ABSTRACT
Two polyphosphoesters containing anthracene-derived aminophosphonate and hydrophilic H-phosphonate repeating units, poly[oxyethylene(aminophosphonate-co-H-phosphonate)]s (1 and 2), were tested for the in vitro antitumour activity on cell cultures derived from ascitic form of Ehrlich mammary adenocarcinoma by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)-dye reduction assay. The in vitro safety testing of the copolymers was performed by BALB/c 3T3 neutral red uptake assay. A study on their uptake and subcellular distribution in non-tumourigenic and tumour cells was performed by means of fluorescence microscopy. Both copolymers showed significant antitumour activity towards Ehrlich ascites carcinoma (EAC) cells. However, the in vitro safety testing revealed significant toxicity of polymer 2 to BALB/c 3T3 mouse embryo cells. In contrast, polymer 1 showed complete absence of cytotoxicity to BALB/c 3T3 cells. The fluorescent studies showed that the substances were diffusely distributed in the cytoplasm in both cell culture systems. As opposed to BALB/c 3T3 cells, in EAC cells, intense fluorescent signal was observed in the nuclei and in the perinuclear region. The tested polyphosphoesters are expected to act under physiological conditions as prodrugs of aminophosphonates.
Introduction
Neoplastic diseases are one of the major problems of modern biomedical science because of their high and continuously increasing incidence and mortality. Systemic chemotherapy is the main approach used in cancer treatment. However, some drawbacks of chemotherapy, such as the toxicity to healthy tissues and the insufficient concentration of the anticancer agent at the tumour site, define the need for development and implementation of new therapeuticals and treatment methods.[Citation1] The search for novel strategies for targeted delivery of drugs to the tumour cells is a major trend of contemporary anticancer research. The recent advances in the development of new drug delivery systems for controlled drug release in tumour tissues with reduced side effects are quite promising. In this field, the use of biodegradable polymer drugs has attracted the most attention.[Citation2,Citation3]
Polyphosphoesters comprise a group of biodegradable and biocompatible phosphorus-containing polymers, which have found important biomedical applications, including as drug and gene delivery systems.[Citation4,Citation5] Under physiological conditions, these polymers are degraded into nontoxic products through hydrolysis and enzymatic cleavage of their phosphoester bonds.[Citation4,Citation6] The poly(alkylene H-phosphonate)s are one of the most promising drug carriers among this class of polymers.[Citation7] The immobilization of aminophosphonate moieties bearing a DNA-intercalating anthracene ring to these polymer carriers attracts particular attention in the development of new antitumour therapeuticals with improved properties. The versatile biological activities (including antitumour, antibacterial and antiviral) of aminophosphonic acid derivatives, are commonly known.[Citation7–11] Anthracene-based compounds have been used as cytostatic drugs, inhibitors of tubulin polymerization and some of them have found a bioanalytical application as fluorescent probes.[Citation12–17]
The synthesis of the poly[oxyethylene(aminophosphonate-co-H-phosphonate)]s 1 and 2 and their in vitro antitumour activity testing on a panel of seven human epithelial cancer cell lines have been previously described.[Citation18] Both compounds showed high antiproliferative activity against human cell lines from ductal carcinoma of the breast (MCF-7 and MDA-MB-231), with low and high metastatic potential, respectively.[Citation18] In this study, in vitro cultures of Ehrlich ascites carcinoma (EAC) cells were used to confirm the antineoplastic effect of these polymers on mammary adenocarcinoma cells. The EAC is a transplantable, poorly differentiated, rapidly growing tumour with very aggressive behaviour, derived from spontaneous mouse mammary adenocarcinoma and hence growing in solid and ascitic forms. EAC closely resembles metastatic human breast tumours and is used worldwide as an experimental model to study the antitumour effects of natural and synthetic chemical substances, showing precise and reproducible results.[Citation19] In addition, the results from the in vitro safety testing of the poly[oxyethylene(aminophosphonate-co-H-phosphonate)]s are also presented. The fluorescent properties of the anthracene moieties were used for studies on the subcellular distribution of the polymers in tumour and non-cancer cell culture systems.
