ABSTRACT
We investigated Hopi/Osr27 (gypsy) and Houba/Tos5/Osr13 (copy) retrotransposon movements in 10-day-old roots and leaves of Oryza sativa cvs. Ipsala, Beser and Osmancik-97. Seeds from these three cultivars were germinated between filter papers in Petri dishes for 10 days. Three biologically independent (nonrelated) seeds were germinated for each cultivar. Then, roots and leaves grown from the same rice plant were harvested and used for genomic DNA isolation. Inter-retrotransposon amplified polymorphism–polymerase chain reaction with suitable primers was performed with each DNA template to analyze the movements of Hopi/Osr27 and Houba/Tos5/Osr13 retrotransposons. Polymorphism ratios were evaluated both among cultivars and among roots and leaves from the same cultivar. The polymorphism ratios ranged from 0% to 17% for Hopi/Osr27 and from 10% to 87% for Houba/Tos5/Osr13. The obtained results at retrotransposon and varietal levels indicated that the retrotransposon type and genotype dependence are responsible for the occurrence of different variations. Transposable elements are very important for understanding the relationship between cultivars and evolution. Our findings are expected to contribute to the understanding of spontaneous genomic insertion events and their effects on the genetic and epigenetic changes during rice development.
Introduction
Rice is the third important crop plant produced after wheat and maize. It is an important food and at the same time is used as a model organism for genetic studies (genome evolution, etc.). The diploid chromosome number of rice is 24 and this plant's genome project is one of the first to be finished. A large part of the eukaryotic genomes consists of transposable elements (TEs).[Citation1] Previous studies identified that maize has 75% retrotransposons in a 2800 Mb genome,[Citation2–4] barley has 75% retrotransposons in a 5300 Mb genome,[Citation5] cabbage has 28% retrotransposons in a 600 Mb genome [Citation6] and Vicia species have 45% retrotransposons in a 1300 Mb genome.[Citation7] Depending on the order of the genes encoded, retrotransposons are further classified into Ty1-copy and Ty3-gypsy retrotransposons. The gene order of Ty1-copy retrotransposons is PR-INT-RT-RH (protease, integrase, reverse transkriptase, RNase H, respectively) whereas that of Ty3-gypsy retrotransposons is PR-RT-INT-RH.[Citation8] Based on a unified classification system for eukaryotic TEs,[Citation1] 32,370 elements were classified into 510 distinct families, including 353 gypsy-like families (19,052 elements) and 157 copy-like families (13,318 elements), of which, approximately 95% were reported.[Citation9,Citation10] The ratio of gypsy-like to copy-like elements in soybean is 1.4:1 which is slightly lower than that in maize (1.6:1) [Citation4,Citation11] and much lower than that in rice (4.9:1).[Citation12,Citation13]
The transposition of TEs can generate genome plasticity by inducing various chromosomal mutations, allelic diversity and genome expansion.[Citation14–21] For instance, the genome size of Oryza australiensis, a wild relative of rice, was doubled within the last 3 million years (3 Myr) by aggressive proliferation of long terminal repeat-retrotransposons (LTR-RTs) belonging to three families.[Citation22] Moreover, approximately one-quarter of the rice genome is composed of LTR-RTs.[Citation6,Citation11,Citation12,Citation23–25] Due to their variation capacity between species, retrotransposons are usually studied for the detection of genetic relationships between varieties and related species, and even between different plant organs in the same plants.[Citation26–35]
TEs have been used for genetic markers because of their genome-wide distribution.[Citation36–38] One of these markers is the inter-retrotransposon amplified polymorphism (IRAP). In this method, sequences between two adjacent LTR-RTs are amplified by primers that are complementary to the 3'-end of the LTR sequence.[Citation39] The LTR sequences between adjacent retrotransposons can be arranged as (1) head-to-head, (2) tail-to-tail or (3) head-to-tail.[Citation38] If the arrangement between two identical tandem duplicate LTR-RTs is either head-to-head or tail-to-tail, a single primer can amplify the spacer. If the adjacent retrotransposons are from different lineages (which is usually the case), two different primers, derived from each LTR sequence, are needed to amplify the IRAP. Each IRAP reaction produces multiple amplicons, ranging in size from 300 to 3000 bp.[Citation40,Citation41] Hopi/Osr27 is a gypsy retrotransposon with a size of 12,892 bp, LTR sequence of 1103 bp and copy number of 1332. Moreover, Houba/Tos5/Osr13 is a copy-like retrotransposon with a length of 6437 bp, LTR size of 968 bp and copy number of 563, in the rice genome.[Citation42] The objective of this study was to compare Hopi/Osr27 and Houba/Tos5/Osr13 retrotransposons’ movements in 10-day-old roots and leaves in Oryza sativa cvs. Osmancik-97, Beser and Ipsala, which were obtained from the same seed, by using IRAP molecular marker technique to find out the possible differences in transposon-mediated polymorphisms.
