ABSTRACT
The aim of this study was to investigate the effects of calcium silicate-based products on cytotoxicity in the 3T3 fibroblast and gelatinolytic activity of matrix metalloproteinases (MMPs). 3T3 fibroblasts were incubated directly with Ortho Mineral trioxide aggregate (MTA), BioAggregate, Biodentine, MTA Plus, MTA Angelus and MTA Cerkamed for 24 hours and seven days. The cytotoxicity was determined using an MTT assay. Supernatants were collected to determine MMP-2 and MMP-9. Data were analysed using IBM SPSS 22. Seventh day extracts of Ortho MTA and Biodentine showed reduced cell viability. Specific characterization of MMPs in cell culture demonstrated that MMP-2 (62 kPa) in the cell culture supernatants by gelatin zymography showed induced expression in four out of seven groups by 3T3 cells. No MMP-9 expression was observed. The cytotoxicity of materials revealed a significant difference in cell viability between the groups on the first and seventh days. The results of this study revealed minor cytotoxic effects for Ortho MTA and Biodentine. This study suggests that endodontic sealers induced production of MMP-2. MMP-9 might be expressed in small amounts when compared with MMP-2.
Introduction
The selection of root repair material is a key point in performing a successful long-term root perforation treatment. For this purpose, the use of new commercially available calcium silicate-based products has become popular in dentistry; however, these materials have not met the requirements of an ideal root repair material, and efforts to develop new materials continue.
Mineral trioxide aggregate (MTA, Loma Linda University, Loma Linda, CA, USA) was developed by Dr Mahmoud Torabinejad in the early 1990s as a root repair material for lateral root perforations.[Citation1] MTA has become one of the standard materials for the treatment of perforations, pulp capping and retrograde filling because of its ability to induce the formation of mineralized tissue.[Citation2] However, MTA also has undesirable physical and chemical properties, such as solubilization,[Citation3] difficult handling properties, long setting time [Citation4] and the potential to discolour tooth structures.[Citation5] Recently, endodontic materials, BioAggregate (Innovative Bioceramix, Vancouver, BC, Canada) and Biodentine (Septodont, Saint-Maur-des-Fosses, France) were developed based on tricalcium silicate with Active Biosilicate Technology attempting to improve on the physicochemical and biological properties.[Citation6]
Cell culture techniques are useful for the evaluation of the biocompatibility of different materials. Amongst in vitro assays, in vitro cytotoxic tests are simple, reproducible, relevant and suitable for evaluation of basic biologic properties.[Citation7] Cytotoxicity can be determined using the MTT-based colorimetric assay. MTT is 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide and exists as a yellow tetrazolium salt. This test indicates the number of viable cells and the level of metabolic activity in a sample.[Citation8]
Several studies have reported that endodontic materials can activate matrix metalloproteinases (MMPs), which play an important role in the pathogenesis of periapical inflammation.[Citation9,Citation10] Degeneration of matrix proteins is thought to occur in periapical inflammation, and matrix turnover requires the activity of many different endopeptidases. MMPs are a family of zinc-dependent enzymes that play an important role in the process of degrading extracellular matrix components.
MMP-2 [Citation11] and MMP-9,[Citation12,Citation13] sometimes referred to as gelatinases, are of particular interest because some studies suggest that these MMPs play an important role in the pathogenesis of chronic inflammatory and in pulp, periodontal and periapical tissue destruction.[Citation14]
Although there are some studies in literature that evaluate the cytotoxicity of different endodontic materials on fibroblasts, to date, the interactions of root repair materials and fibroblasts, as well as MMP expression under these conditions, are still not well elucidated.[Citation9,Citation10] However, no reports have evaluated the gelatinolytic activity induced by these new materials. Thus, the aim of this study was to assess and compare the cytotoxicity and the gelatinolytic activity of MMP-2 and MMP-9 produced by 3T3 fibroblasts after stimulation with seven different tricalcium silicate-based endodontic materials. The null hypothesis tested was that root repair materials have no effect on the expression of MMP-2 and MMP-9 in 3T3 fibroblasts.
Materials and methods
Sample preparation
The materials were prepared in accordance with the manufacturer's instructions. The test materials evaluated in this study and their compositions are listed in . Samples (2 mm in diameter, 8 mm in height) were prepared under aseptic conditions and inserted into sterile stainless steel plates. Subsequently, the disc-shaped specimens were incubated at 37 °C for 48 hours in 100% humidity. After complete setting, sterilization was achieved by UV light for 20 minutes and the materials were placed in serum-free Dulbecco's Modified Eagle's Medium (DMEM/F-12) (Gibco, Grand Island, NY, USA) using a 1.25 cm2/mL ratio between the sample surfaces and the medium volume. Undiluted extracts were used for the test.
