?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A total of 17 actinomycetes were isolated and screened against five bacterial pathogens. Forty-one per cent of the isolates were active against the tested pathogens. The most potent isolate was identified as Streptomyces parvus by using a 16S rRNA sequence analysis. S. parvus produced active compound(s) against a number of Gram negative and Gram positive bacteria. The obtained inhibition zones were 14, 19, 20 and 20 mm against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Aeromonas hydrophila, respectively. Moreover, the anticancer activity of S. parvus was tested against four different cell lines: human liver cancer cell line, mouse lymphoma cell line, breast cancer cell line and human colon cancer cell line. The inhibition activities were 53%, 56%, 57% and 42%, respectively. To achieve a maximum production of the bioactive compound, Plackett–Burman design was applied. The productivity increased up to 1.3-fold, when S. parvus was grown in optimized medium composed of: 10 g L−1 starch, 1.5 g L−1 KNO3, 0.75 g L−1 K2HPO4, 0.75 g L−1 MgSO4∙7H2O, 0.015 g L−1 FeSO4, 2 mL (103 colony-forming units mL−1) inoculum size with pH 8 for 7 d of incubation. The main constitutes of S. parvus crude extract were determined by gas–liquid chromatography mass spectrometry. They were found to be ethane, 1,1-diethoxy; di-n-octyl phthalate; ethanol, 2,2-diethoxy; 9,12-octadecadienoic acid; methyl ester (E,E) and benzoic acid.
Introduction
Marine microorganisms, as a new and promising source of biologically active compounds, are currently of considerable interest.[Citation1] They produce a variety of metabolites, some of which can be used for drug development.[Citation2] These microorganisms are virtually unlimited sources of novel compounds with many therapeutic applications.[Citation3] Actinomycetes hold a prominent position because they have a wide diversity and proven ability to produce new compounds. Further, the discovery of novel antibiotic and non-antibiotic lead molecules through microbial secondary metabolite screening is becoming increasingly important.[Citation3]
The majority of actinomycetes can be found in both aquatic and terrestrial habitats.[Citation4,Citation5] The recent researchers screened intensively the marine plants, medicinal plants, sediments and soil environments of actinomycetes, in order to elucidate their bioactive molecules.[Citation4,Citation6–9] Although soils have been screened by the pharmaceutical industry for about 50 years, only a miniscule fraction of the surface of the globe has been sampled, and only a small fraction of actinomycetes taxa has been discovered.[Citation10] Actinomycetes are the most economically and biotechnologically valuable prokaryotes. About half of the discovered bioactive secondary metabolites are produced by them.[Citation11] Microorganisms have been reported to produce around 23,000 bioactive secondary metabolites and over 10,000 of these compounds are produced by actinomycetes, representing 45% of all bioactive microbial metabolites discovered.[Citation12] Among actinomycetes, around 7600 compounds are produced by Streptomyces species.[Citation8,Citation13] Approximately 289 secondary metabolites from the marine-derived genus of Streptomyces are reported in the Marinlit database, covering a wide variety of chemical structures, including peptides, macrolides, lactones, indoles, terpenes and quinones.[Citation14] These compounds show an extensive range of industrially useful activities, such as cytotoxic, antibacterial, antifungal, antimalarial,[Citation15,Citation16] anticancer,[Citation16] immunosuppressant,[Citation16,Citation17] anti-inflammatory, anthelmintic, herbicide, enzyme and others.[Citation6,Citation8]
The bioactive secondary metabolites from marine-derived Streptomyces have thus attracted increasing interest during the last decades.[Citation18] Because of the excellent record of accomplishment of actinomycetes in this regard, a significant effort has been focused on the successful isolation of novel actinomycetes from terrestrial sources for drug screening programmes in the past 50 years. Recently, there has been a decrease in the rate of discovery of new compounds from terrestrial actinomycetes. On the other hand, there has been an increase in the rate of re-isolation of known compounds. Thus it is crucial for new groups of actinomycetes from unexplored or underexploited habitats to be pursued as sources of novel bioactive secondary metabolites.[Citation19] Although there is an extraordinary diversity of life in the terrestrial environment, the greatest biodiversity is localized in the oceans.[Citation11,Citation20] Marine actinomycetes are chemically rich sources of structurally diverse secondary metabolites.[Citation14,Citation21]
Looking at the wide importance of actinomycetes in various fields, the present study was undertaken to isolate actinomycetes from seawater and sediment samples along the sea shore of Suez Bay, Egypt. Also, an identification of the most potent isolate was conducted to assess its antibacterial and anticancer activities.
Materials and methods
Actinomycetes isolation and growth conditions
A total of 17 actinomycetes' isolates representing different colony morphologies were isolated from seawater and sediment samples along the sea shore of Suez Bay, Egypt, from winter to autumn of 2012. Actinomycetes strains were cultivated and purified on starch–nitrate medium,[Citation22] which had the following constituents: 20 g L−1 starch, 1 g L−1 KNO3, 0.5 g L−1 K2HPO4, 0.5 g L−1 MgSO4∙7H2O, 0.05 g L−1 FeSO4 and 15 g L−1 agar (in case of solid medium), pH was adjusted to 7 and the incubation was conducted at 30–32 °C for 7 d.[Citation23]
Pathogenic indicators
The bacterial pathogens used as target strains in the present investigation were Aeromonas hydrophila, Vibrio damsela, Pseudomonas aeruginosa ATCC 6739, Escherichia coli and Staphylococcus aureus ATCC 6538. These pathogens were kindly provided by the staff members of The National Institute of Oceanography and Fisheries, Egypt.
Bacterial identification
The promising actinomycetes' isolate was cultured in starch–nitrate liquid medium for 7 d and genomic DNAs were extracted with the genomic DNA extraction protocol of GeneJET genomic DNA Purification Kit (Fermentas). The polymerase chain reaction (PCR), using Maxima Hot Start PCR Master Mix (Fermentas), was carried out in a thermal cycler (Multigene Optimax, Labnet International, Inc.). The PCR thermocycler was programmed as follows: 95 °C for 5 min for initial denaturation, 30 cycles at 95 °C for 1 min, 55 °C for 1 min, 72 °C for 2 min and a final extension at 72 °C for 10 min. The PCR mixture contained 25 pmol of each primer (Sigma Scientific Services Company, Egypt, 2013), 10 ng chromosomal DNA, 200 mmol L−1 dNTPs and 2.5 U of Taq polymerase in 50 μL of Taq polymerase buffer (10X standard Taq reaction buffer).
