ABSTRACT
Abiotic stresses may seriously affect plant growth and development. In order to explore new stress tolerance genes in rice (Oryza sativa L.), expression profiles were obtained for leaf and panicle tissues. They were taken at seedling, booting, heading and flowering stages of indica cultivar Pei'ai 64S plants under cold, drought and heat stresses by using the GeneChip Rice Genome Array (Affymetrix), which includes 51,279 transcripts from japonica and indica rice. O. sativa L. cytosolic sulphotransferase-like gene (OsSOT9) was highly induced in leafs and panicles during different developmental stages, in response to all stresses, especially at booting stage under cold stress. The quantitative real-time polymerase chain reaction analysis showed that this result was almost consensus with GeneChip Rice Genome Array. cDNA of OsSOT9 was cloned to study its function in stress tolerance through reverse transcription PCR. Sequence analysis showed that the cDNA encoded a protein with molecular weight of about 37.78 kDa and pl of about 6.6, which was composed of 343 amino acid residues. Bioinformatics data showed that the protein contained a conservative domain of the sulphotransferase (SOT) family gene. OsSOT9 was found to be closely related to the cytosolic sulphotransferase after comparison of the protein sequences. Analysis of the putative promoter region found 12 kinds of cis-elements related to stress response. On the basis of the above analyses, we suggested that OsSOT9 is a novel candidate gene involved in stress tolerance in rice.
Introduction
Rice (Oryza sativa L.) is an important crop and source of carbohydrates for more than one-third of the world's population, and it is cultivated worldwide on arable lands.[Citation1,Citation2] Various abiotic stresses, such as drought and low and high temperatures, impact negatively on plant growth and crops productivity.[Citation3] We need to understand the mechanism by which rice perceives environmental signals and transmits those signals to activate adaptive responses and isolate stress-related genes. For this, stress-tolerant rice should be developed. This is significant for ensuring stable and high yield, raising the utilization efficiency of low-yield fields and expanding rice planting area. Therefore, we investigated the gene expression levels of rice at different stresses.[Citation4] Studies have shown that plants subjected to stress conditions do not take passive defence, but proactive measures to deal with stress. Hence, it is necessary to develop high-quality and high-yielding varieties.[Citation5–7]
Sulphonation is an important reaction, involved in the biotransformation of various endogenous molecules and xenobiotics.[Citation8] This biological phenomenon has a crucial role in cell growth, development and defence and is presented in various organisms.[Citation8,Citation9] Sulphonation is the transfer of a sulphonate group (SO3−1) from the universal sulphonate donor 3'-phosphoadenosine 5'-phosphosulphate (PAPS) to an appropriate acceptor molecule.[Citation9] They have conserved amino acid motives, which seem to be involved in PAPS binding (regions I and IV).[Citation10–12] Sulphotransferases (SOTs) catalyse the sulphonation of a wide range of compounds, producing sulphate esters and sulphate conjugates.[Citation13–15] SOT proteins are classified based on their affinity for different classes of substrates.[Citation11,Citation16] Mainly, there are two groups of SOT proteins. The first group accepts macromolecules, for instance, proteins, peptides and glycosaminoglycans and the second group is combined with small organic molecules, such as steroids, flavonoids and xenobiotics.[Citation16,Citation17] Sulphonation via SOTs is hypothesized to affect the biological activities of certain compounds and also the regulation of physiological processes, for example, growth, development and adaptation to stress.[Citation11,Citation18–20]
Members of the SOT family have been found in most organisms investigated to date, except in archaea.[Citation11,Citation12,Citation16,Citation21] Mammalian SOTs catalyse the sulphate conjugation of many hormones, neurotransmitters, drugs and xenobiotic compounds.[Citation10–12] Like mammals, plants contain a large number of sulphotransferase genes.[Citation8,Citation11] Sequence analysis has identified 18 cytosolic sulphotransferases in Arabidopsis and 35 in rice.[Citation8] Two cDNA clones, coding for flavonol 3'- and 4'-sulphotransferases from Flaveria chloraefolia, were first isolated.[Citation8,Citation22] These two SOTs exhibit strict substrate and hydroxyl position specificity.[Citation8,Citation22,Citation23] However, the biological functions of SOTs in plants are still largely unknown.[Citation8] Some studies found that the mRNA levels of Arabidopsis SOTs were low in normal growth conditions.[Citation24–26] The mRNA levels in Brassica napus control seedling were similar to those in Arabidopsis.[Citation18,Citation27] The expression pattern of B. napus increased after treatment with salicylic acid and ethanol, which further showed the potential function of these enzymes in the stress response.[Citation18,Citation28]
In this paper, we screened a stress tolerance candidate gene OsSOT9 from cultivar Pei'ai 64S (photo-thermo-sensitive genic male sterile line), which is the maternal parent of the super hybridization rice Liang-You-Pei-Jiu (LYP9) by GeneChip Rice Genome Array and quantitative real-time polymerase chain reaction (qRT-PCR). The OsSOT9 gene was highly induced in leafs and panicles under abiotic stresses during all of the developmental stages and may play an important role in response to diverse abiotic stresses in rice.