Table 1. Materials tested and their composition.
Cell culture
Mouse 3T3 fibroblast cells from the American Type Culture Collection were cultured in DMEM/F-12 and supplemented with 15% foetal bovine serum (Gibco), penicillin (100 units/mL) and streptomycin (100 units/mL) at 37 ºC in 5% CO2. Cells (3 × 105 per well) were distributed in each well of the six-well plates and were allowed to become 80% confluent. The cells in the control group (3 × 105 cells), which was not exposed to the test materials, were cultured for 24 hours. After this time, a fresh DMEM/F-12 without serum was added to the cells. The cells in the test group were cultured for 24 hours, at which time the culture medium was replaced with fresh DMEM/F-12 without serum. At the end of 24 hours, the undiluted extracts were collected and used for gelatinase zymography and MTT assay for cytotoxicity testing.
Cytotoxicity testing
Cell viability was determined with MTT assay. 3T3 cells were seeded in DMEM/F-12 containing 15% foetal bovine serum (complete culture medium), at a density of 1 × 105 cells/cm2, in 24-well culture plates. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) stock solution was prepared as 1 mg/mL in complete medium. Then, 0.6 mL of this solution was added to each well, and cells were incubated in a humidified atmosphere of 5% CO2 in air at 37 ºC for four hours. After the incubation period, the supernatant was removed, the dark blue formazan crystals were dissolved in 0.6 mL of ethanol and the plates were shaken for five minutes. The blue solution was transferred to a 96-well plate, and the optical densities were read at 570 nm in an enzyme-linked Immunosorbent assay (ELISA) multi-well spectrophotometer. The relative cell viability of the test materials were calculated using the following formula: relative cell viability of the test materials (%) = (optical density of the test material/optical density of the negative control) × 100.
Zymography
Gelatinase zymography was performed with 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) containing 0.1% gelatin to determine the activities of MMP-2 and MMP-9 galactosidase (Mr 116 kDa), phosphorylase b (Mr 97 kDa), conalbumin (Mr 75 kDa), ovalbumin (Mr 45 kDa), carbonic anhydrase (Mr 29 kDa) and ribonuclease (Mr 13.7 kDa), which were used as molecular standards. Aliquots of 15 µL supernatants were mixed with sample buffer without reducing agents and were loaded on the gel. Following electrophoresis, the gel was incubated with 10 mL of renaturating buffer (Biorad) for 30 minutes and afterward with the 10 mL developing buffer (Biorad) for 40 minutes. The developing buffer was removed and a fresh 10 mL of new developing buffer was added and allowed to develop overnight at 37 ºC. After incubation, the gel was washed with distilled water 3 × 5 minutes each and stained with 0.5% Coomassie brillant blue in 50% methanol and 10% glacial acetic acid for 30 minutes. Molecular weights of the bands were determined with the mobilities of the standard proteins. Gelatinolytic activities were visualized as unstained bands against the background of Coomassie blue stained gelatin. After staining, the gel was scanned and the intensities of the bands were measured using ImageJ software.[Citation15]
Microscopic evaluation
3 × 105 cells were exposed to 400 µL undiluted extract for 24 hours. After incubation, the cells were analysed and photographed to observe cell morphology and cell density close to the biomaterials under a microscope at ×40 (Eclipse TS100, Nikon, Tokyo, Japan).
Statistical analysis
The experiment was repeated three times independently and the data were analysed using the statistical software IBM SPSS 22 (IBM SPSS, Turkey). The Kruskal–Wallis test was used to compare the results between groups. The Mann–Whitney post hoc multiple comparisons U test was used to assess the causes of differences between groups. Data were analysed for each group using the Wilcoxon sign test. The significance level used was p < 0.01.