The PCR clean-up of the PCR product was performed by using GeneJET™ PCR Purification Kit (Fermentas) at Sigma Scientific Services Company, Egypt, 2013. The sequencing of the PCR product was made by the GATC Company by using ABI 3730xl DNA sequencer with universal primers (16S 27F and 16S 1492R), (5′-AGAGTTTGATCCTGGCTCAG-3′ and 5′-GGTTACCTTGTTACGACTT-3′). Genotypic characterization was performed using 16S sequence analysis. Multiple alignments with sequences of the most closely related members and calculations of levels of sequence similarity were carried out using BioEdit (software version 7).[Citation24] Sequences of rRNA genes, for comparison, were obtained from the National Center for Biotechnology Information database.
Electron microscopy studies
For the electron microscopy, starch–nitrate agar medium was inoculated with spores of S. parvus strain and incubated for 14 d at 32 °C. A plug of the culture was removed and fixed in glutaraldehyde (2.5%, v/v), washed with water and post-fixed in osmium tetroxide (1%, w/v) for 1 h. The sample was washed twice with water, dehydrated in ascending ethanol concentrations (30%, 50%, 70%, 90% and 100%, v/v) and finally coated in gold and examined at 15–20 kV in JEOL JSM 5400 LV scanning electron microscope.
Antibacterial activity
The actinomycetes' isolates that were studied were inoculated in flasks (250 mL) containing 50 mL of starch–nitrate medium. Seeded flasks were incubated at 30–32 °C on a rotary shaker at 200 rpm for 7 d. At the end of the incubation, the cultures were harvested, filtered and sterilized by ultrafiltration by using 0.22 µL sterilized filters and used as antimicrobial agents. The antagonistic activity of actinomycetes' supernatants was detected by using well-cut diffusion technique, in which, 5-mm diameter wells were punched in marine nutrient agar (Oxoid Ltd, England) plates inoculated with bacterial pathogenic strains. Fifty microlitre of the tested supernatant was pipetted into each well. The plates were incubated at 30 °C for 24 h. Each set was prepared in duplicate. After incubation, the radius of the clear zone around each well was linearly measured in mm.[Citation25,Citation26]
Optimization of culture conditions
Plackett–Burman design
The Plackett–Burman experimental design [Citation27] was used to evaluate the relative importance of the various factors involved in the production of antimicrobial agents by the selected actinomycete isolate S. parvus against the bacterial pathogen A. hydrophila. Seven independent variables were examined in this experiment and their settings are shown in . Each variable was tested in four runs at low level (-1) and in four trials at high level (+1). The eight different runs and the basal control (trial number 9 in ) were performed in duplicate. The main effect of each variable on the inhibition zones' diameter was determined by using Equation Equation (1)(1)
(1) :
(1)
(1) where Exi is the variable main effect, Mi+ and Mi- are the radiuses of the clear zones around each well in the runs. The independent variable (xi) is present in the high and low concentrations, respectively, and N is the number of runs, divided by 2. By using Microsoft Excel, statistical t-values for equal unpaired samples were calculated [Citation28] to determine the variable's significance. From the main effect results, an optimized medium was predicted.
Table 1. Independent variables affecting the production of antimicrobial agent(s).
Table 2. Experimental results of the applied Plackett–Burman design for seven cultural variables.
Verification experiment
A verification experiment was carried out in duplicate. The predicted optimum levels of the independent variables were examined and compared to the basal conditions setting (trial number 9 in ) and the secondary metabolites production was detected by measuring the average of the inhibition zones' diameter (mm).
Optimization of the incubation time and temperature
The effect of different incubation temperatures (25–35 °C) and different incubation periods (6–8 d) on the production of antimicrobial agent(s) by S. parvus strain against bacterial pathogen A. hydrophila was determined by using the optimized medium. The production of the antimicrobial agent(s) was detected by measuring the inhibition zones' diameter (mm).
Immobilization technique
The immobilization was performed by using adsorption techniques, as described by Eikmeier and Rehm,[Citation29] to enhance the production of the antimicrobial agents. Erlenmeyer flasks (250 mL capacity) containing 50 mL of the optimized medium and porous supporting materials (luffa pulp, pumice, sponge and tails), were inoculated with the selected actinomycete isolate. The flasks were then shaken slowly at 120 rpm for 7 d of incubation. The production of the antimicrobial agents was compared with control (free cells), by using well-cut diffusion technique. The production of the antimicrobial agent(s) was detected by measuring the inhibition zones' diameter (mm).
Extraction of the active compound(s)
A 7-d culture of the selected strain was centrifuged (Centurion Scientific, United Kingdom) at 10,000 rpm for 15 min. The supernatant was extracted with equal volume of chloroform, n-butanol or ethyl acetate. After soaking and vigorously shaking for a complete extraction, the organic phase was collected and evaporated by using a rotary evaporator (Stuart, BIBBY Scientific Limited, United Kingdom), and then dissolved in the appropriate solvent. The antagonistic activity of the crude extract was tested by using the well-cut diffusion method.[Citation30] The activity of the extracted antimicrobial agent(s) was detected by measuring the inhibition zones' diameter (mm).
Estimation of the crude extract's antibacterial activity
The antimicrobial activity of the crude extract was estimated via comparison with some standard commercial antibiotics and was determined by disc diffusion method according to Bauer et al.[Citation31] About 250 μg of the actinomycete crude extract was impregnated on sterile disc (Whatman No.1 filter paper was punched into discs) and dried under sterile conditions at room temperature. Three standard commercial antibiotic discs were used for the comparative study. The used antibiotics were 5 μg/disc methicillin, 2 μg/disc lincomycin and 30 μg/disc chloramphenicol (Oxoid Ltd, England). The discs were assayed onto the surfaces of the plates seeded with A. hydrophila (1 of the McFarland scale) as a target strain. The plates were incubated at 30 °C overnight. After incubation, the inhibition zone around each disc was linearly measured (mm). The assays were performed in duplicate.