Materials and methods
Plant materials preparation and abiotic stresses treatment
The cultivar Pei'ai 64S is a kind of conventional variety in China and belongs to ‘photo-thermo-sensitive genic male sterile line.’ The seeds of cultivated indica cultivar Pei'ai 64S (Oryza sativa L.) were sterilized with 0.1% HgCl2 for 10 min and then washed three times under running water. The seeds were immersed for 3 d at 25 ℃ and their water was changed every day. Then they germinated for 2–3 d at 37 °C in distilled water. In this study, rice seedlings were divided into three groups of two treatments and one control group. The control group was maintained under normal growth conditions and the treatment groups were exposed to heat, cold and drought stresses, which are closely related to rice production. At five-leaf stage, some plants of the treatment groups were taken for seedling test. The rest were transplanted to other pots (five plants for each pot) in greenhouse under natural conditions of regular water and fertilizer management, pest and disease control and were kept for tests at booting, heading and flowering stages. For the cold tests, the rice seedling at five-leaf stage was left at 4 °C for 12 h. The materials from the booting, heading and flowering stages were treated for 16 h at 12 °C. For the heat tests, the treatment group was put at 45 °C for 2 h, and then the plants were harvested. All the treated rice seedlings were put into a US Percival produced PGC15.5 artificial climate chamber, whereas the control group was in another chamber at 28 °C. Both the control and treatment groups were under dark conditions. For the drought tests, the water was poured away from the basin and the pots were put in dry shed. The leaves were collected after 16 h, when they started curling. The control and treatment groups were under the same conditions, except that the control group received water. We used leaf and panicle tissue samples in the whole genome expression profiling.
Isolation of total RNA, GeneChip Rice Genome Array (Affymetrix) and quantitative real-time polymerase chain reaction (qPCR)
The process was performed according to previously described protocols.[Citation4,Citation29–32] Microarray analysis was performed according to the GeneChip® Expression Analysis Technical Manual (2005 version) provided by Affymetrix (Affymetrix Inc., Santa Clara, CA, USA). The main operating procedures were as follows: (1) extraction and purification of total RNA: total RNA was isolated from the frozen samples by extraction method using TRIzol reagent (Invitrogen). The samples that were kept at −70 ℃ were taken out and vortexed. Chloroform (200 μL) was added and then was vigorously shaken for 15 s. Centrifugation followed at 12,000 × g for 15 min at 4 ℃. The upper layer was carefully removed from each tube and transferred to another centrifuge tube. Isopropanol (500 μL) was added and precipitated for at least 1 h at −40 ℃. After this, centrifugation followed to separate the RNA. The RNA pellets were washed twice with 75% ethanol, air dried and dissolved in an appropriate volume of RNase-free water. The RNA purity was determined by A260/280 absorbance ratio (1.9–2.0). The isolated RNAs were stored at −70 ℃, after checking the purity and integrity of 5S, 18S and 28S rRNA bands on a 1.5% agarose gel; (2) synthesis and