Materials and methods
Tested substances
Poly[oxyethylene(aminophosphonate-co-H-phosphonate)]s (1 and 2) have been synthesized from poly(oxyethylene H-phosphonate)s and the Schiff base, 9-anthrylidene-p-toluidine.[Citation18] Poly(oxyethylene H-phosphonate)s were synthesized via a polytransesterification reaction of dimethyl H-phosphonate and poly(ethylene glycol)s (PEGs) with an average molecular weight of 600 Da (PEG 600) and 200 Da (PEG 200). Copolymers 1 and 2 were prepared from poly(oxyethylene H-phosphonate)s obtained on the basis of PEG 600 and PEG 200, respectively. The content of the aminophosphonate units in the studied copolymers is 43% and 13% degree of polymerization for polymers 1 and 2, respectively.[Citation18]
Cells and culture conditions
The Ehrlich mouse mammary adenocarcinoma cells and BALB/c 3T3 (clone 31) mouse embryo fibroblasts were used for the in vitro antitumour activity and the in vitro safety assessment, respectively. The cells were grown in monolayers in Dulbecco's modified Eagle's medium (DMEM) (Sigma), supplemented with 10% (v/v) fetal bovine serum (FBS), 100 U/mL penicillin and 100 μg/mL streptomycinin (Sigma) in 75 cm2 tissue culture flasks (Orange Scientific). Cultures were maintained in a humidified atmosphere with 5% CO2 at 37.5 °C.
In vitro antitumour activity
To test the in vitro antitumour activity, the viability (%) of Ehrlich ascites tumour cells was studied by using the standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)-dye reduction assay, described by Mosmann.[Citation20] Cultures treated with the referent antineoplastic drug Oxaliplatin and vehicle-treated cultures were used as positive and negative controls, respectively. The trypsinized tumour cells were adjusted to a density of 1 × 105 cells/mL culture medium and plated (100 μL/well) in 96-well flat-bottomed microplates (Orange Scientific). The cells were allowed to adhere for 24 h before treatment with test compounds dissolved in dimethyl sulfoxide (DMSO) (Sigma) and further diluted in culture medium to reach the desired test concentrations. The DMSO concentration never exceeded 0.5% (v/v). A concentration ranging from 1 to 0.0681 mg/mL (six wells per concentration) was applied for 24 h. Oxaliplatin was applied in a concentration range from 0.0100 to 0.0001 mg/mL with a dilution factor of 2. The MTT solution (5 mg/mL in phosphate-buffered saline (PBS)) was added (100 μL/well), and plates were incubated for 3 h at 37.5 °C in a humidified atmosphere and 5% CO2. The MTT-formazan crystals were dissolved by adding 100 μL/well of an absolute ethanol / DMSO (1:1 v/v) solution and the absorption was registered using a microplate reader (TECAN, Sunrise TM, Groedig/Salzburg, Austria) at 580 nm. All experiments were performed in triplicate.
Safety assessment (BALB/c 3T3 neutral red uptake assay)
The cytotoxicity testing was performed as described by Borenfreund and Puerner [Citation21] and the latest modification of the BALB/c 3T3 neutral red uptake assay (3T3 NRU test) [Citation22] for the cytotoxicity/phototoxicity testing. Cells were plated at a density of 1 × 104 cells in 100 μL culture medium DMEM in each well of 96-well flat-bottomed microplates and allowed to adhere for 24 h before treatment with the test compounds, dissolved in DMSO and further diluted in the culture medium. A wide concentration range was applied (from 1 to 0.0681 mg/mL, with a dilution factor of 6√10 = 1.47) for 24 h. Sodium dodecyl sulphate was used as a positive control substance. After treatment with neutral red-containing medium DMEM, washing and application of the ethanol/acetic acid desorbing solution (50% ethanol, 49% water and 1% acetic acid), the absorption was measured with microplate reader (TECAN, Sunrise TM, Groedig/Salzburg, Austria) at 540 nm.
Fluorescent studies
EAC and BALB/c 3T3 cells were seeded on sterile 12 ring diagnostic slides and treated with the poly[oxyethylene(aminophosphonate-co-H-phosphonate)]s in concentrations lower than the mean IC50 values (concentrations inducing 50% inhibition of the cell growth) for 24 h. After fixation in cold acetone (20 °C) the slides were air-dried, covered and examined with a fluorescent microscope Leica 5000 DM.
Data analysis
The relative cell cytotoxicity/viability, expressed as a percentage of the untreated negative controls, was calculated for each concentration. The statistical analysis included one-way analysis of variance followed by Bonferroni's post hoc test. p < 0.05 was accepted as the lowest level of statistical significance. The IC50 values were calculated using non-linear regression analysis (GraphPad Prizm5 Software). The values in the figures are presented as mean ± standard deviation (SD).
Results and discussion
In vitro antitumour activity
The poly[oxyethylene(aminophosphonate-co-H-phosphonate)]s showed the concentration-dependent antiproliferative effect on the EAC cells after 24 h of exposure ().
Figure 1. Antitumour activity of poly[oxyethylene(aminophosphonate-co-H-phosphonate)]s 1 (A) and 2 (B) and the positive control substance Oxaliplatin (C) on Ehrlich ascites carcinoma cells.