Antifouling activity
Fouling bacteria (1 mL of seawater) were incubated with cover glass in 50 mL conical flask containing 20 mL of nutrient broth medium (Oxoid Ltd, England) at 28 °C for 24 h. About 0.1 g of the crude extracted substance was added into the flask as an antifouling agent. After dying with 0.4% crystal violet solution for 10 min, the cover glass was washed with water, air dried and observed under a microscope (OPTIKA microscopes, model: B-182, Italy).[Citation32] A control flask (without crude extract) was used for comparison.
Anticancer activity
In order to evaluate the anticancer activity of S. parvus culture supernatant, several steps were undertaken: lyophilization (Telstar, Spain), cytotoxicity test and effect of the median inhibitory dose (IC50) on four different tumour cell lines: human liver cancer cell line (HepG2), mouse lymphoma cell line (EI-4), breast cancer cell line (MCF-7) and human colon cancer cell line (Caco-2).
The lyophilization process can be summarized in three general steps: freezing, primary drying and secondary drying. Filtrate of S. parvus culture (10 mL) was lyophilized and converted into powder by using a Telson lyophilizer (Spain). Different concentrations (5, 25, 50, 75 and 100 µg mL−1) of the powder were prepared and used in the second step (cytotoxicity test). To measure the cytotoxicity of the examined compounds, 5 × 104 lymphocyte cells were seeded in 96-well plates and incubated in RPMI (Roswell Park Memorial Institute) 1640 medium supplemented with 200 µmol L−1 L-glutamine and 10% faetal bovine serum (FBS) containing different concentrations (5, 25, 50, 75 and 100 µg mL−1) of the tested compound and incubated for 72 h in 5% CO2 incubator. Wells were washed with Hank's balanced salt solution. Fraction of viable lymphocytes was measured by the 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.
In the MTT assay, yellow MTT is reduced to purple formazan in the mitochondria of viable cells. A quantity of 100 μL of the MTT working solution (0.5 mg mL−1) was added to each well and incubated at 37 °C for 4 h. Next, media were removed, wells were washed with phosphate buffer saline and 100 μL of dimethyl sulphoxide was added to solubilize the formazan crystalline product. The absorbance was measured with a plate reader (Cell Star, USA) at 570 nm. The viable cells' fraction was determined by dividing the absorbance of the cells treated with a test compound (test well) to that of the cells unexposed to any compounds (control well). Safe doses of different test compounds were determined from the GraphPad InStat software, as the compound concentration that caused 100% viability of normal cells (EC100) and was calculated from the equation of cell viability as follows: (absorbance of wells containing cells treated with the compound at different concentrations/absorbance of control well) × 100.
Cytotoxicity assay was performed on different human and mouse cancer cell lines by using the described method. The anticancer activity of the tested compound was assayed using four different cell lines (EI-4, HepG2, Caco-2 and MCF-7). Cells (15 × 103, 4 × 103, 3 × 103 and 3 × 103) were seeded in 96-well plates and incubated in 200 μL of RPMI 1640, RPMI 1640 high-glucose formulation Dulbecco's modified eagle's medium and RPMI 1640 media/10% FBS (Lonza, Switzerland), respectively, for 24 h in 5% CO2 incubator for cell attachment. Next, the media were changed with the same media containing the safe dose of the tested compound and cells were incubated in that media for 72 h. Medium without any chemicals was used as a negative control. Fractions of viable cells were measured with MTT assay.[Citation33] The inhibition of growth rate of tumour cells for each compound with different concentrations was calculated as follows: [100 − (absorbance of cells that were exposed to the compound at different concentrations/absorbance of control well)] × 100.
Antiviral activity
Qualitative in vitro anti-hepatitis C virus screening
HepG2 cells were washed twice with RPMI 1640 medium supplemented with 200 µmol L−1 L-glutamine (Lonza, Switzerland) and 25 µmol L−1 N-[2-hydroxyethyl] piperazine-N9-[2-ethanesulphonic acid] buffer (Lonza, Switzerland). The cells were suspended to 2 × 105 cells mL−1 in RPMI 1640 medium containing 10% FBS (Gibco-BRL). The cells were seeded in six-well plates and incubated for 24 h in an incubator (37 °C, 5% CO2, 95% humidity) for cell attachment.
Cytotoxicity assay was performed on HepG2 cell line by using the previously described method. HepG2 cell culture was prepared as described by El-Hawash et al.[Citation34] The cells were infected with 2% hepatitis C virus (HCV)-infected serum in RPMI culture medium containing 8% FBS. The tested compound was added at its non-lethal dose (IC50 dose of the compound in HEPG2 cells). Positive (infected untreated cells) and negative (uninfected untreated cells) control cultures were included. After 96 h of incubation at 37 °C, 5% CO2 and 95% humidity, a second dose of the test compound was added. The cells were incubated for another 96 h, followed by a total RNA extraction. The positive strand and its replicating form (negative strand) were detected by reverse transcription polymerase chain reaction (RT-PCR) using HCV specific primers to the 5' untranslated region of the virus.
RNA extraction and RT-PCR of HCV RNA
Total RNA was extracted from HepG2 HCV-infected cells using the method described by El-Awady et al.[Citation35] Briefly, culture cells were mixed with 500 µL of 4 mol L−1 guanidinium isothiocyanate containing 25 mmol L−1 sodium citrate, 0.5% (w/v) sarcosyl, 0.1 mol L−1 β-mercaptoethanol and 100 µL sodium acetate. The lysed cells were mixed with an equal volume of phenol, chloroform and isoamyl alcohol (25:24:1, pH 4). After vortexing (Thermolyne, USA) of the sample, the mixture was centrifuged at 14,000 rpm for 10 min at 4 °C. The aqueous layer was collected and mixed with an equal volume of isopropanol. After an overnight incubation at -20 °C, RNA was precipitated by centrifugation at 14,000 rpm for 30 min at 4 °C and the precipitated RNA was washed twice with 70% ethanol.