purification of cDNA: RNA was reverse transcribed by priming and reverse transcriptase. An amount of 1 μg total RNA was incubated with 0.5 μg random primer (Gibco BRL) for 10 min at 70 °C in 12 μL. The samples were then cooled on ice, and the following components were added: 1 μL deoxyribonucleotides (10 mmol/L each), 4 μL RTbuffer (supplied with the reverse transcriptase kit), 1.5 μL MgCl2 (25 mmol/L), 2 μL dithiothreitol (100 mmol/L), 0.5 μL RNase inhibitor (20 IU/μL) and 1 μL reverse transcriptase (200 U/μL; SuperScript II, Gibco BRL). The samples were incubated for 1 h at 42 °C and the reaction was stopped by heating to 70 °C for 15 min. After cooling for 10 min at 4 °C, 1 μL RNase (2 U/μL, RNase H, Gibco BRL) was added and the solution was heated at 37 °C for 20 min. The cDNA was stored at −20 °C until further use; (3) cRNA synthesis and cRNA transcription purification in vivo: in brief, we used an aliquot of 5 µL stranded cDNA using the Super Script Double-Stranded cDNA Synthesis Kit (Invitrogen) and poly (T)-nucleotide primers that contained a sequence recognized by T7 RNA Polymerase. A portion of the resulting double-stranded cDNA was used as a template to generate biotin-tagged cRNA from an in vitro transcription reaction using the Bio-Array High-Yield RNA Transcript Labeling Kit. The resulting biotin-tagged cRNA was fragmented to strands of 35–200 bases in length, according to the protocols from Affymetrix; (4) cRNA fragmentation and preparation of hybridization solution: 10 µg of this fragmented target cRNA was hybridized to an Affymetrix rice array; (5) chip hybridization and elution chip: hybridization was performed at 45 °C with rotation for 16 h. The GeneChip arrays were washed and then stained on an Affymetrix Fluidics Station 400, followed by scanning on a Gene Array scanner; (6) scan chips and data analysis: the hybridization data were analysed using GeneChip operating software GCOS 1.4. A logistic scaling procedure and comparison analysis were performed on different arrays with GCOS 1.4 and Excel. The reagent of total RNA extraction (Fermentas, Waltham, MA, USA) was used for removing genomic DNA with DNase, following the operational method suggested by the manufacturer. Three biological replications were used for real-time qRT-PCR. Real-time PCR primers were designed by Primer Expression 3.0 software. The primers for the target gene (OsSOT9) were OsSOT9-F: 5'-CATGTGGGCCATGCAAAGTG-3', OsSOT9-R: 5'-AACAGCAGCAAAATCACAATGAA-3', 18S-F: 5'-CGTCCCTGCCCTTTGTACAC-3' and 18S-R: 5'-CGAACACTTCACCGGATCATT-3'. The primers were synthesized in Invitrogen (Biotechnology Limited Company, Shanghai, China). Before the qRT-PCR, the primers were tested by conventional RT-PCR and agarose gel electrophoresis. All qRT-PCR assays were carried out on ABI7900 using QuantiTect SYBR Green RT-PCR Kit (Cat. No.204243, QIAGEN). Each reaction mixture (10 μL) contained 5 μL Master Mix (2×), 0.2 μL reverse transcriptase, 0.5 μL of each primer (10 mmol/L), 2 μL template RNA sample (40 ng) and 2.3 μL RNase-free water. PCR system without RT mix (containing reverse transcriptase) was used to detect possible residual DNA. The thermal cycle was programmed as follows: incubation for 30 min at 48 °C, initial denaturation for 10 min at 95 °C and 40 amplification cycles (15 s at 95 °C, 40 s at 58 °C and 20 s at 72 °C).
