ABSTRACT
Haploid, diploid and tetraploid shoots of Echinacea purpurea L. sharing the same genome were cultured in medium and their rooting response to the composition of the culture medium was investigated. It was found that in medium without growth regulators, haploid shoots could initiate roots quite efficiently with the shortest time required for the emergence of roots and with the highest rooting rate; the response of the diploids was similar to that of the haploids and largely different from that of the tetraploids. The tetraploids obviously required longer time for the initiation of roots and had the lowest rooting rate. Supplementing the medium with 0.05 and 0.15 mg/L naphthaleneacetic acid or 0.1 and 0.3 mg/L indole-3-butyric acid (IBA) had little positive effect on the rooting of diploid shoots and, in some cases, had even negative effect on the rooting of haploid shoots, but enhanced effectively the rooting of the tetraploid shoots. By supplementing the medium with 0.3 mg/L IBA, the time required for the emergence of roots from the tetraploid shoots was shortened and the rooting rate was increased largely. As a result, healthy tetraploid plantlets with fully developed root system could be efficiently propagated.
Introduction
Purple coneflower (Echinacea purpurea L.) is one of the most important herbs with evident immunoregulatory effects [Citation1] and is now cultivated widely in many countries, as well as in the North American area, from where the plant originates. Only diploid purple coneflower plants used to be found in nature,[Citation2] but now haploid plants have been created by the method of in vitro anther culture,[Citation3] and also tetraploids by the methods of in vitro treatment of diploid petiole explants [Citation4] and direct treatment of the seeds [Citation5] with colchicine. Polyploids, such as triploids and tetraploids, with more than two sets of chromosomes, are usually superior to diploids, with respect to genetic adaptability and tolerance to environmental stress.[Citation6] On the basis of the obtained experimental results on colchicine induced autotetraploid Salvia miltiorrhiza Bge plants, Gao et al. [Citation7] suggested that the functional compounds of the medicinal plants are accumulated in the vegetative parts. Polyploids are more valuable because they may exhibit increased biomass or content of the effective compounds.[Citation4,Citation8–10] The main functional compound of the purple coneflower is chicoric acid, which is accumulated in the roots of the plant. It has recently been confirmed that the purple coneflower's tetraploid plants contain more secondary metabolites than the diploid plants.[Citation5,Citation11] Another merit of polyploid over diploid purple coneflower plant is the observation that the roots of the tetraploid purple coneflower plants are obviously thicker and, therefore, the harvesting of the roots is easier.[Citation4] The purposes of the present study were to establish a culture method for the efficient rooting of tetraploid shoots and to clarify the influence of gene dosage on the rooting of shoots.
Materials and methods
Materials
A haploid clone was obtained by in vitro culturing of anthers, identified by observation of the chromosome number of the root tip cells under a microscope.[Citation3] Diploid and tetraploid clones were developed from the haploid clone by inducing of adventitious bud regeneration by colchicine (China Shenggong Co., Ltd) treated haploid petiole explants (120 mg/L colchicine for 24 d in vitro).[Citation4] These clones were used as experimental materials for the comparison of their culture response of shoots' rooting to various culture conditions. Because all these clones were of the same origin, they shared the same genome and were different only in their gene dosage.
Methods
Shoots were proliferated by repeatedly inducing axillary bud growth on medium containing benzyl adenine by the methods described before.[Citation12] Haploid, diploid and tetraploid shoots with two small leaves were isolated from the mother tissues and inoculated into agar-gelled hormone-free Murashige and Skoog media or MS media containing various concentrations of naphthaleneacetic acid (NAA) or indole-3-butyric acid (IBA); five shoots were inoculated in one culture bottle, each of which was filled with 35 mL medium. All cultures were kept in a culture room with a temperature range of 25–27 °C and under fluorescent light (about 40 μmol/(m2 s)) with a 12-h photoperiod.
Several parameters were set for evaluating the culture results. When roots (at least 0.5 mm long) were observed for the first shoot in any culture bottle, it was recorded as ‘earliest root emergence (d)’; when root initiation became evident for the last shoot in any culture bottle, it was recorded as ‘latest root emergence (d).’ ‘Average root emergence (d)’ was calculated by adding the data of the ‘earliest root emergence (d)’ with the data of the ‘latest root emergence (d)’ and then divided by 2; ‘primary root number per shoot’ includes all roots that were at least 1.0 mm long; ‘total primary root length (mm)’ was obtained as a sum of the lengths of all roots that were at least 1.0 mm long; ‘average primary root thickness (mm)’ was calculated by measuring all the primary root thicknesses at their bases under a microscope, adding the data together and dividing the sum by the number of the primary roots; ‘secondary root number per shoot’ includes the number of all roots developed from the primary ones and at least 1.0 mm long; ‘rooting rate (%)’ was calculated by dividing the number of shoots that developed roots with the total number of shoots in cultures, when the number of shoots that initiated roots seemed to have reached their maximum; ‘fresh weight per plant (mg)’ means not just the roots but the whole plant and was determined at the end of the treatment; ‘duration of the experiment (d)’ is the number of days that allowed the treatment to reach best results.