Materials and methods
Inter-retrotransposon amplified polymorphism–polymerase chain reaction (IRAP-PCR) profiles of root and leaf tissues of the same plantlets were compared with each other. In addition, three plantlets of each cultivar were analyzed in terms of PCR profiles to determine whether there are any naturally occurring polymorphisms among the individuals. Furthermore, the three cultivars were also compared with each other to reveal the polymorphism rates between different cultivars.
Plant materials and DNA isolation
Seeds of three cultivars of O. sativa (Ipsala, Beser and Osmancik-97) were used to investigate the retrotransposon polymorphism between root and leaf tissues. The seeds were germinated at 25 °C in Petri dishes that contained moist filter paper. After 10 days of germination, roots and leaves of each plant were harvested individually. Genomic DNAs were isolated from three roots and three leaves of each cultivar, according to the protocol of Pervaiz et al.[Citation43] The quantity and quality of DNAs were measured by NanoDrop 2000c uv-Vis spectrophotometer (Thermo Scientific, 2000c). Before the IRAP analysis, the DNA’s concentration was equalized to 10 ng/μL.
IRAP analysis
The IRAP technique was used to investigate the retrotransposon polymorphism. For this purpose, we assigned Hopi/Osr27 and Houba/Tos5/Osr13 retrotransposons as candidates. Hopi/Osr27 sequences of O. sativa cv. japonica were obtained from National Center for Biotechnology Information (NCBI, accession number: AF537364.1) and Houba/Tos5/Osr13 sequences O. sativa cv. japonica were also obtained from NCBI (accession number: AF537365.1). The IRAP primers were designed based on the 5' and 3' LTR sequences of Hopi/Osr27 and Houba/Tos5/Osr13. The primer sequences are given in . The IRAP-PCR was performed in a total volume of 20 μL, containing 20 ng template DNA, 10 nmol forward and reverse primers designed by Integrated DNA Technologies (IDT) and SapphireAmp Fast PCR Master Mix (Takara, RR350A). Primer dimer or other contaminations were checked by using no template control (negative control). In this control, the PCR contents were the same as in IRAP-PCR, but without template (water was used instead of template). The PCR conditions were as follows: initial denaturation at 94 °C for 2.5 min, followed by 30 cycles at 94 °C for 30 s, 55 °C for 30 s, 72 °C for 3 min and the reaction was completed with a cycle of final extension at 72 °C for 7 min. The PCR products were resolved in a 8% polyacrylamide (29:1 Acrylamide:Bis) gel electrophoresis (Bio-Rad, Proean II xi Cell) at 150 V for 8 h in 1X TBE buffer (pH 8.0). A molecular weight marker (GeneRulerTM DNA Ladder Mix, SM0331, Thermo Scientific) was also loaded to determine the sizes of the PCR fragments. The gel was stained with ethidium bromide in 1X TBE buffer for 15 min. After staining, the gel was visualized on UV transilluminator, photographed and used for data analyses.