cDNA cloning
In this study, the sequences used for the PCR were from Gramene Basic Local Alignment Search Tool (BLAST) and National Center for Biotechnology Information (NCBI) search database and specific primers were designed by using the Primer Premier 5.0 software. The PCR amplification primers were OsSOT9-F: (5'-AAGCTTTTCTGCATCCATGGCC-3', HindIII upstream restriction site) and OsSOT9-R: (5'-GGATCCAACTGCTCGATCCGTGTCA-3', BamHI downstream restriction site). The PCR amplification assays were carried out on C1000™ Thermal Cycler from Bio-Rad PCR. The full-length OsSOT9 cDNA was amplified using high fidelity HiFi Taq DNA Polymerase (Transgen). Each reaction mixture (50 μL) contained 5μL TransTaq HiFi Buffer (10×), 2 μL of each primer (10 mmol/L), 4 μL dNTPs (2.5 mmol/L), 1 μL template DNA (400 ng), 0.4 μL TransTaq HiFi DNA Polymerase and 35.6 μL of RNase-free water. The PCR cycler was programmed as follows: initial denaturation at 95 °C for 5 min, 30 amplification cycles (denaturation at 95 °C for 30 s, annealing at 58 °C for 30 s and polymerization at 72 °C for 110 s) and final elongation at 72 °C for 10 min. All the PCR products were purified by using Gel Extraction Mini Kit (Biomed, Shanghai, China). The amplified fragment was transformed into vector pMD18-T (TaKaRa, Dalian, China). The positive transformants were screened by using ampicillin selection. Meanwhile, restriction enzymes HindIII and BamHI were used for double cuts for confirmation. Restricted fragments were analysed on 1.0% agarose gel. The positively screened clone was sequenced by Invitrogen (Biotechnology Limited Company, Shanghai, China).
Sequence analysis
The analysis and comparison of the obtained amino acid sequence with published sequences were performed with Standard Protein–Protein Basic Local Alignment Search Tool (BLASTP) on the NCBI server (http://www.ncbi.nlm.nih.gov/). The 1500 bp upstream promoter sequences from the ATG site of OsSOT9 gene were performed with PlantCARE on the web (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/). Information about the secondary structure of OsSOT9 protein was obtained from online protein prediction software (http://www.ebi.ac.uk/InterProScan/). OsSOT9 gene was aligned with other SOT proteins from different species by using DNAMAN V6. Phylogenetic relationship with other SOT proteins from diverse species was constructed using Mega 4.1.[Citation30,Citation33] A phylogenetic tree was generated by the maximum parsimony method using the Mega 6.06 bootstrap (1000 replications) to obtain support values.
Data analyses
The computational method of real-time quantitative PCR was as follows: the expression of relative target genes (Rel. Exp) = 2ΔΔCt, whereas ΔΔCt = (unknown sample ΔCt) − (Calibrator ΔCt). The unknown sample ΔCt = (internal reference gene Ct) − (target gene Ct) and Calibrator ΔCt = (reference sample internal reference gene Ct) − (reference sample of target gene Ct).
Results and discussion
Expression levels of OsSOT9 under different stresses
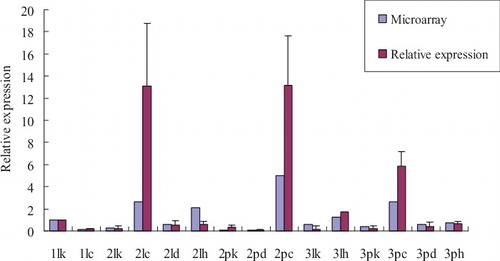
In order to discover and separate stress-responsive genes in rice, we analysed the whole genome expression profiling obtained from leaf and panicle tissues. They were taken at seedling, booting, heading and flowering stages of indica cultivar Pei'ai 64S plants under cold, drought or heat stresses by using the GeneChip Rice Genome Array (Affymetrix) which includes 51,279 transcripts. The microarray analysis revealed that numerous genes were up- and down-regulated under diverse stresses in the rice genome. The results showed that the expression levels of OsSOT9 were obviously up-regulated in different tissues of rice at booting and flowering stages under stress conditions. Under low temperature, the expression levels were down-regulated in leafs at seedling stage and were also down-regulated under drought stress in the panicles at booting stage (). Under cold treatment, the expression level of OsSOT9 increased by 10.79-fold and by 71.44-fold in leafs and panicles at booting stage, respectively. Also, it increased by 6.26-fold in the panicles at heading and flowering stage, but reduced by 7.69-fold in leafs at seedling stage. Under heat treatment, the expression level of OsSOT9 was up-regulated by 8.62-fold and by 2.13-fold in leafs at booting and heading and flowering stages, respectively, and by 1.75-fold in panicles at heading and flowering stage. Under drought conditions, the expression level of OsSOT9 was up-regulated by 2.33-fold and by 1.32-fold in leafs at booting stages and in panicles at heading and flowering stage, respectively, but reduced by 0.79-fold in the panicles at booting stage. To verify the reliability of microarray data, quantitative real-time PCR analysis was conducted (). The results showed that expression levels of OsSOT9 were increased under different stresses, especially at low temperature. The expression pattern was similar to the microarray data, suggesting that OsSOT9 is a multiple stress-responsive gene in rice.