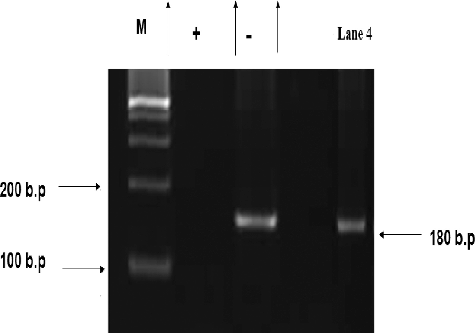
The complimentary DNA (cDNA) and the first PCR reaction of the nested PCR detection system for the HCV RNA was performed in a 50 µL volume single-step reaction using the Ready-To-Go RT-PCR beads (Amersham Pharmacia Biotech, USA), 10 µmol L−1 from each of the RT-downstream primers (PCR forward primer and reverse primer P2) (5′-GGTGCAC GGTCTACGA GACCTC-3′, 5′-AAC TAC TGT CTT CAC GCA GAA-3′ and 5′-TGC TCA TGG TGC ACG GTC TA-3). The thermal cycling protocol was as follows: 30 min at 42 °C for cDNA synthesis, followed by 5 min at 95 °C and 30 cycles of 1 min at 94 °C, 1 min at 55 °C and 1 min at 72 °C. The nested PCR amplification was performed in 50 µL reaction mixture containing 0.2 mmol L−1 from each dNTP, 10 mmol L−1 from each of the reverse and forward nested primers (5′-ACT CGG CTA GCA GTC TCG CG-3′ and 5′-GTG CAG CCT CCA GGA CCC-3′), 2 U Taq DNA polymerase (Promega, USA) and 10 µL from the RT-PCR reaction product in a 1 × Tag buffer (10 mmol L−1 Tris-HCl, 50 mmol L−1 KCl and 1.5 mmol L−1 MgCl2, pH 8.3). The PCR product was separated by agarose gel electrophoresis (OWI Scientific-INC, USA) by using 2.5% agarose gel that was impregnated with ethidium bromide at 90 V. A fragment of 174 bp length was identified in the positive samples and analysed electrophoretically using an ultraviolet (UV) transilluminator (Herolab, UVT-20 mlw, Germany).[Citation36]
The gas–liquid chromatography mass spectra of the crude extract
Identification of the crude extract's chemical constituents of the selected actinomycete isolate was done using high performance 5890 gas–liquid chromatography (GLC) (Hewlett Packard) coupled with 5989 B series mass spectrometer (MS) (Shimadzu (EI), Japan).The percentage of each compound was calculated as the ratio of the peak area to the total chromatographic area.[Citation37] The gas–liquid chromatography mass spectrometer (GC-MS) peaks were identified by comparison with several reported data, their profiles and similarity percentages were detected from the Wiley 275 libraries.
Results and discussion
Screening for antibacterial activity
The screening for antagonistic activity revealed that 7 out of the 17 isolated marine actinomycetes isolates (41%) exhibited antagonistic effect against the tested bacterial pathogens (). Three isolates (18%) were active only against one bacterial pathogen and 23% of actinomycetes isolates were capable of inhibiting the growth of most of the tested bacterial pathogens. Isolate 8 exhibited good activities against S. aureus ATCC 6538, E .coli, P. aeruginosa ATCC 6739 and A. hydrophila and the inhibition zones were 14, 19, 20 and 20 mm, respectively. Isolate 8 was selected as a representative of this group and was subjected to further studies.
Table 3. The antibacterial activity of the actinomycetes isolates against some bacterial pathogens.
The results obtained in the present experiment confirmed the bioactive potential of marine actinomycetes' strains. Patil et al. [Citation38] reported that 77 isolates out of 104 were found to be inhibitory to at least one of the pathogens. Also, Patel et al. [Citation39] reported that 79% of the actinomycetes isolates were active against at least one of the 18 tested pathogens. In a recent study, Rana and Salam [Citation9] characterized 23 actinomycetes isolates, 7 of which showed significant inhibition of different Gram positive and Gram negative bacteria and also some fungi, with zones of inhibition up to 20 mm. Actinomycetes isolates have a broad spectrum of antimicrobial activities, and this is why it is of major importance to conduct further studies for the isolation of novel antimicrobial compounds. So far, quite a number of reports are available, in which such novel compounds have been obtained and studied.[Citation4,Citation9]
Molecular identification
The obtained sequence of the 16S rRNA gene indicated the promising actinomycete isolate from the present study (isolate 8 from sediment in Suez Bay, Egypt), which was similar to S. parvus with a maximum identity of 96%. The nucleotide sequence was deposited to GenBank sequence database with the following accession number: KP675949. shows the spore formation of S. parvus.
Figure 1. Scanning electron micrograph showing spore formation of S. parvus on starch–nitrate agar medium after 14 d of incubation.
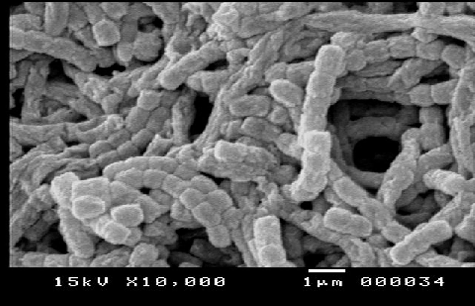
Patil et al. [Citation38] found that the highest incidence of inhibitory isolates was isolated from sediment samples. All the isolates of antagonistic marine actinomycetes were identified to be Streptomyces. The genus Streptomyces seems to provide a wide variety of new antibiotics more than any other genus; hence, it is of great importance for both industrial applications and human health care.[Citation40]
Optimization of culture conditions
In order to maximize the antimicrobial agent production by marine S. parvus, an experimental design and immobilization techniques were employed to optimize the culture conditions. Plackett–Burman design, an efficient technique for medium component optimization, was employed to identify the significant variables that enhance the antimicrobial agent production.[Citation41] The results were achieved in an economical manner.[Citation42] One of the advantages of the Plackett–Burman design is that it may rank the effects of different variables on the measured response, independently of their nature (either nutritional or physical factor) or sign (whether contributes positively or negatively).[Citation43]
Plackett–Burman design
The components of the starch–nitrate agar medium, in addition to some other cultural factors, including inoculum size and pH, were examined according to a design matrix (), with respect to the production of antimicrobial agent(s).