Statistical analyses
All of the data were analysed statistically using the Student Newman–Keuls means separation test (SAS software, SAS Institute, Cary, NC 1995). Significant differences among means were determined using Duncan's multiple range tests. P ≤ 0.05 was considered significant.
Results and discussion
Rooting of shoots in hormone-free medium
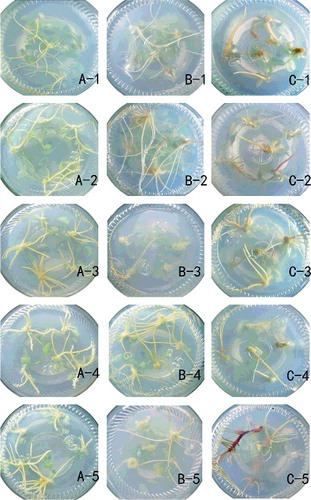
Shoots of different gene dosages were inoculated in hormone-free medium and their rooting culture response was recorded in . Haploid shoots initiated roots much faster than the diploid and tetraploid ones, with tetraploid shoots requiring much longer time for root initiation. Diploid shoots had the largest number of primary roots and the biggest total primary root length. However, the differences between the diploids and haploids were much smaller than the differences between the haploids and tetraploids. On the other hand, tetraploid shoots had thicker roots, lower rooting rate and less fresh weight than haploids and diploids. It could be also seen from A-1), 1(B-1), 1(C-1) that the rooting response was similar between haploids and diploids and was largely different between diploids and tetraploids.
Table 1. Rooting of shoots with different gene dosages in a hormone-free medium.
Figure 1. Rooting of shoots with different gene dosages in various media. Haploid (A); diploid (B); tetraploid (C); hormone-free (1); 0.05 mg/L NAA (2); 0.15 mg/L NAA (3); 0.1 mg/L IBA (4); 0.3 mg/L IBA (5).
The rooting of in vitro propagated shoots is a necessary step for the production of intact plantlets, because only intact plantlets can be cultivated in the field. There have been many reports on shoot bud regeneration [Citation13–15] and micropropagation of diploid purple coneflower,[Citation16,Citation17] but in all these studies, rooting of the shoots did not seem to be a problem and has not been investigated in detail. Although there are quite a few merits of tetraploid over the diploid purple coneflower plants,[Citation5,Citation11] tetraploid plants may encounter low seed setting rate or completely lose the seed setting ability. In these cases, an in vitro culture technique would become a necessary mean for micropropagation of tetraploid plantlets, in order to meet the demand of large area cultivation. The present study revealed that the rooting of the tetraploid purple coneflower was more difficult than the haploid and diploid ones.
Rooting of shoots in medium containing NAA
Shoots of different gene dosages were inoculated in medium containing NAA and their rooting culture response is recorded in . In comparison with the data shown in , it became clear that NAA supplementation promoted the rooting of the tetraploid shoots; the average root emergence time was reduced from 46.2 to 38.2 d with 0.05 mg/L NAA and to 30.5 d with 0.15 mg/L NAA. NAA also increased largely the primary and secondary root numbers, total primary root length, fresh weight per plant and especially the rooting rate of the tetraploids (C-1)–(C-3)). In comparison with the evident positive effect of NAA on rooting of the tetraploids, the effects of NAA on the rooting of haploids and diploids were weak and, in some cases, such as in the root emergence times, the effects were even negative (A-2), (A-3), (B-2), (B-3)).
Table 2. Rooting of shoots with different gene dosages in medium containing NAA.
In previous experiments, we have observed different responses to the in vitro culture conditions for the regeneration of adventitious buds and for the multiplication of axillary buds among explants sharing the same genome but with different gene dosages.[Citation18,Citation19] The present studies, in addition, revealed the difference in culture response for rooting; enlarged gene dosage required higher concentration of NAA for earlier and better root initiation.
Rooting of shoots in medium containing IBA
Shoots with different gene dosages were inoculated in medium containing IBA and their rooting culture response was recorded in . The result of the IBA supplementation was quite similar to the NAA supplementation, with only one clear difference – IBA induced much more secondary roots. In C-2)–(C-5), it is clearly seen that C-5) presented the best medium composition for root induction of the tetraploid shoots. Tetraploid plantlets with healthy roots taken from the bottle shown in C-5) are presented in
Table 3. Rooting of shoots with different gene dosages in medium containing IBA.
NAA and IBA are two of the most frequently used auxins for enhancing adventitious root initiation in many plant species. In the present experiments, different effects of these two types of auxin were observed. For example, IBA induced more secondary roots than NAA at all tested concentrations. Healthy tetraploid plantlets with well-developed roots could be efficiently produced by culturing shoots in medium supplemented with 0.3 mg/L IBA.