Cloning and sequence analysis of OsSOT9
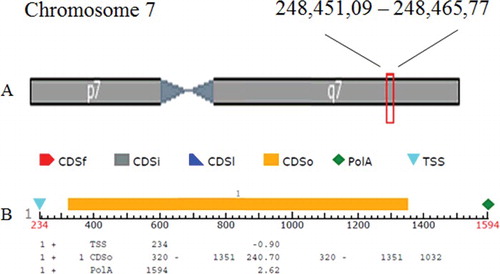
Sequence analysis from NCBI and Gramene BLAST revealed that OsSOT9 consisted of 1469 bp with 50% G + C contents and had an open reading frame of 1032 bp, which encoded a protein of 343 amino acids with M.W. ≈ 37.78 kDa and pI ≈ 6.6, corresponding to Nipponbare LOC_Os07g41460. To further analyse the OsSOT9 gene, we designed two specific primers and cloned the complete coding DNA sequence through reverse transcription PCR from rice Pei'ai 64S.
OsSOT9 is located in chromosome 7 and contains only one coding exon with no introns (). We analysed the possible promoter region, 1500 bp upstream from the ATG site of OsSOT9, which may contain several putative cis-elements related to stress responses. There were 32 CAAT boxes (common cis-acting elements in the promoter and enhancer regions), 18 TATA boxes, 2 5' untranslated region (5′UTR) Py-rich stretches (cis-acting elements conferring high transcription levels), 3 ABA-responsive elements (cis-acting elements involved in the abscisic acid responsiveness, ABRE), 2 ACE (cis-acting elements involved in light responsiveness), ATC-motif (part of a conserved DNA module involved in light responsiveness), Box II (part of a light responsive element), 7 G-Box (cis-acting regulatory elements involved in light responsiveness), GAG-motif (part of a light responsive element), GARE-motif (gibberellin-responsive element), HES (cis-acting element involved in heat stress responsiveness), I-box (part of a light responsive element), LTR (cis-acting element involved in low-temperature responsiveness), NON-box (cis-acting regulatory element related to meristem specific activation), o2-site (cis-acting regulatory element involved in zein metabolism regulation), Skn-1-motif (cis-acting regulatory element required for endosperm expression), Sp1 (light responsive element), TC-rich repeats (cis-acting element involved in defence and stress responsiveness), TCCACCT-motif, TCCC-motif (part of a light responsive element), W-box, chs-CMA2a (part of a light responsive element) and circadian (cis-acting regulatory element involved in circadian control). The 12 kinds of cis-elements found in the predicted promoter region were related to stress responses (). The existence of these stress-related cis-elements provided evidence that the OsSOT9 promoter region responded to various kinds of stress signals. The expression of OsSOT9 is regulated by several stress factors.
Bioinformatics data showed that the OsSOT9 protein contained a conservative domain of the sulphotransferase family gene, which is consistent with the sequence alignment result ().
Phylogenetic analysis of OsSOT9
The database search and analysis with BLASTP through the NCBI website showed that the deduced amino acid sequence of OsSOT9 had higher homology (99%) with hypothetical protein OsI_EAZ04648.1 from indica and also had homology (94%) with OsJ_NP_001060227 from japonica. It shared high identity (78%) with cytosolic sulphotransferase (Ob_XP_006658788.1) from Oryza brachyantha. BLASTP also showed that OsSOT9 shared high identity with sulphotransferase from other plant species. The percentages were from 69% to 41%. Such examples were 69% (At_EMT24568.1), 67% (Zm_NP_001146492.1), 47% (Pd_XP_008777580.1) and so on (S in the Online Supplementary Appendix). Genes encoding members of the SOTs family have been recently reported in a number of plants, including Oryza sativa L.,[Citation16] Arabidopsis thaliana,[Citation34] Brassica rapa,[Citation35,Citation36] Brassica napus L.,[Citation18,Citation27] Flaveria chloraefolia [Citation22] and Turnera krapovickasii.[Citation37] Comparison of OsSOT9 with several similar proteins from rice and other plant species revealed that sulphotransferase domain is highly conserved in these proteins, which suggested that they have the same functions, associated with response to stress.[Citation21]
A list of putative sulphotransferases was compiled by searching databases for ORFs (open reading frames) with characteristic sequences that are highly conserved among SOT domains. To elucidate the phylogenetic relationships between the deduced protein sequences of OsSOT9 and several known plant SOT genes and the SOT family genes in rice, a phylogenetic analysis was performed using full-length protein sequences (). The phylogenetic tree showed that OsSOT9 had the highest homology with cytosolic sulphotransferase (Ob_XP_006658788.1), indicating that OsSOT9 is a putative cytosolic sulphotransferase gene.