Table 1. Primer sequences used for IRAP analyses.
Data analyses
The well-resolved bands were scored as a binary value, ‘1’ for presence and ‘0’ for absence of bands. The binary matrix (1/0) was used to calculate the similarity between root and leaf tissues by Jaccard's coefficient.[Citation44] The Jaccard's similarity index was calculated using the formula: NAB/(NAB + NB + NA), where NAB is the number of bands shared by two samples, NA indicates the amplified fragments in sample A and NB represents the amplified fragments in sample B.
Results and discussion
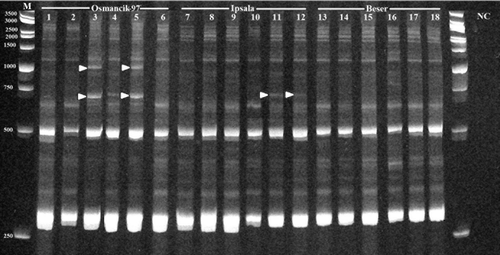
In the present study, Hopi/Osr27 and Houba/Tos5/Osr13 retrotransposon mediated polymorphism between root and leaf tissues of three O. sativa cultivars (Ipsala, Beser and Osmancik-97) was investigated. For this purpose, Hopi/Osr27 band profiles produced 10 homomorphic IRAP bands, which were observed in each sample (roots and leaves), with a variable length between 250 and 3000 bp (). In Beser cultivar, the root and leaf tissues of all three plants were homomorphic (; lanes 13–18). This result showed that there may be no transposition events of Hopi/Osr27 retrotransposon during germination. Because all individuals had common band profiles, it may be concluded that Beser cultivar does not have any naturally occurring polymorphisms in terms of this retrotransposon. IRAP-PCR analyses of Ipsala cultivar root and leaf also showed the same band profile, similar to Beser cultivar. However, the third individual (; lanes 11 and 12) of Ipsala had one polymorphic band that was not observed in the other two individuals. This result might prove that Ipsala cultivar has a natural polymorphism, with respect to Hopi/Osr27 retrotransposon profile, although there are no transposition events during or following germination. The third cultivar, Osmancik-97, had a different profile than Ipsala and Beser because it had both homomorphic and polymorphic profiles between root and leaf tissues of individuals. The first individual of Osmancik-97 was homomorphic based on its root and leaf tissues' IRAP profiles. Also, it had the same band profile as Beser's and as the first two samples of Ipsala. However, the second individual of Osmancik-97 had 8% polymorphism between root and leaf tissues (). While the root profile of the second individual was the same as Ipsala's third plant sample, the leaf profile was same as the leaf profile of the third individual of Osmancik-97. The last sample of Osmancik-97 (; lanes 5 and 6) had the highest polymorphism rate between root and leaf tissues (17%). Hopi/Osr27 is one of the gypsy type LTR-RTs that is represented with high copy number (1332 copies) in the O. sativa’s genome. This might show that Hopi/Osr27 is an active retrotransposon through the evolutionary processes.[Citation42] However, it was epigenetically silenced as other retrotransposons. Epigenetic mechanisms provide a control system to retrotransposon burst.[Citation21] There are various explanations about the somatic activities of TEs, such as stress and developmental stages.[Citation45,Citation46]
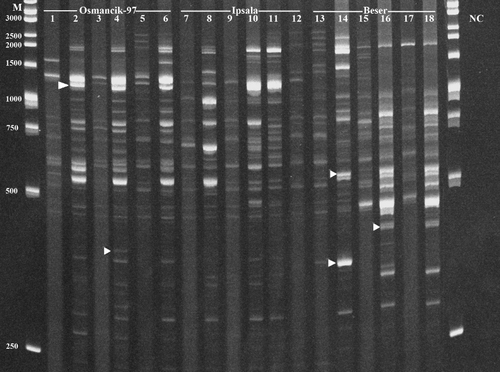
Table 2. Polymorphism ratios of Hopi/Osr27 and Houba/Tos5/Osr13.