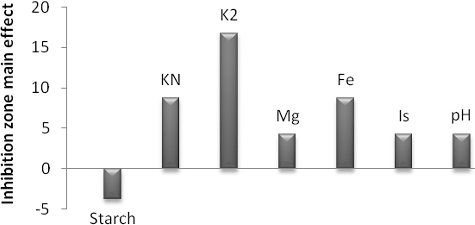
In the analysis of these designs, the main effects of the examined factors on the inhibition zone's diameter were calculated (). Based on these results, the low (−) level of starch and the high (+) levels of K2HPO4, KNO3, MgSO4, FeSO4, inoculum size and pH encouraged the production of antimicrobial agent(s) formed by S. parvus strain (). Therefore, decreasing the starch concentration and simultaneously increasing the K2HPO4 and KNO3 concentrations in the culture medium can increase the suppression of A. hydrophila with respect to production of the antimicrobial agent(s) by S. parvus. Moreover, the significant variables were identified by statistical analysis of the Plackett–Burman experiment by using the t-test supported by Excel Microsoft Office to determine the statistical significance of the measured response and calculate the main effects for S. parvus ().
Table 4. Statistical analyses of the Plackett–Burman experimental results.
Verification experiment
To verify the obtained results from the statistical analysis of Plackett–Burman design, verification tests were performed in duplicate by using the predicted optimized medium against the basal conditions media. The growth of S. parvus on the optimized medium with the following composition: 10 g L−1 starch, 1.5 g L−1 KNO3, 0.75 g L−1 K2HPO4, 0.75 g L−1 MgSO4∙7H2O, 0.015 g L−1 FeSO4, 2 mL (103 colony-forming units (CFU) mL−1) inoculum size with pH 8 for 7 d of incubation, recorded larger inhibition zones (27 mm) than that in the case of the basal conditions, with an increase by 1.3-fold. This result confirmed the validity of the optimized medium.
Another data obtained by Wefky et al. [Citation44] found that the production of the bioactive compounds by Enterococcus faecium was positively affected by increasing the incubation period, peptone, inoculum size, temperature, pH and seawater concentration; the optimized medium increased the inhibition zone by about 1.6-fold.
Optimization of incubation time and temperature
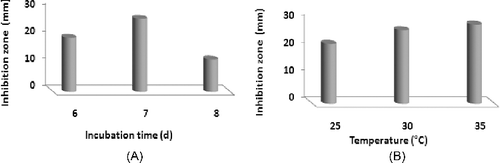
The effect of different incubation times (6–8 d) and incubation temperatures (25–35 °C) on the growth of S. parvus strain and the enhancement of the antimicrobial agent(s) production were evaluated. The optimum activity was detected after 7 d of incubation. On the other hand, the increase in the incubation temperatures enhanced the antimicrobial agent(s) production by S. parvus. The highest activity exhibited the largest inhibition zone of 29 mm and was observed in cultures previously incubated at 35 °C ((A) and 3(B)).
Figure 3. Effect of different incubation times (A) and temperatures (B) on the production of antimicrobial agent(s) by S. parvus strain (grown on verified medium).
El-Sersy and Abou-elela [Citation45] reported that a marine strain, identified as Nocardia brasiliensis, produced a bioactive compound that exhibited the largest inhibition zone and the highest activity against the fish pathogen V. damsel, when the culture's conditions were adjusted to pH 8 and the incubation was performed at 40 °C under static conditions. The increase in the incubation temperature led to an increase in the productivity and a higher production of the antagonistic compound.
Cell immobilization
In the most natural environments, the prevailing microbial lifestyle is in association with a surface, in a structure known as biofilm.[Citation46] There are multiple advantages of using immobilized microbial cells to enhance productivity and recovery. Although whole cell immobilization is achieved by various methods of chemical coupling and cell entrapment, the only method that has been applied to a large scale is via biofilm formation on the surface of a solid carrier.[Citation47]
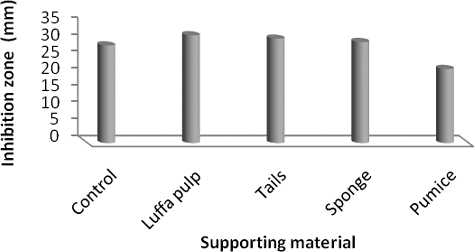
It was observed that the activity of the immobilized living cells of S. parvus was higher than that of the free cells (except in the case of pumice). The results in revealed that biofilm of S. parvus on luffa pulp showed the highest activity in the production of the antimicrobial agent(s). It gave the highest diameter of the inhibition zone (32 mm), i.e. raised up the productivity by 1.45-fold. This result coincided with Abou-elela et al.,[Citation48] who reported that the production of bioactive compounds by immobilized Nocardiopsis aegyptia cells on luffa pulp was the highest and its yield was increased from 1.12- to 1.16-fold.
Cell immobilization biotechnology is a multidisciplinary area, shown to have an important impact on many scientific sub-disciplines, including biomedicine, pharmacology, cosmetology, food and agricultural sciences, beverage production, industrial waste treatment and analytical applications.[Citation49]
Antibacterial activity of S. parvus
The crude extracts of S. parvus resulting from each solvent were tested for their antimicrobial activities against A. hydrophila. The results revealed that n-butanol extract exhibited the highest inhibition zone (26 mm), followed by ethyl acetate extract (24 mm) and chloroform (23 mm) and thus n-butanol was chosen as the most efficient solvent for extracting the desired compounds.
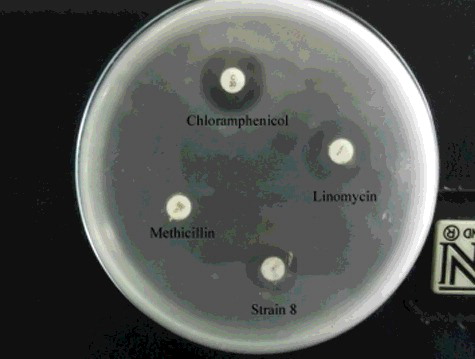
The antibacterial activity of S. parvus crude extract against A. hydrophila was estimated via comparison with commercial standard antibiotics (). The crude extract disc exhibited an inhibition zone of 15 mm, whereas the other antibiotic discs (30 μg chloramphenicol, 2 μg lincomycin and 5 μg methicillin) exhibited inhibition zones of 24, 22 and 0 mm, respectively.