Results and discussion
Cytotoxicity results
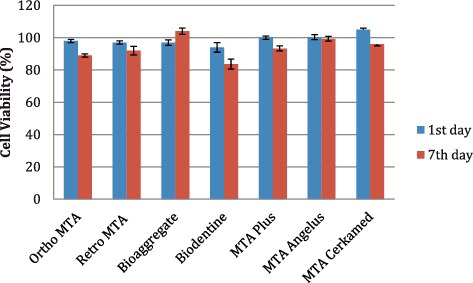
The results of the MTT assay on the cell viability of 3T3 fibroblasts derived from different materials after the first and seventh days of incubation are summarized in . The absorption value obtained with the control cells was adjusted to 100% viability. Cytotoxicity was rated based on cell viability relative to the control group: non-cytotoxic >90%, slightly cytotoxic 60%--90%, moderately cytotoxic 30%--59% and severely cytotoxic <30% cell viability.
Table 2. Assessing the cell viability of 3T3 cells after culture with one and seven days of materials (AVG: average, SD: standard deviation).
As a result of the MTT assays on the first day, there were significant differences in cell viability between the groups (p:0.008; p < 0.01). Whilst MTA Cerkamed showed the highest cell viability among the materials, Biodentine had the least cell viability. After seven days, there were significant differences in the results between the groups (p:0.004; p < 0.01). The most cell viability was observed in BioAggregate, MTA Angelus and MTA Cerkamed. Ortho MTA (89%) and Biodentine (83%) materials were rated as slightly cytotoxic. The difference of mean cell viability after one day and seven days was significantly different (p:0.003; p < 0.01) (, ). Biodentine revealed the lowest level of cell viability compared with the other materials on both days. Ortho MTA, MTA Cerkamed and MTA Plus induced a significant reduction in cell viability. However, BioAggregate showed increased cell viability.
Figure 1. Cytotoxic effects of Ortho MTA, Retro MTA, BioAggregate, Biodentine, MTA Plus, MTA Angelus and MTA Cerkamed upon cultured 3T3 fibroblast cells, one and seven days after. Results are shown in mean percentage from three independent experiments. The error bars represent the standard deviation (SD) (*p < 0.05).
Zymography results
As shown in , gelatinolytic activity of MMP-9 expression was not detectable following treatment with materials. However, MMP-2 (62 kPa) in the cell culture supernatants by gelatin zymography showed induced expression in four out of seven groups by 3T3 cells (). There was a statistically significant difference between the groups in the MMP-2 expression (p:0.006; p < 0.01). The average of the MMP-2 expression of MTA Cerkamed was the highest and Ortho MTA was the lowest between the material groups. There were no other differences between materials group (p > 0.05).
Figure 2. Effect of the Ortho MTA, Retro MTA, BioAggregate, Biodentine, MTA Plus, MTA Angelus and MTA Cerkamed on the production of MMP-2 and MMP-9 expression as determined gelatin zymogram.
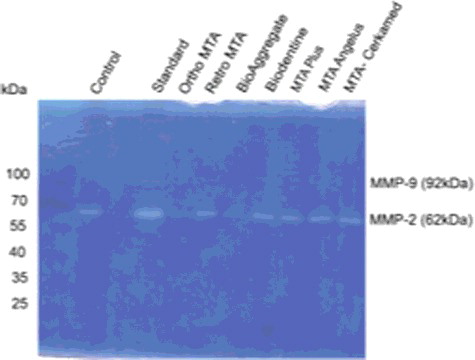
Table 3. MMP-2 levels of the conditioned medium from the 3T3 cells contact with materials (AVG: average, SD: standard deviation).
Microscopy results
The morphology of control and 3T3 cells with eluates from the materials were utilized under light microscopy (). It was observed that there was no significant alteration in the cell morphology of 3T3 cells compared with the controls.
Figure 3. Microscopic pictures of 3T3 cell culture following exposure of the Ortho MTA (a), Retro MTA (b), BioAggregate (c), Biodentine (d), MTA Plus (e), MTA Angelus (f), MTA Cerkamed (g) and control group (h).
Root repair materials used in endodontics are frequently placed in direct contact with periodontal tissues and, thus, these materials should be biocompatible and have non-toxic characteristics.[Citation2] Endodontic materials can release soluble components that may be diluted by tissue fluids and then carried to the surrounding cells and tissues, which can cause degeneration of the periapical tissue and delay wound healing.[Citation16] For this purpose, we evaluated and compared the cytotoxicity and gelatinolytic activity of seven commercially available root repair materials (Ortho MTA, Retro MTA, BioAggregate, Biodentine, MTA Plus, MTA Angelus and MTA Cerkamed) that are widely used in endodontics practice.
