ABSTRACT
Fusarium graminearum and Fusarium culmorum are among the major causal agents of Fusarium head blight, which reduces both crop yield and grain quality in wheat worldwide. The present study was conducted with 57 isolates collected from 23 different locations across four provinces in the 2011/2012 growing season. Out of the 57 Fusarium isolates, 32 isolates were identified as F. graminearum and 25 isolates were identified as F. culmorum. Both pathogens are of particular importance, since they produce several mycotoxins. Among these, deoxynivalenol (DON) and nivalenol (NIV) are well known for their toxicity towards human and animal health. Genetic chemotyping of F. graminearum and F. culmorum species indicated that both DON and NIV chemotypes were present in the surveyed area. Of the 32 F. graminearum isolates, the primer sets Tri13DON and Tri13NIV identified 87.5% as DON chemotypes and 12.5% as NIV chemotypes. Similarly, the 25 F. culmorum isolates displayed 88% DON and 12% NIV chemotypes. In addition, DON acetylated derivatives, 3-acetyldeoxynivalenol (3-AcDON) and 15-AcDON, were identified by polymerase chain reaction based methods. It was determined that 15-AcDON sub-chemotype was dominant in F. graminearum populations, whereas 3-AcDON was dominant in F. culmorum populations. This is the first report demonstrating the presence of F. graminearum and F. culmorum isolates and the distribution of 3-AcDON and 15-AcDON chemotypes in both Fusarium species in wheat fields of eastern Mediterranean region of Turkey.
Introduction
Wheat (Triticum aestivum L.) is one of the most important cereal crops of the world in terms of both areas cultivated and amount of grain produced. Approximately one quarter of the total arable land is cultivated with wheat. The area under wheat cultivation during 2013/14 was 218.5 million hectares with a production of 713.2 million tons.[Citation1] Compared with other cultivated crops, it ranks first in terms of both growing area and production share.
A number of different Fusarium spp., F. graminearum, F. culmorum, F. avenaceum, F. poae, Microdochium nivale and Microdochium majus, are commonly associated with Fusarium head blight (FHB) of wheat crops. Of these, F. graminearum and F. culmorum are potentially the most destructive species in terms of yield loss and mycotoxin production.[Citation2–4] These Fusarium species are widely found in nature and are well known as pathogenic for plants and as producers of mycotoxins, which can have an adverse effect on human and animal health. The FHB disease in wheat inhibits the formation of grains by degrading starch granules in the kernels and producing wrinkled, hollow and coarse grains, which results in seed yield reduction.[Citation5] In addition to yield reduction, the disease also reduces the quality of the grain by contaminating it with harmful mycotoxins, such as trichothecenes.[Citation6] These mycotoxins are secondary metabolites produced by several genera of fungi, including Fusarium, causing great concern for animal and human health.[Citation6] The incidences of B trichothecene chemotypes and their acetylated derivatives have been reported by different authors.[Citation7] B trichothecene chemotypes, deoxynivalenol (DON) and nivalenol (NIV) differ only in their C-4 position; NIV has a hydroxyl group at this position and DON does not have a hydroxyl group.[Citation8] These changes can affect greatly the toxicity.[Citation9] NIV was reported as more toxic than DON.[Citation10] The NIV chemotype was reported in some of the African, Asian, European and South American countries,[Citation11–14] but not in the North American countries. Therefore, the NIV chemotype is restricted to specific regions of the world, as opposed to the DON chemotype, which is common all over the world.[Citation13]
Because of the global importance of FHB, F. graminearum and F. culmorum have been intensively studied. The mycotoxin, produced by related Fusarium ssp. in cereal grains, is of increasing concern in the world, especially in EU and USA, where regulatory limits for mycotoxins permitted in grains and food are set by a range of directives and commission regulations.[Citation15–17]
Both DON and NIV mycotoxins can be observed in the same geographical regions and usually one of the chemotypes is predominant. Thus, it is important to obtain information about the relationship between chemotype and geographical distribution, and to determine the dominant chemotype in the population in order to reduce the contamination risk of food or feed.