Figure 5. Phylogenetic alignment of the OsSOT9 amino acid sequence with other plant sulphotransferase proteins and corresponding sequences from rice.
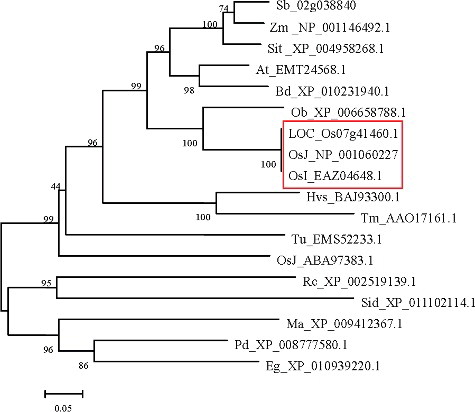
Up to now, 35 SOTs in rice have been identified, but little is known about their molecular mechanism.[Citation10] However, 343 amino acids were found in OsSOT9 gene in our study. The number of amino acids in this gene was similar to that in SOT in Arabidopsis.[Citation11] By integrating this result with the research on protein-binding domains () and phylogenetic tree in this paper (), it can be seen that OsSOT9 should be one of the SOTs in rice.
A few of AtSOT have been studied, such as AtSOT12/At2g03760.[Citation8] Its gene expression could be strongly induced by salt, osmotic stress and hormone treatments. Overexpressing plants were more resistant to pathogen infection.[Citation8] The transcriptional level of OsSOT9 showed that it responded strongly to abiotic stresses (). This was also discovered by the results of promoter sequences of abiotic stress responding genes that OsSOT9 contains cis-regulatory elements responding to abiotic stress ().
The SOTs can be divided into membrane-bound proteins and soluble cytosolic proteins,[Citation21] but only a few membrane-bound SOTs have been characterized in plants up to now.[Citation21] They are either bound to the cell membrane, as shown for the gallic acid glucoside SOT from Mimosa pudica L.,[Citation19] or localized in the Golgi apparatus, as shown for the tyrosylprotein SOTs (TPSTs) from Asparagus officinalis L. [Citation38] and Arabidopsis thaliana (L.) Heynh.[Citation39] A study showed that the size of the membrane SOT protein was 42 kDa in plants, whereas most soluble plant SOT proteins have only a molecular mass of about 35 kDa.[Citation40] The difference might reflect the addition of a transmembrane domain.[Citation19] However, the accurate localization of most cytosolic plant SOTs still remains unknown.[Citation40,Citation41] Therefore, the intracellular localization should be investigated experimentally.
Conclusions
This paper described the identification and molecular characterization of a new stress responsive gene OsSOT9 from Pei'ai 64S, whose expression in leafs at booting and flowering stage was up-regulated after cold treatment. The complete Pei'ai 64S cDNA was cloned and its length was 1469 bp with 50% G + C contents. The longest open reading frame encoded a putative protein of 343 amino acids, with a calculated molecular mass of 37.78 kDa and an isoelectric point of 6.6. The 12 kinds of cis-elements that were found in the predicted promoter region and were related to stress responses further certified that OsSOT9 is related to stress tolerance. All these results lay a foundation for future function analysis of OsSOT9 gene.
SUPPLEMENTARY_APPENDIX.pdf
Download PDF (439.9 KB)Acknowledgments
We thank research assistant Xiaomei Jia for offering help for revising this manuscript.
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/13102818.2015.1136237.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Jain M, Nijhawan A, Arora R, et al. F-box proteins in rice. Genome-wide analysis, classification, temporal and spatial gene expression during panicle and seed development, and regulation by light and abiotic stress. Plant Physiol. 2007;143(4):1467–1483.
