SW, Chung YS, et al. Molecular cloning of low-temperature-inducible ribosomal proteins from soybean. J Exp Bot. 2004;55:1153–1155.
- Ludwig A, Tenhaken R. Suppression of the ribosomal L2 gene reveals a novel mechanism for stress adaptation in soybean. Planta. 2001;212:792–798.
- Saez-Vasquez J, Gallois P, Delseny M. Accumulation and nuclear targeting of BnC24: a Brassica napus ribosomal protein corresponding to a mRNA accumulating in response to cold treatment. Plant Sci. 2000;156:35–46.
- Brosché M, Strid A. The mRNA-binding ribosomal protein S26 as a molecular marker in plants: molecular cloning, sequencing and differential gene expression during environmental stress. Biochim Biophys Acta. 1999;1445:342–344.
- Gao J, Kim SR, Chung YY, et al. Developmental and environmental regulation of two ribosomal protein genes in tobacco. Plant Mol Biol. 1994;25:761–770.
- Rogalski M, Schöttler MA, Thiele W, et al. Rpl33: a nonessential plastid-encoded ribosomal protein in tobacco is required under cold stress conditions. Plant Cell. 2008;20:2221–2237.
- Mukhopadhyay P, Singla-Pareek SL, Reddy MK, et al. Stress-mediated alterations in chromatin architecture correlate with down-regulation of a gene encoding 60S rpL32 in rice. Plant Cell Physiol. 2013;54(4):528–540.
- Mahfouz MM, Kim S, Delauney AJ, Verma DP. Arabidopsis target of rapamycin interacts with raptor, which regulates the activity of S6 kinase in response to osmotic stress signals. Plant Cell. 2006;18:477–490.
- Tatematsu K, Ward S, Leyser O, et al. Identification of cis-elements that regulate gene expression during initiation of axillary bud outgrowth in Arabidopsis. Plant Physiol. 2005;138:757–766.
- Wullschleger S, Loewith R, Oppliger W, et al. Molecular organization of target of rapamycin complex 2. J Biol Chem. 2005;280:30697–30704.
- Kawaguchi R, Girke T, Bray EA, et al. Differential mRNA translation contributes to gene regulation under non-stress and dehydration stress conditions in Arabidopsis thaliana. Plant J. 2004;38:823–839.
- Turck F, Zilbermann F, Kozma SC, et al. Phytohormones participate in an S6 kinase signal transduction pathway in Arabidopsis. Plant Physiol. 2004;134:1527–1535.
- Trémousaygue D, Garnier L, Bardet C, et al. Internal telomeric repeats and ‘TCP domain’ protein-binding sites co-operate to regulate gene expression in Arabidopsis thaliana cycling cells. Plant J. 2003;33:957–966.
- Liu X, Baird WV. The ribosomal small-subunit protein S28 gene from Helianthus annuus (Asteraceae) is down-regulated in response to drought, high salinity, and abscisic acid. Am J Botany. 2003;90:526–531.
- Giannino D, Frugis G, Ticconi C, et al. Isolation and molecular characterisation of the gene encoding the cytoplasmic ribosomal protein S28 in Prunus persica [L.] Batsch. Mol Gen Genet. 2000;263:201–212.
- Beltrán-Peña E, Ortíz-López A, de Jiménez ES. Synthesis of ribosomal proteins from stored mRNAs early in seed germination. Plant Mol Biol. 1995;28:327–336.