Figure 5. Effect of S. parvus' (strain 8) crude extract against A. hydrophila in comparison with some standard commercial antibiotic discs.
Actinomycetes produce most of the roughly 10,000 known antibiotics and 75%–80% of the antibiotics prescribed today are derived from the genus Streptomyces.[Citation8] Marine derived actinomycetes have become recognized as a source of novel antibiotics.[Citation50] Furthermore, marine natural products exhibit a wide range of biological activities.[Citation51]
Anti-biofouling activity of S. parvus
shows the inhibitory activity of S. parvus crude extract on bacterial biofilm formation. The crude extract reduced the density of the bacterial cells and acted as an anti-biofouling agent.
Figure 6. Photographs illustrating the antifouling effect of S. parvus' crude extract on the fouling bacteria (A) and the effect without crude extract (B).
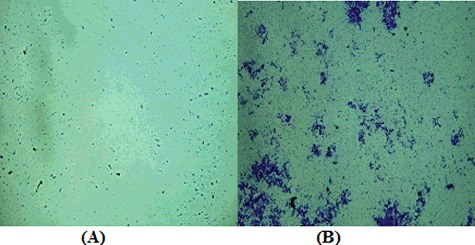
The present study concluded that the marine S. parvus' crude extract might be a potential source for the development of eco-friendly antifouling compounds, which might be a better alternative to the pollution causing synthetic antifoulants. This result agreed with the studies of Bavya et al. [Citation52] on the antifouling effect of S. filamentosus, which reduced the biofilm formation on glass.
Anticancer activity of S. parvus
As clearly shown in , the effect of S. parvus' culture supernatant on HepG2 tumour cell line exhibited a reasonable degree of anticancer activity, causing almost 53% inhibition after 72 h ((A) and 7(B)). Upon using the EI-4 tumour cell line and MCF-7 cell line, the supernatant of the tested compound showed almost 56% ((C) and 7(D)) and 57% ((E) and 7(F)) inhibition, respectively, after 72 h by using the MTT assay. On the other hand, the supernatants of the tested compound showed the weakest anticancer activity (42%) towards Caco-2 cell line, as detected by the MTT assay ((G) and 7(H)). showed the lowest IC50 (the highest anticancer activity) against lymphoma cells and the highest IC50 (the lowest anticancer activity) against Caco-2 cell line.
Table 5. IC50 and percentage of growth inhibition of the tested compound's safe dose against different cancer cell lines after 72 h using the MTT assay.
Figure 7. Growth inhibition effect of the tested S. parvus' compound on different cancer cell lines. Comparison of treated (A) vs. untreated (B) HepG2 cancer cells; treated (C) vs. untreated (D) EI-4 cancer cells; treated (E) vs. untreated (F) MCF-7 cancer cells; treated (G) vs. untreated (H) Caco-2 cancer cells.
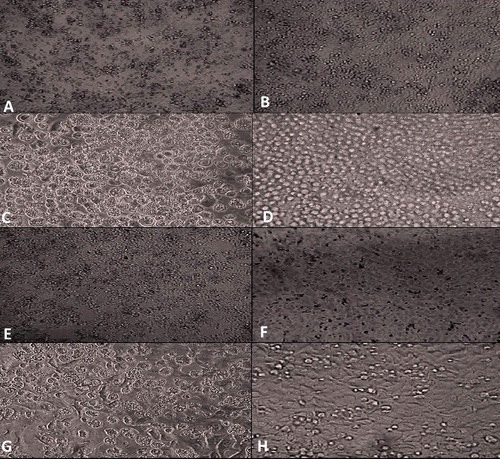
In a similar study, Vijayabharathi et al. [Citation53] reported that Sterptomyces strain, isolated from humus soils in the Western Ghats, has an anticancer activity against HepG2 (hepatic carcinoma) and HeLa (cervical carcinoma) in vitro. Secondary metabolites from actinomycetes, especially the genus Streptomyces, may be one of the most important sources for novel anticancer agents.[Citation54] Also, Yip et al. [Citation54] isolated three purified fractions from a novel strain, Streptomyces sp. H7372. In MCF-7 and MDA-MB-231 breast cancer cells, 31-2 (one of the fractions) induced a cytostatic (anti-proliferative) effect without causing cytotoxicity (cell death).
Antiviral activity of S. parvus
As shown in , the supernatant of the actinomycete strain S. parvus failed to inhibit the HCV replication. A fragment of 174 bp length was identified in the positive samples.
GC mass spectra of the crude extract
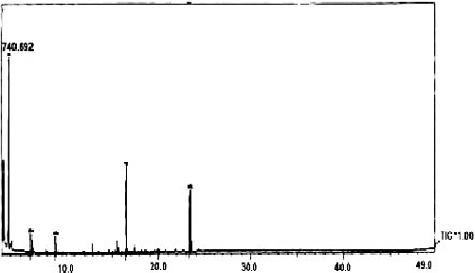
The GC-MS of S. parvus extract determined the main constitutes and the relative percentage of the identified compounds (). It was found that, the main constituents were ethane, 1,1-diethoxy; di-n-octyl phthalate; ethanol, 2,2-diethoxy; 9,12-ctadecadienoic acid, methyl ester (E,E) and benzoic acid ().
Table 6. The chemical composition of S. parvus' crude extract obtained by using GC-MS.