Diagnosis and genetic chemotyping of Fusarium isolates are very important. The most common methods used for genetic chemotyping are high performance liquid chromatography coupled with mass spectroscopy (HPLC/MS) or gas chromatography–mass spectrometry (GC/MS) analyses of extracts from cultures inoculated onto substrates, such as wheat, maize or rice.[Citation18–20] HPLC comprises several methods with different normal-phase or reversed-phase columns, distinctive elution mixtures and gradients, detection methods, sample preparation and purification procedures. The mycotoxins are categorized by the retention time. In the case of GC/MS analyses, volatile mycotoxins at the column temperature are converted into volatile derivatives and MS sorts them according to their mass-to-charge ratio. Chemical analyses and chemical chemotyping by HPLC/MS and GC/MS are laborious and time-consuming processes.[Citation21] However, various methods for molecular identification have been applied, such as conventional polymerase chain reaction (PCR), real‐time PCR, multiplex PCR, DNA microarray and sequencing, in order to study the molecular characterization of trichothecene. However, the mycotoxin biosynthesis pathway via PCR is a useful method, as opposed to the conventional chemical methods, because of its sensitivity and potential specificity.[Citation14,Citation22–24] The genetic chemotyping in Fusarium is based on the sequences of the Tri7 and Tri3 highly conserved genes in F. graminearum.[Citation25,Citation26] Several PCR assays have been developed for these genes in order to enable the detection of fungi with potential to produce trichothecenes.[Citation27–29]
The purposes of the present study were to investigate the distribution of F. graminearum and F. culmorum species' complex chemotypes in wheat fields grown in the eastern Mediterranean part of Turkey, to evaluate the possible mycotoxin contamination risks by focusing on species and mycotoxin composition, and to advise the industry of seasonal and regional risks of disease incidence and mycotoxin contamination.
Materials and methods
Collected isolates
The naturally infected mature wheat heads were collected during the 2011–2012 growing season covering 57 isolates in 4 provinces, including Adana (Kadirli, Yüreğir, Ceyhan, Karataş, Yumurtalık, İmamoğlu, Kozan and Feke), Osmaniye (Merkez, Toprakkale and Düziçi), Hatay (Antakya, Reyhanlı, Serinyol, Kırıkhan, İskenderun, Samandag and Altınözü) and Mersin (Silifke, Tarsus, Tuzla, Merkez and Erdemli) locations. Kernels were surface disinfected in 3% (v/v) sodium hypochlorite (NaOCl) for 3 min, rinsed with sterile distilled water and placed on potato dextrose agar (PDA, Merck, Darmstadt, Germany) plates. The plates were incubated at 25 °C under alternating light and dark periods (12 h photoperiod) for 6 d to induce sporulation. All Fusarium isolates were purified and the shape and size of macroconidia, nature of conidiogeneous cells, septations, absence of microconidia and chlamidospores on PDA were identified according to the morphological criteria.[Citation30] Fusarium isolates were further identified using an optical microscope (Olympus, USA) at 400×–500× magnification, according to the morphological criteria on PDA.[Citation31,Citation32]
DNA extraction
Total genomic DNA was extracted from the mycelia of 6-day-old culture of each isolate grown on the PDA medium. The mycelia were freeze-dried (Edwards Modulyo freeze dryer) and grounded to a fine powder in liquid N2. Fifty milligram of the powder was transferred to a 1.5-mL Eppendorf tube and mixed with 700 µL 2× cetyltrimethylammonium bromide, hexadecyltrimethylammonium bromide (CTAB) buffer (pH 8.0). The Eppendorf tubes were incubated at 65 °C for 30 min. After the incubation, an equal volume of phenol:chloroform:isoamyl alcohol (25:24:1; v:v:v) was added into the mixture and centrifuged (Heraeus Biofuge centrifuge, Germany) at 13,000g for 15 min. The supernatant was transferred into a new tube, mixed with 600 µL isopropanol and chilled to –20 °C, followed by another centrifugation step for 5 min at 15,000g at 4 °C. After drying at room temperature for 30 min, the DNA was dissolved in 100 μL of 10 mmol/L Tris, 1 mmol/L ethylenediaminetetraacetic acid (EDTA) (TE, pH 7.5) buffer.