Houba/Tos5/Osr13 retrotransposon bands showed different profiles among cultivars with length between 250 and 2500 bp (). IRAP analyses of Houba/Tos5/Osr13 retrotransposon resulted in higher polymorphism ratio (10%–87%) than Hopi/Osr27 (0%–17%) (). This showed that Houba/Tos5/Osr13 might have a more effective role than Hopi/Osr27 in tissue differentiation. However, the results from Ipsala and Beser cultivars were not consistent with the Osmancik-97 results. Polymorphism rates of different tissues in the same individuals and of different individuals in the same cultivar were variable. As opposed to Hopi/Osr27, there was a 14%–79% polymorphism rate in Beser cultivar (; lanes 13–18) and 23%–74% in Ipsala cultivar. Retrotransposons are commonly studied with different plant species and even with different plant organs in the same individual. Vukich et al. [Citation47] examined the expression differences between copy and gypsy elements in sunflower (Helianthus annuus L.) root, leaf and flower tissues. In another study, Marakli et al. [Citation28] concluded that movements of the BAGY2 retrotransposon were more stable compared to those of BARE1 in roots and leaves derived from the same barley embryo. Cakmak et al. [Citation48] reported that some SIRE1 retrotransposition events occured not only in different 10-day-old roots and leaves, but also in roots and leaves derived from the same embryo in barley. There are two common perspectives about the effects of retrotransposons' movements. Some researchers believe that the role of TEs for the germline differentiation is insignificant, whereas others think that transposition events can be beneficial or harmful for the organisms.[Citation49]
Conslusions
In our study, we compared three different rice cultivars (Osmancik-97, Beser and Ipsala) with two different rice-specific retrotransposons (Hopi/Osr27 and Houba/Tos5/Osr13). We observed differences in retrotransposon movements not only among cultivars, but also among different plant organs in the same individual. Despite the importance of retrotransposons for the genome dynamics and gene activity, our understanding of their biology is still in a primitive state. Our results with rice retrotransposons could be helpful for the understanding of the mechanisms responsible for polymorphism.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Wicker T, Sabot F, Hua-Van A, et al. A unified classification system for eukaryotic transposable elements. Nat Rev Genet. 2007;8:973–982.
- Bennetzen JL, SanMiguel P, Chen M, et al. Grass genomes. P Natl Acad Sci USA. 1998;95:1975–1978.
- Meyers BC, Tingey SV, Morgante M. Abundance, distribution, and transcriptional activity of repetitive elements in the maize genome. Genome Res. 2001;11:1660–1676.
- Baucom RS, Estill JC, Chaparro C, et al. Exceptional diversity, non-random distribution, and rapid evolution of retroelements in the B73 maize genome. Plos Genet. 2009;5:1–13.
- The International Barley Genome Sequencing Consortium. A physical, genetic and functional sequence assembly of the barley genome. Nature. 2012;491:711–717.
- Zhang X, Wessler SR. Genome-wide comparative analysis of the transposable elements in the related species Arabidopsis thaliana and Brassica oleracea. P Natl Acad Sci USA. 2004;101:5589–5594.
- Hill P, Burford D, Martin DMA, et al. Retrotransposon populations of Vicia species with varying genome size. Mol Genet Genomics. 2005;273:371–381.
- Roy NS, Choi JY, Lee SI, et al. Erratum to: marker utility of transposable elements for plant genetics, breeding, and ecology: a review. Genes Genomics. 2015;37:487.
- Jurka J, Kapitonov VV, Pavlicek A, et al. Repbase update, a database of eukaryotic repetitive elements. Cytogenet Genome Res. 2005;110:462–467.
- Du J, Grant D, Tian Z, et al. SoyTEdb: a comprehensive database of transposable elements in the soybean genome. BMC Genomics. 2010;11:113.
- Schnable PS, Ware D, Fulton RS, et al. The B73 maize genome: complexity, diversity, and dynamics. Science. 2009;326:1112–1115.