The major chemical component (ethane, 1,1-diethoxy) is reported to have a known biomedical value in the pharmacological fields, which is known to demonstrate valuable therapeutic uses, including anti-inflammatory, antipyretic, antithrombotic and analgesic effects.[Citation55] Also, chemical compounds, such as hexadecanoic acid, octadecatrienoic acid, pentadecanoic acid, heneicosanoic acid and oleic acid may be responsible for the antimicrobial properties of Andrographis paniculata's extract.[Citation56] In addition, Kumar et al. [Citation57] concluded that compounds like hexadecanoic acid, methyl ester and 9,12-octadecadienoic acid (Z,Z)-, methyl ester have anticancer properties. Jaina et al. [Citation58] reported that the 1,2-benzenedicarboxylic acid has insecticidal, pesticide and antitumour effects; the hexadecanoic acid has antioxidant, nematicide, hypocholesterolemic and pesticide effects and the 9,12-octadecadienoic acid has anticarcinogenic, antiatherogenic, antioxidant and anti-inflammatory effects. Furthermore, the synthetic methyl and propyl esters of p-hydroxybenzoic acid, the so-called parabens, are on the generally recognized as safe list for use in food at a maximum concentration of 0.02%.[Citation59]
Conclusions
The marine environment is a good source for valued species that need to be explored. The aim of the present study was to explore our natural environments, searching for actinomycetes producing bioactive compounds. The findings of the present study suggested that the antagonistic marine Streptomyces isolates, especially S. parvus or the antibacterial compounds produced by it, could be used as antibiotics and antifoulants, which might have future applications in the aquaculture systems. In addition, the S. parvus' compounds may be applied as anticancer agents. Future studies are required for the identification of the product.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- De Rosa S, Mitova M, De Caro S, et al. New peptide from a bacterium associated with marine sponge Ircinia muscarum. In: Sener B, editor. Biodiversity: biomolecular aspects of biodiversity and innovative utilization. Springer Science & Business Media; 2002. p. 335–340.
- Pietra F. Secondary metabolites from marine microorganisms: bacteria, protozoa, algae and fungi. Achievements and prospects. Nat Prod Rep. 1997;14:453–464.
- Gorajana A, Venkatesan M, Vinjamurim S, et al. Resistoflavine, cytotoxic compound from a marine actinomycete, Streptomyces chibaensis AUBN1/7. Microbiol Res. 2007;162:322–327.
- Isik K, Gencbay T, Özdemir-Kocak F, et al. Molecular identification of different actinomycetes isolated from East Black Sea region plateau soil by 16S rDNA gene sequencing. Afr J Microbiol Res. 2014;8(9):878–887.
- Macagnan D, Romeiro R, de Souza J, et al. Isolation of actinomycetes and endospore-forming bacteria from the cacao pod surface and their antagonistic activity against the witches' broom and black pod pathogens. Phytoparasitica. 2006;34:122–132.
- Naine J, Srinivasan MV, Devi SC. Novel anticancer compounds from marine actinomycetes: a review. J Pharm Res. 2011;4:1285–1287.
- Kaewkla O, Franco CM. Rational approaches to improving the isolation of endophytic actinobacteria from Australian native trees. Microb Ecol. 2013;65:384–393.
- Antunes TC, Borba MP, Spadari CC, et al. Screening of actinomycetes with activity against clinical isolates of gram positive cocci with multiresistant profile. J Adv Sci Res. 2014;5(1):13–17.
- Rana S, Salam MD. Antimicrobial potential of actinomycetes isolated from soil samples of Punjab. Indian J Microbiol Exp. 2014;1(2).
- Duraipandiyan V, Sasi AH, Islam VIH, et al. Antimicrobial properties of actinomycetes from the soil of Himalaya. J Med Mycol. 2010;20:15–20.
- Lam K. Discovery of novel metabolites from marine actinomycetes. Curr Opin Microbiol. 2006;9:245–251.
- Shin HJ. Anticancer compounds from marine microorganisms. In: Kim SK, editor. Marine pharmacognosy: trends and applications. CRC Press, Taylor & Francis Group; 2013. p. 409–419.
- Sharma M. Actinomycetes: source, identification, and their applications. Int J Curr Microbiol Appl Sci. 2014;3(2):801–832.
- Han Z, Xu Y, McConnell O, et al. Two antimycin A analogues from marine-derived cctinomycete Streptomyces lusitanus. Mar Drugs. 2012;10(3):668–676.
- Marinlit Database [ Internet]. Christchurch: University of Canterbury; 2011. [cited 2015 Apr 9]. Available from: http://www.chem.canterbury.ac.nz/marinlit/marinlit.shtml.
- Kurtböke DI, French JR, Hayes RA. et al. Eco-taxonomic insights into actinomycete symbionts of termites for discovery of novel bioactive compounds. Adv Biochem Eng Biotechnol. 2015;147:111–135.
- Mann J. Natural products as immunosuppressive agents. Nat Prod Rep. 2001;18:417–430.
- Blunt JW, Copp BR, Munro MHG, et al. Marine natural products. Nat Prod Rep. 2011;28:196–268.
- Attimarad SL, Gaviraj EN, Nagesh C, et al. Screening, isolation and purification of antibiotic(s) from marine actinomycetes. Int J Res Ayurveda Pharm. 2012;3(3):447–453.
- Donia M, Hamann MT. Marine natural products and their potential applications as anti-infective agents. Lancet Infect Dis. 2003;3:338–348.
- Fenical W, Jensen PR. Developing a new resource for drug discovery: marine actinomycete bacteria. Nat Chem Biol. 2006;2:666–673.
- Mostafa SA. Studies on certain actinomycetes from Egyptian soil with special reference to their metabolites [dissertation]. Egypt: Alexandria University; 1985.
- Jensen PR, Dwight R, Fenical W. Distribution of actinomycetes in near shore tropical marine sediments. Appl Environ Microbiol. 1991;57:1102–1108.
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–98.
- Ventosa A, Quesad F, Rodríguez-Varela F, et al. Numerical taxonomy of moderately Gram negative rods. J Gen Microbiol. 1982;128:1959–1968.
- El-Masry MH, Khalil AI, Hassouna MS, et al. In situ and in vitro suppressive effect of agricultural composts and their water extracts on some phytopathogenic fungi. World J Microbiol Biotechnol. 2002;18:551–558.
- Plackett RL, Burman JP. The design of optimum multi factorial experiments. Biometrica. 1946;33:305–325.
- Cochran WG, Snedecor GW. Statistical methods. Ames (IA): Iowa State University Press; 1989.
- Eikmeier H, Rehm HJ. Stability of calcium alginate during citric acid production of immobilized Aspergillus niger. Appl Microbiol Biotechnol. 1987;26:105–111.
- Eladly AM. Ecological and physiological studies on some actinomycetes isolated from soil [MSc dissertation]. Assiut: Al-Azhar University; 2008.
- Bauer RW, Kirby MDK, Sherris JC, et al. Antibiotic susceptibility testing by standard single disc diffusion method. Am J Clin Pathol. 1966;45:493–496.