![Figure 1. Antitumour activity of poly[oxyethylene(aminophosphonate-co-H-phosphonate)]s 1 (A) and 2 (B) and the positive control substance Oxaliplatin (C) on Ehrlich ascites carcinoma cells.](/cms/asset/aa0d41a5-07d9-41b9-b5f7-dc3967a24914/tbeq_a_1088402_f0001_b.gif)
Copolymer 1 exhibited lower antiproliferative activity than copolymer 2. The mean IC50 values from three consecutive experiments were 0.248 mg/mL ± 0.020 mg/mL and 0.138 mg/mL ± 0.016 mg/mL for copolymer 1 and copolymer 2, respectively. Although the tested copolymers were found to be cytotoxic towards the EAC cells, both polymers were found to have significantly lower antiproliferative activity when compared to the positive control substance.
In vitro safety testing
The in vitro safety testing of copolymer 1 revealed a complete absence of cytotoxicity to BALB/c 3T3 cell line. In contrast, copolymer 2 induced significant reduction of viability of BALB/c 3T3 cells after 24 h of treatment in a concentration ranging from 1 to 0.1 mg/mL ().
The mean IC50 value from three consecutive experiments was 0.179 ± 0.018 mg/mL. The toxic effect of copolymer 2 on mouse embryo fibroblasts in the concentration range 0.318–0.068 mg/mL was lower than the cytotoxicity of the positive control substance – sodium dodecyl sulphate (data not shown).
Fluorescent studies
The tested polyphosphoesters containing anthracene-derived aminophosphonate units showed similar patterns of subcellular distribution. The fluorescent signal of polymers 1 and 2 was observed mainly in the cytoplasm of BALB/c 3T3 (clone 31) non-tumorigenic mouse embryo cells. A representative image for polymer 2 is shown in (a)). In contrast, the EAC cells revealed intensive fluorescence in the nucleus and in the perinuclear region (b)). Chromatin condensation and chromatin margination in the nuclei of the treated carcinoma cells were the main morphologic alterations which are indicators of the nuclear phase of the apoptotic process.
Figure 3. Fluorescence microscopy of subcellular distribution of copolymer 2, applied at a concentration of 0.1 mg/mL, in (A) BALB/c 3T3 cells and (B) Ehrlich ascites carcinoma cells, after 24 h of treatment.
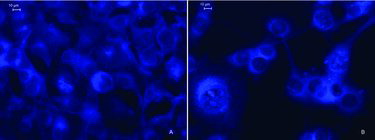
The results from the fluorescent microscopic morphological study correlated well with the data of the in vitro antitumour activity of the tested polymers and revealed apoptotic alterations in the EAC cells. The latter observation implies that the aminophosphonate moiety bearing a DNA-intercalating anthracene ring is the main pharmacophoric fragment of the studied poly[oxyethylene(aminophosphonate-co-H-phosphonate)]s and points to the mechanism of tumour cell death. The analysis of the results from our initial study indicated that the tested compounds bearing anthracene-derived aminophosphonate units 1 and 2 were active cytotoxic agents in the EAC cells. Polymer 2 was more active than polymer 1 in its cytotoxic activity after binding to the DNA of the mouse carcinoma cells, but, unlike copolymer 1, was toxic to non-tumorigenic BALB/c 3T3 mouse embryo cells. Copolymer 1 showed no signs of toxicity to BALB/c 3T3 mouse fibroblasts, which is probably a consequence of its inability to pass through the nuclear membrane. In tumour cells, however, the anthracene-containing polymer 1 exerted toxic activity, as shown from the results of the MTT-dye reduction assay.
The results from this and our previous study [Citation18] reveal similarity of the cytotoxic activities of copolymers 1 and 2 in human breast cancer cells and animal mammary carcinoma cells, and underline the significance of the EAC model for studies on the antitumour activity of different compounds. In addition, similar subcellular distribution pointing to a similar mode of biological activity has been established. Therefore, both of the tested polymers, belonging to the low-toxicity group of DNA intercalators, appear promising for the development of active antineoplastic agents for chemotherapy of mammary gland malignancies.
Conclusions
The polyphosphoesters containing anthracene-derived aminophosphonate units induced concentration-dependent antiproliferative effects on the EAC cells. Copolymer 2 showed the higher antitumour activity. However, the in vitro safety testing revealed significant toxicity of this polymer to non-tumorigenic BALB/c 3T3 mouse embryo cells. In contrast, polymer 1 showed a complete absence of cytotoxicity to BALB/c 3T3 cells. The results obtained imply that these copolymers could be considered as promising leads for further development of active agents in chemotherapy of malignant breast disease. In addition, the fluorescent properties of the anthracene ring allow adequate and precise studies on the cellular uptake and intracellular distribution of these substances in the malignant and normal cells.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- De Souza R, Zahedi P, Allen C, et al. Polymeric drug delivery systems for localized cancer chemoterapy. Drug Deliv. 2010;17:365–375.