- Liu SB, Cao XF, Liao YR, et al. Identification and characterization of a novel abiotic stress responsive ATPase gene from rice. Plant Omics. 2015;8(2):169–177.
- Nakashima K, Ito Y, Yamaguchi-Shinozaki K. Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiol. 2009;149(1):88–95.
- Chen RJ, Qiu JD, Jiang YY, et al. Isolation of a novel MYB transcription factor OsMyb1R from rice and analysis of the response of this gene to abiotic stresses. Afr J Biotechnol. 2012;11(16):3731–3737.
- Yokoi S, Bressan RA, Hasegawa PM. Salt stress tolerance of plants. JIRCAS Work Rep. 2002;23(1):25–33.
- Beck EH, Fettig S, Knake C, et al. Specific and unspecific responses of plants to cold and drought stress. J Biosci. 2007;32(3):501–510.
- Duan ZQ, Bai L, Zhao ZG, et al. Drought-stimulated activity of plasma membrane nicotinamide adenine dinucleotide phosphate oxidase and its catalytic properties in rice. J Integr Plant Biol. 2009;51(12):1104–1115.
- Baek D, Pathange P, Chung JS, et al. A stress-inducible sulphotransferase sulphonates salicylic acid and confers pathogen resistance in Arabidopsis. Plant Cell Environ. 2010;33(8):1383–1392.
- Strott CA. Sulfonation and molecular action. Endocr Rev. 2002;23(5):703–732.
- Hernández-Sebastià C, Varin L, Marsolais F. Sulfotransferases from plants, algae and phototrophic bacteria, Chapter 6. In: Hell R, Dahl C, Knaff D, and Leustek T, editors. Sulfur metabolism in phototrophic organism: Advances in photosynthesis and respiration, vol 27. Dordrecht: Springer; 2008. p. 111–130.
- Klein M, Papenbrock J. The multi-protein family of Arabidopsis sulphotransferases and their relatives in other plant species. J Exp Bot. 2004;55(404):1809–1820.
- Klein M, Papenbrock J. Sulfur assimilation and abiotic stress in plants. Berlin: Springer; 2008. Chapter 7, Sulfotransferases and their role in glucosinolate biosynthesis; p. 149–166.
- Gidda SK, Varin L. Biochemical and molecular characterization of flavonoid 7-sulfotransferase from Arabidopsis thaliana. Plant Physio Biochem. 2006;44(11–12):628–636.
- Hempel F, Bozarth A, Sommer MS, et al. Transport of nuclear-encoded proteins into secondarily evolved plastids. Biol Chem. 2007;388(9):899–906.
- Nowell S, Falany CN. Pharmacogenetics of human cytosolic sulfotransferases. Oncogene. 2006;25(11):1673–1678.
- Chen RJ, Jiang YY, Dong JL, et al. Genome-wide analysis and environmental response profiling of SOT family genes in rice (Oryza sativa). Genes Genomics. 2012;34(5):549–560.
- Ananvoranich S, Varin L, Gulick P, et al. Cloning and regulation of flavonol 3-sulfotransferase in cell-suspension cultures of Flaveria bidentis. Plant Physiol. 1994;106(2):485–491.
- Marsolais F, Gidda SK, Boyd J, et al. Chapter Fourteen Plant soluble sulfotransferases: structural and functional similarity with mammalian enzymes. Recent Adv Phytochem. 2000;34:433–456.
- Varin L, Chamberland H, Lafontaine JG, et al. The enzyme involved in sulfation of the turgorin, gallic acid 4‐O‐(β‐D‐glucopyranosyl‐6′‐sulfate) is pulvini‐localized in Mimosa pudica. Plant J. 1997;12(4):831–837.
- Yang HP, Matsubayashi Y, Nakamura K, et al. Oryza sativa PSK gene encodes a precursor of phytosulfokine-α, a sulfated peptide growth factor found in plants. Proc Natl Acad Sci. 1999;96(23):13560–13565.
- Hirschmann F, Krause F, Papenbrock J. The multi-protein family of sulfotransferases in plants: composition, occurrence, substrate specificity, and functions. Front Plant Sci. 2014;5:556.