MTA has been advocated to be used in various clinical applications, such as root resorption, root perforations, apexification, retrograde filling, pulp capping and pulpotomy in paediatric dentistry.[Citation1–4] In addition, BioAggregate and Biodentine materials have recently been developed that can also be used as a dentine replacement both in the coronal and root regions.[Citation17,Citation18] Biological compatibility of these materials have a great importance as cytotoxic materials may cause inflammatory reactions when in direct contact with both soft and hard tissues.[Citation1,Citation9,Citation10] Therefore, the success of root canal therapy relies on the cytocompatibility of the repair materials.[Citation19]
Several methods are available for cytotoxicity testing of materials. The MTT assay is a standard assay for evaluating the cytotoxicity of new biomaterials.[Citation17,Citation20] 3T3 fibroblasts are routinely used for cytotoxicity tests; cultures of these cells have been used in previous studies.[Citation21] In this study, we first examined cultures on the first and seventh days of the incubation periods. One-day exposure allowed for the immediate biologic behaviour of the material, which characterizes the setting process and its adverse effects. Seven-day exposure period highlights the cell behaviour over a regular interval time to characterize a degenerative or reparative process.[Citation17] 3T3 cells were chosen to demonstrate the toxicity potential assessment of selected materials as they are frequently used for such testing.[Citation10,Citation22,Citation23] Moreover, in this study, the gelotinase activities were evaluated using zymography. MMPs seem to be an important group for the progression of the inflammatory process. MMP-2 and MMP-9 are of particular interest because they are synthesized by fibroblasts and pulp cells, and have been implicated in the pathogenesis of periodontitis, oral carcinogenesis and pulpal inflammation.[Citation9–12,Citation24] Researchers stated that endodontic materials could induce the production of MMP-2.[Citation9,Citation10,Citation25] Our results revealed that 3T3 cells did not produce MMP-9, but they did produce MMP-2. The average of the MMP-2 expression of Ortho MTA and BioAggregate was lower than other groups. This MMP-2 expression may be related to the induction of extracellular matrix proteolysis, and it seems to be associated with the initiating event that allows for the progression of the inflammatory process.[Citation25] Recently, Silva et al. have reported that fibroblasts do not secrete MMP-9,[Citation9] and previous studies have shown that MMP-9 can be expressed in little amounts when compared to MMP-2.[Citation11,Citation12]
Statistical analyses of the MTT assay data revealed a significant difference amongst the materials for the first and seventh day (p:0.008; p:0.004; p < 0.01). However, there was no significant difference revealed between the first and seventh day for the same material. Ortho MTA and Biodentine showed slight cytotoxicity on 3T3 fibroblast cells. These results are in agreement with those found in previous cytotoxicity studies.[Citation26,Citation27] BioAggregate showed no cytotoxic effect in the first day extracts. Seventh day extracts of BioAggregate showed higher cell viability than the first day extracts. This result also has agreement with those found in previous studies.[Citation18,Citation28,Citation29] These differences between materials are explained by the differences in the initial amount of various ions released from these materials. One possible explanation of better cell viability and low MMP-2 expression of BioAggregate could be related to the different ingredients of the materials. The major difference between MTA is that BioAggregate is aluminium-free and contains a significant amount of tantalum oxide instead of bismuth oxide and calcium phosphate.[Citation30,Citation31] This aluminium-free property might increase its biocompatibility.
Many in vitro studies have also shown that BioAggregate exhibits potent antimicrobial action,[Citation32] excellent biocompatibility [Citation33] and significant induction of bone and periodontal regeneration.[Citation34] Even though MTA and BioAggregate have similar compositions and usages, BioAggregate was recently shown to display superior local and systemic biocompatibility in vivo compared with MTA.[Citation35]
With respect to root repair materials, the cytotoxicity of materials used during treatment is of great concern because damage or irritation could cause degeneration of the dental pulp and periradicular tissues since they directly contact the pulpal tissues. To promote healing and functional recovery of the pulp, these materials should either stimulate cell viability or be biologically neutral.[Citation36] Our results show that MTA-derived materials could enhance the cell viability of 3T3 cells in vitro. Recent studies showed that the cytotoxic effects imposed by Ortho MTA,[Citation26,Citation27] Retro MTA,[Citation37] BioAggregate,[Citation18,Citation28,Citation29] Biodentine,[Citation26,Citation28,Citation38,Citation39] MTA Plus [Citation40] and MTA Angelus exhibited negligible cytotoxicity.[Citation41,Citation42] While the extracts of BioAggregate promoted cell viability, Biodentine showed a reduced number of cells. The overall effects of BioAggregate seemed to be better than those of MTA and Biodentine, and to indicate a better cytocompatibility pattern. The microscopy findings of this study revealed that the tested materials did not induce cytotoxicity on 3T3 fibroblast cells.