- Taghizadeh B, Taranejoo S, Monemian S, et al. Classification of stimuli-responsive polymers as anticancer drug delivery systems. Drug Deliv. 2014;22:145–155.
- Troev KD. Polyphosphoesters chemistry and application: poly(alkylene H-phosphonate)s. Amsterdam: Elsevier; 2012. p. 1–122.
- Luten J, Nostrum C, De Smedt S, et al. Biodegradable polymers as non-viral carriers for plasmid DNA delivery. J Control Release. 2008;126:97–110.
- Zhao Z., Wang J, Mao H, et al. Polyphosphoesters in drug and gene delivery. Adv Drug Deliv Rev. 2003;55:483–499.
- Steinbach T, Alexandrino E, Wurm F. Unsaturated poly(phosphoester)s via ring-opening metathesis polymerization. Polym Chem. 2013;4:3800–3806.
- Bloemink M, Diederen J, Dorenbos J, et al. Calcium ions do accelerate the DNA binding of new antitumour-active platinum aminophosphonate complexes. Eur J Inorg Chem. 1999;10:1655–1657.
- Kafarski P, Lejczak B. The biological activity of phosphono - and phosphinopeptides. In: Kukhar VP, Hudson HR, editors. Aminophosphonic and aminophosphinic acids: chemistry and biological activity. Chichester: Wiley; 2000. p. 407–435.
- Kraicheva I, Tsacheva I, Vodenicharova E, et al. Synthesis, antiproliferative activity and genotoxicity of novel anthracene-containing aminophosphonates and a new anthracene-derived Schiff base. Bioorg Med Chem. 2012;20:117–24.
- Sonar S, Sadaphal S, Labade V, et al. An efficient synthesis and antibacterial screening of novel oxazepine α-aminophosphonates by ultrasound approach. Phosphorus Sulfur. 2010;185:65–73.
- Zhou J, Fan H, Song B, et al. Synthesis and antiviral activities of α-aminophosphonate derivatives containing a pyridazine moiety. Phosphorus Sulfur. 2010;168:81–87.
- Bowden G, Garcia D, Peng Y, et al. Molecular pharmacology of the anthracycline drug 9,10-anthracenedicarboxaldehyde bis[(4,5-dihydro-1 H imidazol-2-yl)hydrazone] dihydrochloride. Cancer Res. 1982;42:2660–2665.
- Bowden G, Roberts R, Alberts D, et al. Comparative molecular pharmacology in leukemic L1210 cells of the anthracene anticancer drugs mitoxantrone and bisantrene. Cancer Res. 1985;45:4915–4920.
- Herrmann U, Tűmmler B, Maass G, et al. Anthracenoyl crown ethers and cryptands as fluorescent probes for solid-phase transitions of phosphatidylcholines: synthesis and phospholipid membrane studies. Biochemistry. 1984;23:4059–4067.
- Martinez R, Chacon-Garcia L. The search of DNA-intercalators as antitumoural drugs: what it worked and what did not work. Curr Med Chem. 2005;12:127–151.
- Nickel H, Schmidt P, Böhm K, et al. Synthesis, antiproliferative activity and inhibition of tubulin polymerization by 1,5- and 1,8-disubstituted 10H-anthracen-9-ones bearing a 10-benzylidene or 10-(2-oxo-2-phenylethylidene) moiety. Eur J Med Chem. 2010;45:3420–3438.
- Prinz H, Ishii Y, Hirano T, et al. Novel benzylidene-9(10H)-anthracenones as highly active antimicrotubule agents. Synthesis, antiproliferative activity, and inhibition of tubulin polymerization. J Med Chem. 2003;46:3382–3394.
- Kraicheva I, Vodenicharova E, Shenkov S, et al. Synthesis, characterization, antitumour activity and safety testing of novel polyphosphoesters bearing anthracene-derived aminophosphonate units. Bioorg Med Chem. 2014;22:874–882.
- Ozaslan M, Karagoz1 I, Kilic I, et al. Ehrlich ascites carcinoma. Afr J Biotechnol. 2011;10:2375–2378.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
- Borenfreund E, Puerner J. Toxicity determined in vitro by morphological alteration and neutral red absorption. Toxicol Lett. 1985;24:119–124.
- 3T3 Neutral Red Uptake (NRU) Phototoxicity Assay. Invittox protocol no. 78 [Internet]. [cited 2015 Jul 17]. Available from: http://ecvam-dbalm.jrc.ec.europa.eu.

![Figure 2. Cytotoxicity of poly[oxyethylene(aminophosphonate-co-H-phosphonate)] 1 (A) and 2 (B).](/cms/asset/9d5396d0-080c-48a9-a92c-c6c2f5f97f09/tbeq_a_1088402_f0002_b.gif)