- Varin L, DeLuca V, Ibrahim RK, et al. Molecular characterization of two plant flavonol sulfotransferases. Proc Natl Acad Sci. 1992;89(4):1286–1290.
- Varin L, Marsolais F, Brisson N. Chimeric flavonol sulfotransferases define a domain responsible for substrate and position specificities. J Biol Chem. 1995;270(21):12498–12502.
- Gidda SK, Miersch O, Levitin A, et al. Biochemical and molecular characterization of a hydroxyjasmonate sulfotransferase from Arabidopsis thaliana. J Biol Chem. 2003;278(20):17895–17900.
- Lacomme C, Roby D. Molecular cloning of a sulfotransferase in Arabidopsis thaliana and regulation during development and in response to infection with pathogenic bacteria. Plant Mol Biol. 1996;30(5):995–1008.
- Piotrowski M, Schemenewitz A, Lopukhina A, et al. Desulfoglucosinolate sulfotransferases from Arabidopsis thaliana catalyze the final step in the biosynthesis of the glucosinolate core structure. J Biol Chem. 2004;279(49):50717–50725.
- Rouleau M, Marsolais F, Richard M, et al. Inactivation of brassinosteroid biological activity by a salicylate-inducible steroid sulfotransferase from Brassica napus. J Biol Chem. 1999;274(30):20925–20930.
- Michèle R, Frédéric M, Martine R, et al. Inactivation of brassinosteroid biological activity. J Biol Chem. 1999;274:20925–20930.
- Xu M, Chen RJ, Rocha P, et al. Expression and cloning of a novel stress responsive gene (OsMSR1) in rice. Acta Agronomica Sinica 2008;34(10):1712–1718.
- Dong JL, Jiang YY, Chen RJ, et al. Isolation of a novel xyloglucan endotransglucosylase (OsXET9) gene from rice and analysis of the response of this gene to abiotic stresses. Afr J Biotechnol. 2011;10(76):17424–17434.
- Jiang YY, Dong JL, Chen RJ, et al. Isolation of a novel PP2C gene from rice and its response to abiotic stresses. Afr J Biotechnol. 2011;10(37):7143–7154.
- Chen RJ, Dong JL, Liu SB, et al. Isolation of a novel abscisic acid stress ripening (OsASR) gene from rice and analysis of the response of this gene to abiotic stresses. Afr J Biotechnol. 2012;11(74):13873–13881.
- Zhao Y, Wang JW. Characterization of duck enteritis virus US6, US7 and US8 gene. Intervirology. 2010;53(3):141–145.
- Hashiguchi T, Sakakibara Y, Hara Y, et al. Identification and characterization of a novel kaempferol sulfotransferase from Arabidopsis thaliana. Biochem Biophys Res Commun. 2013;434(4):829–835.
- Zang YX, Kim HU, Kim JA, et al. Genome‐wide identification of glucosinolate synthesis genes in Brassica rapa. FEBS J. 2009;276(13):3559–3574.
- Wang H, Wu J, Sun S, et al. Glucosinolate biosynthetic genes in Brassica rapa. Gene. 2011;487(2):135–142.
- Labonne JJ, Goultiaeva A, Shore JS. High-resolution mapping of the S-locus in Turnera leads to the discovery of three genes tightly associated with the S-alleles. Mol Genet Genomics. 2009;281(6):673–685.
- Hanai H, Nakayama D, Yang H, et al. Existence of a plant tyrosylprotein sulfotransferase: novel plant enzyme catalyzing tyrosine O-sulfation of preprophytosulfokine variants in vitro. FEBS Lett. 2000;470(2):97–101.
- Komori R, Amano Y, Ogawa-Ohnishi M, et al. Identification of tyrosylprotein sulfotransferase in Arabidopsis. Proc Natl Acad Sci. 2009;106(35):15067–15072.
- Bauer M, Dietrich C, Nowak K, et al. Intracellular localization of Arabidopsis sulfurtransferases. Plant Physiol. 2004;135(2):916–926.
- Nowak K, Luniak N, Meyer S, et al. Fluorescent proteins in poplar: a useful tool to study promoter function and protein localization. Plant Biol. 2004;6(1):65–73.