PCR amplification
The PCR amplification was carried out by using 2.5 µL Fg16 F/R and Fc01 F/R specific fungal primers for F. graminearum and F. culmorum, respectively.[Citation33] The primers and their properties, used to identify F. graminearum and F. culmorum, are listed in . The PCR was performed in a 50-µL reaction mixture containing 25 µL of 2× PCR master mix (0.05 U/µL Taq DNA Polymerase in reaction buffer (20 mmol/L Tris-HCl, 10 mmol/L (NH4)2SO4, 10 mmol/L KCl, 2 mmol/L MgSO4, 0.1% Triton X-100, pH 8.8), 0.4 mmol/L of each dNTPs, Fermentas, Lithuania), 8 µL (25–50 ng) fungal DNA, 2.5 µL (300 nmol/L) of each of the primers and 12 µL nuclease-free water.
Table 1. Species- and chemotype-specific primers and the sizes of the expected PCR products.
The PCR conditions used for F. culmorum and F. graminearum detection were as follows: 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for 30 s and extension at 72 °C for 40 s, followed by a final extension at 72 °C for 5 min.
The genetic chemotyping of the collected isolates was done by PCR assays using five specific primer sets, Tri13F/Tri13DONR, Tri7F/Tri7R, Tri315F/Tri315R, Tri303F/Tri303R and Tri13NIVF/Tri13R (). The same as the above mentioned PCR conditions were applied to these specific primers with the exception that the denaturation temperature was 94 °C. The PCR conditions for the five primer sets was 94 °C for 4 min, followed by 35 cycles of 94 °C for 1 min, 60 °C for 40 s, 72 °C for 40 s and a final extension at 72 °C for 6 min.
The PCR products were electrophoresed on 2% agarose gels (1 × 40 mmol/L Tris base, 1 mmol/L EDTA, 20 mmol/L acetic acid (TAE) buffer adjusted to pH 8.0), stained with ethidium bromide (0.05 mg per 100 mL TAE buffer) and photographed under UV light with a Bio-Imaging System (Bio-RAD, Hercules, USA). All assays were repeated at least twice.
Results and discussion
F. graminearum and F. culmorum are causal agents of FHB disease of wheat. A total of 32 isolates from 20 different locations, belonging to Adana, Hatay, Mersin and Osmaniye provinces, were identified as F. graminearum and 25 isolates from 14 different locations, belonging to Adana, Hatay and Mersin, were identified as F. culmorum by using traditional identification techniques (). Both F. graminearum and F. culmorum were confirmed by traditional identification techniques and by species-specific PCR analyses. Based on morphological characteristics, the isolates produced canoe-shaped macroconidia at the initial stages of growth. At later stages, they developed macroconidia. The colonies of F. culmorum developed yellow aerial mycelia with red base on PDA. However, microconidia did not have short thick macroconidia with flattened apical cells. Based on the growth rate and colours of the colonies, F. graminearum and F. culmorum were identified from the samples. The conformation of 32 isolates as F. graminearum and 25 isolates as F. culmorum was made by using two sets of primers – Fg16F/Fg16R (producing 450 bp) and Fc01F/Fc01R (producing 570 bp), respectively. The samples that produced a common band, ranging from 450 bp, confirmed that the studied isolates were F. graminearum. The isolates that produced a 570 bp product were F. culmorum.
Table 2. Code, origin, identification and chemotyping of F. graminearum and F. culmorum isolates obtained from infected wheat tissues in the eastern Mediterranean provinces of Turkey.