Conclusions
The results of the present study show that, among the tested materials, Ortho MTA and Biodentine had mild cytotoxicity. No cytotoxicity was observed with Retro MTA, BioAggregate, MTA Angelus, MTA Plus and MTA Cerkamed. However, BioAggregate showed better cell viability compared with MTA-derived materials. Thus, BioAggregate appears to be a possible alternative to MTA for root repair treatment. There was no effect on the MMP-9 in 3T3 fibroblasts.
Disclosure statement
The authors deny any conflicts of interest related to this study.
Funding
This research was supported by the Research Fund of Istanbul University [project number 39686].
References
- Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999;25:197–205.
- Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review-part II: leakage and biocompatibility. J Endod. 2010;36:190–202.
- Bodanezi A, Carvalho N, Silva D, et al. Immediate and delayed solubility of mineral trioxide aggregate and Portland cement. J Appl Oral Sci. 2008;16:127–131.
- Zapf AM, Chedella SCV, Berzins DW. Effect of additives on mineral trioxide aggregate setting reaction product formation. J Endod. 2015;41:88–91.
- Berger T, Baratz AZ, Gutmann JL. In vitro investigations into the etiology of mineral trioxide tooth staining. J Conserv Dent. 2014;17:526–530.
- Jefferies SR. Bioactive and biomimetic restorative materials: a comprehensive review. Part I. J Esthet Restor Dent. 2014;26:14–26.
- Ribeiro DA, Matsumoto MA, Duarte MA, et al. In vitro biocompatibility tests of two commercial types of mineral trioxide aggregate. Braz Oral Res. 2005;19:183–187.
- Kasugai S, Hasegawa N, Ogura H. A simple in vitro cytotoxicity test using the MTT (3-(4,5)-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) colorimetric assay: analysis of eugenol toxicity on dental pulp cells (RPC-C2A). Japan J Pharmacol. 1990;52:95–100.
- Silva EJ, Accorsi-Mendonça T, Almeida JF, et al. Evaluation of cytotoxicity and up-regulation of gelatinases in human fibroblast cells by four root canal sealers. Int Endod J. 2012;45:49–56.
- Silva EJ, Herrera DR, Almeida JF, et al. Evaluation of cytotoxicity and up-regulation of gelatinases in fibroblast cells by three root repair materials. Int Endod J. 2012;45:815–820.
- Chang YC, Yang SF, Hsieh YS. Regulation of matrix metalloproteinase-2 production by cytokines and pharmacological agents in human pulp cell cultures. J Endod. 2001;27:679–682.
- Tsai CH, Chen YJ, Huang FM, et al. The upregulation of matrix metalloproteinase-9 in inflamed human dental pulps. J Endod. 2005;31:860–862.
- Zehnder M, Wegehaupt FJ, Attin T. A first study on the usefulness of matrix metalloproteinase 9 from dentinal fluid to indicate pulp inflammation. J Endod. 2011;37:17–20.
- Corotti MV, Zambuzzi WF, Paiva KB, et al. Immunolocalization of matrix metalloproteinases-2 and -9 during apical periodontitis development. Arch Oral Biol. 2009;54:764–771.
- Kupai K, Szucs G, Cseh S, et al. Matrix metalloproteinase activity assays: importance of zymography. J Pharmacol Toxicol Methods. 2010;61:205–209.
- De Deus G, Ximenes R, Gurgel-Filho ED, et al. Cytotoxicity of MTA and Portland cement on human ECV 304 endothelial cells. Int Endod J. 2005;38:604–609.
- Attik GN, Hallay F, Pradelle-Plasse N, et al. In vitro biocompatibility of a dentine substitute cement on human MG63 osteoblast cells: Biodentine™ versus MTA®. Int Endod J. 2014;47(12):1133–1141.
- Yan P, Yuan Z, Jiang H, et al. Effect of bioaggregate on differentiation of human periodontal ligament fibroblasts. Int Endod J. 2010;43:1116–1121.
- Jiang Y, Zheng Q, Zhou X, et al. A comparative study on root canal repair materials: a cytocompatibility assessment in L929 and MG63 cells. Sci World J. 2014;12:463826. Available from: http://doi:10.1155/2014/463826.
- AlAnezi AZ, Jiang J, Safavi KE, et al. Cytotoxicity evaluation of endosequence root repair material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e122–e125.