Conclusions
Although there have been several reports on shoot bud regeneration and micropropagation in purple coneflower, all these studies were exclusively conducted in diploid plants and the rooting of the shoot buds has been neglected in most of the cases. The present study showed that the development and growth of roots among haploid, diploid and tetraploid shoots were different, especially in the time of root initiation, rooting rate and culture duration required for the shoots to become intact plantlets suitable for transplantation. Since tetraploid purple coneflower can yield more biomass and accumulate higher content of functional compounds, cultivation of tetraploid plants seems a better choice over the conventional practice of cultivating diploid ones. The methods established in the present study highly improved the rooting efficiency of the tetraploid shoots and would contribute to the realization of a large-scale cultivation of tetraploid purple coneflower.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Barnes J, Anderson LA, Gibbons S, et al. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): a review of their chemistry, pharmacology and clinical properties. J Pharm Pharmacol. 2005;57:929–954.
- McGregor RL. The taxonomy of the genus Echinacea (Compositae). Univ Kansas Sci Bull. 1968;11(8):113–142.
- Zhao FC, Nilanthi D, Yang YS, et al. Another culture and haploid plant regeneration in purple cone flower (Echinacea purpurae L.). Plant Cell Tissue Organ Cult. 2006;86:55–62.
- Nilanthi D, Chen XL, Zhao FC, et al. Induction of tetraploids from petiole explants through colchicine treatments in Echinacea purpurea L. J Biomed Biotechnol. 2009;2009:343–485.
- Abdoli M, Moieni A, Badi HN. Morphological, physiological, cytological and phytochemical studies in diploid and colchicine-induced tetraploid plants of Echinacea purpurea (L.). Acta Physiol Plant. 2013;35:2075–2083.
- Lewis WH, editor. Ploidy: biological relevance. New York (NY): Plenum Press; 1980.
- Gao SL, Cai DN, Xu DR. Autotetraploid plants from colchicines-treated bud culture of Salvia miltiorrhiza Bge. Plant Cell Tissue Organ Cult. 1996;47:73–77.
- Comai L. The advantages and disadvantages of being polyploid. Nat Rev. 2005;6:836–846.
- Majdi M, Karimzadeh G, Malboobi MA, et al. Induction of tetraploidy to feverfew (Tanacetum parthenium Schulz-Bip.): morphological, physiological, cytological, and phytochemical changes. HortScience. 2010;45:16–21.
- Lavania UC, Srivastava, S, Lavania S, et al. Autopolyploidy differentially influences body size in plants, but facilitates enhanced accumulation of secondary metabolites, causing increased cytosine methylation. Plant J. 2012;71:539–549.
- Xu CG, Tang TX, Chen R, et al. A comparative study of bioactive secondary metabolite production in diploid and tetraploid Echinacea Purpurea (L.) Moench. Plant Cell Tissue Organ Cult. 2014;116:323–332.
- Chen R, Chen XL, Li QL, et al. Micropropagation by repeatedly inducing axillary bud formation of different gene dosage purple coneflower plants. Proceedings of the International Conference on Biomedical Engineering and Biotechnology; 2012 May 28–30; Macau. p. 1056–1059.
- Choffe KL, Victor JMR, Murch SJ, et al. In vitro regeneration of Echinacea L.: direct somatic embryogenesis and indirect shoot organogenesis in petiole culture. In Vitro Cell Dev Biol Plant. 2000;36:30–36.
- Lakshmanan P, Danesh M, Taji A. Production of four commercially cultivated Echinacea species by different methods of in vitro regeneration. J Hortic Sci Biotechnol. 2002;77:158–163.
- Mechanda SM, Baum BR. Direct shoot regeneration from leaf segments of mature plants of Echinacea purpurea (L.) Moench. In Vitro Cell Dev Biol Plant. 2003;39:505–509.
- Harbage JF. Micropropagation of Echinacea angustifolia, E. pallida and E. purpurea from stem and seed explants. Hortscience. 2001;36:360–364.
- Nilanthi D, Chen XL, Zhao FC, et al. An efficient in vitro propagation culture protocol for purple coneflower (Echinacea purpurea L.). Proceedings of the 4th International Conference on Bioinformatics and Biomedical Engineering; 2009 June 11–16; Chengdu, China.
- Nilanthi D, Chen XL, Zhao FC, et al. Influence of gene dose on in vitro culture responses of purple coneflower (Echinacea purpurea L.). Proceedings of the 3rd International Conference on Bioinformatics and Biomedical Engineering; 2009 June 11–16; Beijing, China.
- Li QL, Chen R, Chen XL, et al. Estimation of the cloning potential in six selected genotypes of purple coneflower (Echinacea purpurea L.). Biotechnol Biotechnol Equip. 2013;27:3911–3917.