Both F. graminearum and F. culmorum produce different types of trichothecene mycotoxins, principally including nivalenol, deoxynivalenol, 3-acetyldeoxynivalenol (3-AcDON) and 15-acetyldeoxynivalenol (15-AcDON).[Citation34] Those mycotoxins are harmful for people and livestock. Tri13DON and Tri13NIV primer sets were used to determine the DON and NIV chemotypes, respectively, of F. graminearum and F. culmorum isolates. Of the 32 analyzed F. graminearum isolates, the primer sets Tri13DON and Tri13NIV identified 87.5% (28/32) as DON chemotypes and 12.5% (4/32) as NIV chemotypes. Similar DON/NIV rate was obtained among the 25 isolates of F. culmorum – 88% (22/25) were DON chemotypes and 12% (3/25) were NIV chemotypes (). In both F. graminearum and F. culmorum populations, the most frequently isolated chemotype was DON and the less isolated chemotype was NIV. Of the 32 locations of F. graminearum, the NIV chemotype was found in 1 location in Adana and 3 locations in Hatay, however, DON chemotype was found in 7 locations in Adana, 11 locations in Hatay, 4 locations in Mersin and 6 locations in Osmaniye (). DON chemotype was further divided into 15-AcDON and 3-AcDON sub-chemotypes. In DON chemotypes of F. graminearum and F. culmorum, 15-AcDON producing isolates were dominant in F. graminearum population, whereas 3-AcDON producing isolates were dominant in F. culmorum (). The 3-AcDON sub-chemotype in F. graminearum was found in four locations in Hatay, one location in Mersin and one location in Osmaniye provinces. The 15-AcDON sub-chemotype was found in seven locations in Adana and Hatay, three locations in Mersin and five locations in Osmaniye (). Within the F. culmorum locations evaluated, however, NIV chemotype was found in one location in Hatay and two locations in Mersin, however, DON chemotype was found in nine locations in Adana, eight locations in Hatay and five locations in Mersin. The 3-AcDON sub-chemotype in F. culmorum was found in seven locations in Adana, seven locations in Hatay and five locations in Mersin provinces. The 15-AcDON sub-chemotype was found in two locations in Adana and one location in Hatay provinces ().
Table 3. F. graminearum and F. culmorum chemotype occurrеnce in the surveyed regions.
Figure 1. Trichothecene chemotypes of F. graminearum and F. culmorum isolates collected from four provinces in Turkey.
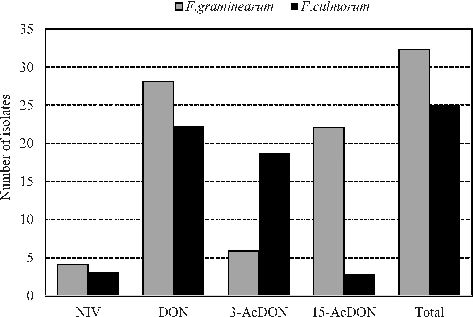
The genetic chemotyping of the 57 F. graminearum and F. culmorum isolates from 23 different locations in four provinces, showed that both DON and NIV chemotypes were present in the eastern Mediterranean region of Turkey. This is consistent with earlier studies of mycotoxin screenings, carried out in the western part of Turkey, which obtained results that both DON and NIV chemotypes were present in wheat fields and the DON chemotype of F. graminearum and F. culmorum were more frequently isolated than the NIV chemotype.[Citation35,Citation36] However, Mert-Türk and Gencer [Citation24] did not detect 3-AcDON and NIV chemotypes in three western provinces of Turkey. DON and NIV chemotypes of F. graminearum and F. culmorum have also been identified in many countries in Europe, North and South America, Africa and Asia.[Citation14,Citation23,Citation37–42] It was reported that the NIV chemotype was more toxic than DON.[Citation10] Therefore, NIV is a serious threat for both people and animals and it is necessary to reduce the toxin production by using resistant cultivars and applying cultural methods.
The analysis of F. graminearum showed that NIV and DON chemotypes coexist in Adana and Hatay provinces, whereas the NIV chemotype was not detected in Osmaniye and Mersin provinces (). When F. culmorum isolates were in consideration, mixed populations of NIV and DON chemotypes were detected in Hatay and Mersin provinces. However, NIV chemotype was not detected in Adana province and neither NIV nor DON chemotypes were detected in Osmaniye province. Similarly, in previous studies, mixed NIV and DON chemotypes have been reported in F. graminearum [Citation14,Citation43] and F. culmorum [Citation44] populations.