- International Rice Genome Sequencing Project. The map-based sequence of the rice genome. Nature. 2005;436:793–800.
- Tian Z, Rizzon C, Du J, et al. Do genetic recombination and gene density shape the pattern of DNA elimination in rice long terminal repeat retrotransposons? Genome Res. 2009;19:2221–2230.
- Oliver KR, Greene WK. Transposable elements: powerful facilitators of evolution. BioEssays. 2009;31:703–714.
- Fedoroff NV. The discovery of transposition. In: Fedoroff NV, editor. Plant transposons and genome dynamics in evolution. Ames (IA): Wiley-Blackwell Inc; 2013. p. 3–14.
- Fedoroff NV, Bennetzen JL. Transposon, genomic shock, and genome expansion. In: Fedoroff NV, editor. Plant transposons and genome dynamics in evolution. Ames (IA): Wiley-Blackwell Inc; 2013. p. 181–201.
- Oliver KR, McComb JA, Greene WK. Transposable elements: powerful contributors to angiosperm evolution and diversity. Genome Biol Evol. 2013;5:1886–1901.
- Lee SI, Kim NS. Transposable elements and genome size variations in plants. Genomics Inform. 2014;12:87–97.
- Wessler SR, Bureau TE, White SE. LTR-retrotransposons and MITEs: important players in the evolution of plant genomes. Curr Opin Genet Dev. 1995;5:814–821.
- Kejnovsky E, Hawkins JS, Feschotte C. Plant transposable elements: biology and evolution. In: Wendel JF, Greilhuber J, Dolezel J, Leitch IJ, editors. Plant genome diversity. Vol. 1. Wien: Springer Verlag; 2012. p. 17–34.
- Fedoroff NV. Presidential address. Transposable elements, epigenetics, and genome evolution. Science. 2012;338:758–767.
- Piegu B, Guyot R, Picault N, et al. Doubling genome size without polyploidization: dynamics of retrotransposition-driven genomic expansions in Oryza australiensis, a wild relative of rice. Genome Res. 2006;16:1262–1269.
- Beló A, Nobuta K, Venu RC, et al. Transposable element regulation in rice and Arabidopsis: diverse patterns of active expression and siRNA-mediated silencing. Trop Plant Biol. 2008;1:72–84.
- Ma J, Devos KM, Bennetzen JL. Analyses of LTR-retrotransposon structures reveal recent and rapid genomic DNA loss in rice. Genome Res. 2004;14:860–869.
- Du J, Tian Z, Hans CS, et al. Evolutionary conservation, diversity and specificity of LTR-retrotransposons in flowering plants: insights from genome-wide analysis and multi-specific comparison. Plant J. 2010;63:584–598.
- Smýkal P, Bacova-Kerteszova N, Kalendar R, et al. Genetic diversity of cultivated flax (Linum usitatissimum L.) germplasm assessed by retrotransposon-based markers. Theor Appl Genet. 2011;122:1385–1397.
- Bayram E, Yilmaz S, Hamat-Mecbur H, et al. Nikita retrotransposon movements in callus cultures of barley (Hordeum vulgare L.). POJ. 2012;5:211–215.
- Marakli S, Yilmaz S, Gozukirmizi N. BARE1 and BAGY2 retrotransposon movements and expression analyses in developing barley seedlings. Biotechnol Biotechnol Equip. 2012;26:3451–3456.
- Gozukirmizi N, Yilmaz S, Marakli S, et al. Retrotransposon-based molecular markers; tools for variation analysis in plants. In: Tashki-Ajdukovic K, editor. Applications of molecular markers in plant genome analysis and breeding. Ontoria: Research Signpost/Transworld Research Network; 2015. p. 19–45.
- Waugh R, McLean K, Flavell AJ, et al. Genetic distribution of Bare-1-like retrotransposable elements in the barley genome revealed by sequence-specific amplification polymorphisms (S-SAP). Mol Gen Genet. 1997;253(6):687–694.