- Kumaran S, Radhakrishnan M, Balagurunathan R. Potential bioactive compound from marine actinomycetes against biofouling bacteria. J Adv Biotechnol. 2011;10:22–26.
- El-Sersy NA, Abdelwahab AE, Abouelkhir, SS, et al. Antibacterial and anticancer activity of ϵ-poly-L-lysine (ϵ-PL) produced by a marine Bacillus subtilis sp. J Basic Microbiol. 2012;52:1–10.
- El-Hawash SA, Abdel Wahab AE, El-Demellawy MA. Cyanoacetic acid hydrazones of 3-(and 4-)acetylpyridine and some derived ring systems as potential antitumor and anti-HCV agents. Arch Pharm. 2006;339(1):14–23.
- El-Awady MK, Ismail SM, El-Sagheer M, et al. Assay for hepatitis C virus in peripheral blood mononuclear cells enhances sensitivity of diagnosis and monitoring of HCV associated hepatitis. Clin Chim Acta. 1999;283:1–14.
- Ghareeb DA, Abd El-Wahab AE, Sarhan EEM, et al. Biological assessment of Berberis vulgaris and its active constituent, berberine: antibacterial, antifungal and anti-hepatitis C virus (HCV) effect. J Med Plant Res. 2013;7(21):1529–1536.
- Kaoutar B, Tarik B, Driss M, et al. Antibacterial activities of the crude ethanol extracts of medicinal plants against Listeria monocytogenes and some other pathogenic strains. Afr J Biotechnol. 2010;9(27):4251–4258.
- Patil R, Jeyasekaran G, Shanmugam SA, et al. Control of bacterial pathogens, associated with fish diseases, by antagonistic marine actinomycetes isolated from marine sediments. Indian J Mar Sci. 2001;30(4):264–267.
- Patel JD, Parmar M, Patel P, et al. Dynamism of antimicrobial activity of actinomycetes a case study from undisturbed microbial niche. Adv Microbiol. 2014;4:324–334.
- Sajid I, Shaaban KA, Hasnain S. Identification, isolation and optimization of antifungal metabolites from the Streptomyces malachitofuscus ctf9. Braz J Microbiol. 2011;2:592–604.
- El-Sharouny EE, El-Toukhy NMK, El-Sersy NA, et al. Optimization and purification of mannanase produced by an alkaliphilic-thermotolerant Bacillus cereus N1 isolated from Bani Salama Lake in Wadi El-Natron. Biotechnol Biotechnol Equip. 2015;29(2):315–323.
- Xiong C, Jinhua W, Dongsheng L. Optimization of solid-state medium for the production of inulinase by Kluyveromyces 5120 using response surface methodology. Biochem Eng J. 2007;34:179–184.
- Youssef GA, Berekaa MM. Improved production of Endoglucanase enzyme by Aspergillus terreus; application of Plackett–Burman design for optimization of process parameters. Biotechnology. 2009;8(2):212–219.
- Wefky SH, Abou-elela, GM, El-Bestawy E. Optimization of fermentation conditions for bioactive compounds production by marine bacterium Enterococcus faecium. J Appl Sci Res. 2009;5(10):1445–1454.
- El-Sersy NA, Abou-elela GM. Antagonistic effect of marine Nocardia brasiliensis against the fish pathogen Vibrio damsela: application of Plackett-Burman experimental design to evaluate factors affecting the production of the antibacterial agent. Int J Oceans Oceanogr. 2006;1(1):141–150.
- Watnick P, Kolter R. Biofilm, city of microbes. J Bacteriol. 2000;182(10):2675–2679.
- Abd-Elnaby HM, Abou-Elela GM, El-Sersy NA. Cadmium resisting bacteria in Alexandria Eastern Harbor (Egypt) and optimization of cadmium bioaccumulation by Vibrio harveyi. Afr J Biotechnol. 2011;10(17):3412–3423.
- Abou-elela GM, Nermeen A, Wefky SH. Statistical optimization of cold adapted á-amylase production by free and immobilized cells of Nocardiopsis aegyptia. J Appl Sci Res. 2009;5(3):286–292.
- Mahmoud DAR, Helmy WA. Potential application of immobilization technology in enzyme and biomass production. J Appl Sci Res. 2009;5(12):2466–2476.
- Faulker DJ. Marine natural products. Nat Prod Rep. 2002;19:1–48.
- Hu Y, Chen J, Hu G, et al. Statistical research on the bioactivity of new marine natural products discovered during the 28 years from 1985 to 2012. Mar Drugs. 2015;13:202–221.
- Bavya M, Mohanapria P, Pazhanimurugan R, et al. Potential bioactive compound from marine actinomycetes against biofouling bacteria. Indian J Geomar Sci. 2011;40(4):578–582.
- Vijayabharathi R, Bruheim P, Andreassen T, et al. Assessment of resistomycin, as an anticancer compound isolated and characterized from Streptomyces aurantiacus AAA5. J Microbiol. 2011;49(6):920–926.
- Yip WK, Cheenpracha S, Chang LC, et al. Anti-proliferative and anti-invasive properties of a purified fraction from Streptomyces sp. H7372. Int J Oncol. 2010;37(5):1229–1241.
- Al-Wathnani H, Ismet A, Tahmaz RR, et al. Bioactivity of natural compounds isolated from cyano-bacteria and green algae against human pathogenic bacteria and yeast. J Med Plants Res. 2012;6(18):3425–3433.
- Mishra US, Mishra A, Kumari R, et al. Antibacterial activity of ethanol extract of Andrographis paniculata. Indian J Pharm Sci. 2009;71(4):436–438.
- Kumar PP, Kumaravel S, Lalitha C. Screening of antioxidant activity, total phenolics and GC-MS study of vitexnegundo. Afr J Biochem Res. 2010;4(7):191–195.
- Jaina SC, Boskey P, Renuka J. Antimicrobial, free radical scavenging activities and chemical composition of Peltophorum pterocarpum Baker ex K. Heyne stem extract. Der Pharma Chemica. 2012;4(5):2073–2079.
- Soni MG, Taylor SL, Greenberg NA, et al. Evaluation of the health aspects of methyl paraben: a review of the published literature. Food Chem Toxicol. 2002;40:1335–1373.