Sub-chemotyping analysis indicated that the majority of F. graminearum isolates displayed more of the 15-AcDON sub-chemotype, whereas F. culmorum isolates displayed more of the 3-AcDON sub-chemotype. Similar results were reported by Quarta et al. [Citation45] and Stêpieñ et al. [Citation46] that the 3-AcDON sub-chemotype was the most frequent chemotype among the F. culmorum isolates, whereas the 15-AcDON sub-chemotype was the most frequent chemotype among F. graminearum isolates. Our finding for F. graminearum was consistent with the finding of Alvarez et al. [Citation42] that the 15-AcDON chemotype had higher frequency than the 3-AcDON. On the other hand, the opposite results were reported by Quarta et al.,[Citation47] whose study reported that the 3-AcDON chemotype was the most dominant chemotype in some parts of Asia and China, Australia, New Zealand and northern Europe. Earlier studies showed that the 3-AcDON chemotype of F. graminearum was more aggressive than the 15-AcDON chemotype in resistant and susceptible wheat cultivars.[Citation12,Citation48] The increase in the incidence of F. graminearum and F. culmorum populations in the eastern Mediterranean region of Turkey could potentially have resulted in increased levels of DON and reduced levels of NIV contamination in wheat grains, depending on the toxin profile of both Fusarium species. However, the high proportion of DON chemotype, found within the F. graminearum and F. culmorum populations, as reported here, indicated that DON contamination was high and NIV contamination was low in wheat grains in the eastern Mediterranean region of Turkey.
Conclusions
The fungal pathogens, F. graminearum and F. culmorum, are among the most common causal agents of FHB disease worldwide. Among the 57 isolates collected from four provinces in the eastern Mediterranean region of Turkey, 32 isolates were identified as F. graminearum and 25 isolates were identified as F. culmorum by traditional identification techniques and PCR assay. Both species produce trichothecenes, most frequently DON and less frequently NIV. DON-producing isolates were further distinguished on the basis of the predominant acetyl DON derivative that they produce – 3-AcDON and 15-AcDON sub-chemotypes. The majority of F. graminearum isolates displayed the 15-AcDON sub-chemotype, whereas F. culmorum isolates displayed the 3-AcDON sub-chemotype. The information of Fusarium chemotypes and their distribution in the wheat cultivation areas could be critical for the projection of disease development, integrated disease control programmes and mycotoxin contamination.
Acknowledgements
The authors would like to thank Tohmas Miedaner and Dr Firas Talas at University of Hohenheim, Stuttgart, Germany for their invaluable help for PCR works.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- FAOSTAT. Food and Agricultural Organization of the United Nations. Rome, Italy: FAO; 2014 [ cited 2015 Jul 10]. Available from: http://faostat.fao.org/site/567/default.aspx#ancor.
- Parry DW, Jenkinson P, McLeod L. Fusarium ear blight (scab) in small grains – a review. Plant Pathol. 1995;44:207–238.
- Jennings P, Turner JA. Towards the prediction of Fusarium ear blight epidemics in the UK – the role of humidity in disease development. Proceedings of the Brighton Crop Protection Conference: Pests and Diseases. Farnham, UK: BCPC Publications; 1996. p.233–238.
- Turner JA, Jennings P. The effect of increasing humidity on Fusarium ear blight and grain quality. Proceedings of the Fifth European Fusarium Seminar, Szeged. Cereal Res Commun. 1997;25:825–826.
- Jackowiak H, Packa D, Wiwart M, et al. Scanning electron microscopy of Fusarium damaged kernels of spring wheat. Int J Food Microbiol. 2005;98:113–123.
- Rotter BA, Prelusky DB, Pestka JJ. Toxicology of deoxynivalenol (vomitoxin). J Toxicol Environ Health. 1996;48:1–34.
- Placinta CM, D'Mello JPF, McDonald AMC. A review of world wide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim Feed Sci Tech. 1999;78:21–37.
- Hye-Seon K, Lee T, Dawlatana M, et al. Polymorphism of trichothecene biosynthesis genes in deoxynivalenol- and nivalenol-producing Fusarium graminearum isolates. Mycol Res. 2003;107:190–197.
- Kimura M, Kaneko I, Komiyama M, et al. Trichothecene 3-O-acetyltransferase protects both the producing organism and transformed yeast from related mycotoxins. J Biol Chem. 1998;273:1654–1661.
- Ryu JC, Ohtsubo K, Izumiyama N, et al. The acute and chronic toxicities of nivalenol in mice. Fundam Appl Toxicol. 1988;11:38–47.
- Mirocha C, Abbas H, Windels C, et al. Variation in deoxynivalenol, 15-acetyldeoxynivalenol, 3-acetyldeoxy- nivalenol, and zearalenone production by Fusarium graminearum isolates. Appl Environ Microbiol. 1989;55:1315–1316.