- Alavi-Kia SS, Mohammadi SA, Aharizad S, et al. Analysis of genetic diversity and phylogenetic relationships in Crocus genus of Iran using inter-retrotransposon amplified polymorphism. Biotechnol Biotechnol Equip. 2008;22:795–800.
- Baumel A, Ainouche M, Kalendar R, et al. Retrotransposons and genomic stability in populations of the young allopolyploid species Spartina anglica C.E. Hubbard (Poaceae). Mol Biol Evol. 2002;19(8):1218–1227.
- Saeidi H, Rahiminejad MR, Heslop-Harrison JS. Retroelement insertional polymorphisms, diversity and phylogeography within diploid, D-genome Aegilops tauschii (Triticeae, Poaceae) Sub-taxa in Iran. Ann Bot. 2008;101:855–861.
- Belyayev A, Kalendar R, Brodsky L, et al. Transposable elements in a marginal plant population: temporal fluctuations provide new insights into genome evolution of wild diploid wheat. Mobile DNA. 2010;1:1–16.
- Gozukirmizi N. Retrotransposon based markers and their applications in barley (Hordeum vulgare L.cvs.) tissue culture. The 5th International Symposium on Sustainable Development. Proceedings; 2014 May 15–18; Sarajevo: International Burch University; 2014.
- Kalendar R, Flavell AJ, Ellis THN, et al. Analysis of plant diversity with retrotransposon-based molecular markers. Heredity. 2011;106:520–530.
- Bonchev G, Parisod C. Transposable elements and microevolutionary changes in natural populations. Mol Ecol Resour. 2013;13:765–775.
- Poczai P, Varga I, Laos M, et al. Advances in plant gene-targeted and functional markers: a review. Plant Methods. 2013;9:6.
- Kalendar R, Grob T, Regina M, et al. IRAP and REMAP: two new retrotransposon-based DNA fingerprinting techniques. Theor Appl Genet. 1999;98:704–711.
- Branco CJS, Vieira EA, Malone G, et al. IRAP and REMAP assessment of genetic similarity in rice. J Appl Genet. 2007;48:107–113.
- Fan F, Cui B, Zhang T, et al. LTR-retrotransposon activation, IRAP marker development and its potential in genetic diversity assessment of masson pine (Linus massoniana). Tree Genet Genomes. 2014;10:2013–2222.
- Vitte C, Panaud O, Quesneville H. LTR retrotransposons in rice (Oryza sativa L.): recent burst amplifications followed by rapid DNA loss. BMC Genomics. 2007;8:218.
- Pervaiz ZH, Turi NA, Khaliq I, et al. Methodology: a modified method for high-quality DNA extraction for molecular analysis in cereal plants. Genet Mol Res. 2011;10:1669–1673.
- Jaccard P. Nouvelles recherches sur la distribution florale [New research on the floral distribution]. Bul Soc Vaudoise Sci Nat. 1908;44:223–270.
- Hamad-Mecbur H, Yilmaz S, Temel A, et al. Effects of epirubicin on barley seedlings. Toxicol Ind Health. 2014;30:52–59.
- Yilmaz S, Marakli S, Gozukirmizi N. BAGY2 retrotransposon analyses in barley calli cultures and regenerated plantlets. Biochem Genet. 2014;52:233–244.
- Vukich M, Schulman AH, Giordani T, et al. Genetic variability in sunflower (Helianthus annuus L.) and in the Helianthus genus as assessed by retrotransposon-based molecular markers. Theor Appl Genet. 2009;119:1027–1038.
- Cakmak B, Marakli S, Gozukirmizi N. SIRE1 retrotransposons in barley (Hordeum vulgare L.). Russ J Genet. 2015;51:661–672.
- O'Donnell K, Burns KH. Mobilizing diversity: transposable element insertions in genetic variation and disease. Mobile DNA. 2010;1:21.