- Sepet E, Pinar A, Ilhan B, et al. Cytotoxic effects of calcium hydroxide and mineral trioxide aggregate on 3T3 fibroblast cell line in vitro. Quintessence Int. 2009;40:55–61.
- Olivier A, Grobler SR, Osman Y. Cytotoxicity of seven recent dentine bonding agents on mouse 3T3 fibroblast cells. OJST. 2012;2:244–250.
- Spagnuolo G, Desiderio C, Rivieccio V, et al. In vitro cellular detoxification of triethylene glycol dimethacrylate by adduct formation with N-acetylcysteine. Dent Mater. 2013;29:e153–e160.
- Shin SJ, Lee JI, Baek SH, et al. Tissue levels of matrix metalloproteinases in pulps and periapical lesions. J Endod. 2012;28:313–315.
- Huang FM, Yang SF, Chang YC. Up-regulation of gelatinases and tissue type plasminogen activator by root canal sealers in human osteoblastic cells. J Endod. 2008;34:291–294.
- Chang SW, Lee SY, Ann HJ, et al. Effects of calcium silicate endodontic cements on biocompatibility and mineralization-inducing potentials in human dental pulp cells. J Endod. 2014;40:1194–1200.
- Lee BN, Son HJ, Noh HJ, et al. Cytotoxicity of newly developed Ortho MTA root-end filling materials. J Endod. 2012;38:1627–1630.
- Jang YE, Lee BN, Koh JT, et al. Cytotoxicity and physical properties of tricalcium silicate-based endodontic materials. J Korean Acad Conserv Dent. 2014;39(2):89–94.
- Yuan Z, Peng B, Jiang H, et al. Effect of Bioaggregate on mineral-associated gene expression in osteoblast cells. J Endod. 2010;46:1145–1148.
- Park JW, Hong SH, Kim JH, et al. X-ray diffraction analysis on white ProRoot MTA and Diadent BioAggregate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(1):155–158.
- Guven Y, Tuna EB, Dincol ME, et al. X-ray diffraction analysis of MTA-Plus, MTA-Angelus and DiaRoot BioAggregate. Eur J Dent. 2014;8(2):211–215.
- Cavdar Tetik EA, Dartar Öztan M, Kıyan M. Comparison of in vitro antimicrobial activities of bioaggregate and mineral trioxide aggregate. Mikrobiyol Bul. 2013;47(3):523–528. Turkish.
- Simsek N, Alan H, Ahmetoglu F, et al. Assessment of the biocompatibility of mineral trioxide aggregate, Bioaggregate, and Biodentine in the subcutaneous tissue of rats. Niger J Clin Pract. 2015;18(6):739–743.
- Tian J, Qi W, Zhang Y, et al. Bioaggregate inhibits osteoclast differentiation, fusion, and bone resorption in vitro. J Endod. 2015;41(9):1500–1506.
- Khalil WA, Eid NF. Biocompatibility of BioAggregate and mineral trioxide aggregate on the liver and kidney. Int Endod J. 2013;46:730–737.
- Modena KC, Casas-Apayco LC, Atta MT, et al. Cytotoxicity and biocompatibility of direct and indirect pulp capping materials. J Appl Oral Sci. 2009;17:544–554.
- Chung CJ, Kim E, Song M, et al. Effects of two fast-setting calcium-silicate cements on cell viability and angiogenic factor release in human pulp-derived cells. Odontology. 2016;104(2):143–151.
- Khedmat S, Dehghan S, Hadjati J, et al. In vitro cytotoxicity of four calcium silicate-based endodontic cements on human monocytes, a colorimetric MTT assay. Restor Dent Endod. 2014;39:149–154.
- Samyuktha V, Ravikumar P, Nagesh B, et al. Cytotoxicity evaluation of root repair materials in human-cultured periodontal ligament fibroblasts. J Conserv Dent. 2014;17:467–470.
- Ashraf A, Gosier JL, Primus CM, et al. In vitro biocompatibility and oxidative stress profiles of different hydraulic calcium silicate cements. J Endod. 2014;40:255–260.
- Hirschman WR, Wheater MA, Bringas JS, et al. Cytotoxicity comparison of three current direct pulp-capping agents with a new bioceramic root repair putty. J Endod. 2012;38:385–388.
- Poggio C, Ceci M, Beltrami R, et al. Biocompatibility of a new pulp capping cement. Ann Stomatol (Roma). 2014;5:69–76.