- Puri KD, Zhong S. The 3ADON population of Fusarium graminearum found in North Dakota is more aggressive and produces a higher level of DON than the prevalent 15ADON population in spring wheat. Phytopathology. 2010;100:1007–1014.
- Desjardins A, Manadhar H, Plattner R, et al. Occurrence of Fusarium species and mycotoxins in Nepalese maize and wheat and the effect of traditional processing methods on mycotoxin levels. J Agr Food Chem. 2000;48:1377–1383.
- Jennings P, Coates ME, Walsh K, et al. Determination of deoxynivalenol and nivalenol-producing chemotypes of Fusarium graminearum isolated from wheat crops in England and Wales. Plant Pathol. 2004;53:643–652.
- FAO. Worldwide regulations for mycotoxins in food and feed in 2003. FAO Food and Nutrition Paper 81. Rome, Italy: Food and Agriculture Organization of the United Nations; 2004. p. 1–180.
- Verstraete F. European Union legislation on mycotoxins in food and feed: overview of the decision-making process and recent and future development. In: Leslie JF, Bandyopadhyay R, Visconti A, editors. Mycotoxins. detection methods, management, public health and agricultural trade. Wallingford (CT): CABI; 2008. p. 77–99.
- EU. Commission Regulation (EC) No 856 ⁄ 2005 of 6 June 2005 amending Regulation (EC) No 466 ⁄ 2001 as regards Fusarium toxins. Official J Eur Union. 2005;L143:3–8.
- Sugiura Y, Watanabe Y, Tanaka T, et al. Occurrence of Gibberella zeae strains that produce both nivalenol and deoxynivalenol. Appl Environ Microbiol. 1990;56:3047–3051.
- Miller JD, Greenhalgh R, Wang YZ, et al. Trichothecene chemotypes of three Fusarium species. Mycologia. 1991;83:121–130.
- Muthomi JW, Schutze A, Dehne HW, et al. Characterisation of Fusarium culmorum isolates by mycotoxin production and aggressiveness to winter wheat. J Plant Dis Prot. 2000;107:113–123.
- Jian-Hua W, He-Ping L, Jing-Bo Z, et al. Development of a generic PCR detection of 3-acetyldeoxynivalenol-, 15-acetyldeoxynivalenol- and nivalenol-chemotypes of Fusarium graminearum. Int J Mol Sci. 2008;9:2495–2504.
- Chandler EA, Simpson DR, Thomsett MA, et al. Development of PCR assays to Tri7 and Tri13 trichothecene biosynthetic genes, and characterization of chemotypes of Fusarium graminearum, Fusarium culmorum and Fusarium cerealis. Physiol Mol Plant Pathol. 2003;62:355–367.
- Shen CM, Hu YC, Sun HY, et al. Geographic distribution of trichothecene chemotypes of the Fusarium graminearum species complex in major winter wheat production areas of China. Plant Dis. 2012;96:1172–1178.
- Mert-Türk F, Gencer R. Distribution of the 3-AcDON, 15-AcDON and NIV chemotypes of Fusarium culmorum in the North-West of Turkey. Plant Prot Sci. 2013;49: 57–64.
- Wang JH, Li HP, Qu B, et al. Development of a generic PCR detection of 3-acetyldeoxy-nivalenol-, 15-acetyldeoxynivalenol- and nivalenol-chemotypes of Fusarium graminearum clade. Int J Mol Sci. 2008;9:2495–2504.
- Yörük E, Albayrak G. Chemotyping of Fusarium graminearum and F. culmorum isolates from Turkey by PCR assay. Mycopathologia. 2012;173:53–61.
- Doohan FM, Parry DW, Nicholson P. Fusarium ear blight of wheat: the use of quantitative PCR and visual disease assessment in studies of disease control. Plant Pathol. 1999;48:209–217.
- Schnerr H, Niessen L, Vogel RF. Real time detection of the tri5 gene in Fusarium species by LightCycler–PCR using SYBR Green I for continuous fluorescence monitoring. Int J Food Microbiol. 2001;71:53–61.
- Edwards SG, Pirgozliev SR, Hare MC, et al. Quantification of trichothecene-producing Fusarium species in harvested grain by competitive PCR to determine efficacies of fungicides against Fusarium head blight of winter wheat. Appl Environ Microbiol. 2001;67:1575–5780.
- Leslie JF, Summerell BA. The Fusarium laboratory manual. Oxford: Blackwell Publishing Ltd; 2006. p.158–159.
- Nirenberg HA. A simplified method for identifying Fusarium spp. occurring on wheat. Can J Bot. 1981;59:1599–1609.
- Nelson PE, Toussoun TA, Marasas WFO. Fusarium species. An illustrated manual for identification. University Park: The Pennsylvania State University Press; 1983.
- Nicholson P, Simpson DR, Weston G, et al. Detection and quantification of Fusarium culmorum and Fusarium graminearum in cereals using PCR assays. Physiol Mol Plant Pathol. 1998;53:17–37.
- Tizaki MA, Sabbagh SK. Detection of 3-acetyldeoxynivalenol, 15-acetyldeoxynivalenol and nivalenol-chemotypes of Fusarium graminearum from Iran using specific PCR assays. Plant Knowledge J. 2013;2:38–42.
- Turner JA, Jennings P, Nicholson P. Investigation of Fusarium infection and mycotoxin levels in harvested wheat grain. London: Home Grown Cereals Authority; 1999. ( Project Report no. 207.)
- Yörük E, Gazdagli A, Albayrak G. Class B trichothecene chemotyping in Fusarium species by PCR assay. Genetika. 2014;46:661–669.
- Gale LR, Ward TJ, Balmas V, et al. Population subdivision of Fusarium graminearum sensu stricto in the upper Midwestern United States. Phytopathology. 2007;97:1434–1439.
- Bakan B, Pinson L, Cahagnier B, et al. Toxigenic potential of Fusarium culmorum strains isolated from French wheat. Food Addit Contam. 2001;18:998–1003.
- Reynoso MM, Ramirez ML, Torres AM, et al. Trichothecene genotypes and chemotypes in Fusarium graminearum strains isolated from wheat in Argentina. Int J Food Microbiol. 2011;145:444–448.
- Yli-Mattila T, Paavanen-Huhtala S, Jestoi M, et al. Real-time PCR detection and quantification of Fusarium poae, F. graminearum, F. sporotrichioides and F. langsethiae in cereal grains in Finland and Russia. Arch Phytopathol Plant Prot. 2008;41:243–260.
- Pasquali M, Giraud F, Brochot C, et al. Genetic Fusarium chemotyping as a useful tool for predicting nivalenol contamination in winter wheat. Int J Food Microbiol. 2010;137:246–253.
- Alvarez CL, Azcarate MP, Pinto VF. Toxigenic potential of Fusarium graminearum sensu stricto isolates from wheat in Argentina. Int J Food Microbiol. 2009;135:131–135.
- Miedaner T, Reinbrecht C, Schilling AG. Association among aggressiveness, fungal colonization, and mycotoxin production of 26 isolates of Fusarium graminearum in winter rye head blight. J Plant Dis Prot. 2000;107:124–234.
- Jennings P, Coates ME, Turner JA, et al. Determination of deoxynivalenol and nivalenol chemotypes of Fusarium culmorum isolates from England and Wales by PCR assay. Plant Pathol. 2004;53:182–190.
- Quarta A, Mita G, Haidukowski M, et al. Assessment of trichothecene chemotypes of Fusarium culmorum occurring in Europe. Food Addit Contam. 2005;22:309–315.
- Stepień Ł, Popiel D, Koczyk G, et al. Wheat-infecting Fusarium species in Poland – their chemotypes and frequencies revealed by PCR assay. J Appl Genet. 2008;49:433–441.
- Quarta A, Mita G, Haidukowski M, et al. Multiplex PCR assay for the identification of nivalenol, 3-, and 15-acetyl deoxynivalenol chemotypes in Fusarium. FEMS Microbiol Lett. 2006;259:7–13.
- Von der Ohe C, Gauthier V, Tamburic-Ilincic L, et al. A comparison of aggressiveness and deoxynivalenol production between Canadian Fusarium graminearum isolates with 3-acetyl and 15-acetyldeoxynivalenol chemotypes in field-grown spring wheat. Eur J Plant Pathol. 2010;127:407–417.
