ABSTRACT
Essential oils generally derived from one or more plant parts have been used throughout history for many great applications. In this study, the flue-cured tobacco flower buds were subjected to hydrodistillation in a Clevenger-type apparatus (4 h). The essential oil was characterized by means of gas chromatography–mass spectrometry (GC–MS). The yield of the oil was 0.57% (w/w). After identification of the components, 34 volatile compounds were identified, which contained 55.0% of the oil. β-Cembrenediol (12.24%), carotol (8.55%), isolimonene (7.37%), thunbergol (4.88%) and 9,12-octadecadienoic acid (4.09%) were the major constituents of the oil. The essential oil was also tested for antimicrobial and antioxidant activities. The essential oil was particularly active against Bacillus subtilis, with the lowest Minimal Inhibitory Concentration and Minimum Bactericidal Concentration value (7 and 7 mg/mL). Furthermore, the essential oil and its main compounds showed a strong potent OH scavenging effect, when compared to butylated hydroxytoluene as a positive control. In conclusion, the tobacco flower bud oil is a potential source of novel antioxidant and antimicrobial agents.
Introduction
Tobacco is one of the most commonly studied plants and a very important economic crop. During cultivation, the removal of the inflorescence (called topping) is necessary to break apical dominance and stimulate the tobacco growth. Hence, how to manufacture the useful produce from the tobacco flower bud biomass, as an agricultural residue, becomes a big issue that needs to be solved.[Citation1]
Essential oils from plants are blends of volatile secondary metabolites and possess antibacterial, antioxidative and anti-ageing activities.[Citation2,Citation3] Essential oils are widely used as flavours and preservatives to prevent growth of bacteria and moulds.[Citation4] The extraction of essential oil from tobacco leaves, and the study of the extract's composition, have been reported previously; however, few reports are currently available concerning the essential oil from tobacco flower bud, as well as its antioxidant and antimicrobial activities.[Citation5–Citation9] The aim of this study was to determine the chemical composition of essential oil from tobacco flower bud. In addition, the antioxidant activities, including the superoxide radical scavenging activity, OH radical scavenging activity and reducing power ability, and the antimicrobial activity against proliferation of selected micro-organisms were evaluated.
Materials and methods
Plant materials and chemicals
The flower buds of flue-cured tobacco (voucher specimen: yunyan87) were collected in flowering stage from the Tobacco Planting Area (Guizhou, China) in 2014. The tobacco flower was dried at 45 °C and sieved using a 40-mesh sieve (pore size 0.42 mm). All other chemicals were of analytical reagent grade.
Essential oil distillation
The resulting powder (30 g) was mixed with 72 g sodium chloride and 600 mL distilled water, which was submitted to water distillation for 4 h using an all-glass Clevenger-type apparatus and 0.57% essential oil (w/w) of dry matters were yielded. After decanting the essential oil, when extracted, was collected and distilled using anhydrous sodium sulphate, and kept in refrigerator at −4 °C until it was tested and analysed.[Citation2]
Essential oil analysis
The oil was diluted in pentane and 1 μL was used for analysis. GC analysis was performed on a gas chromatography–mass spectrometry (GC–MS) (Varian Co., model: Star 3600 CX, Lexington, MA, USA) fitted with flame ionization detector and HP-5 MS column (60 m × 0.32 mm × 0.25 µm) with helium as carrier gas at flow rate of 1.0 mL/min. The injection port was maintained at 250 °C, the detector temperature was 300 °C. The split ratio was 6:1 and ionization voltage maintained at 70 eV. The oven was programmed as follows: at 40 °C for 3 min and then increased to 260 °C at 6 °C/min. At this temperature, the column was maintained for 5 min. Identification of components was determined based on their retention indices determined by reference to a homologous series of n-alkanes, and by comparison of their mass spectral fragmentation patterns with those of authentic samples, or with available library data of the GC–MS system (Wiley 2001 data software) and Adams libraries spectra. The compounds with a match quality index greater than 80% were identified.[Citation5,Citation10]
Antimicrobial activity
The paper disc-diffusion (DD) method was employed for the determination of antimicrobial activity of the essential oil.[Citation3] Test fungi or bacteria strains were suspended in the media and the cell densities were adjusted to 0.5 McFarland standards at 530 nm wavelength using a spectrophotometeric method. A suspension (0.1 mL) of the test organism (5 × 106 cells/mL) was spread on solid media plates. Filter paper discs (6 mm in diameter) were individually impregnated with 15 µL of the diluted oil aliquots (140 µg/mL stock). After solvent evaporation, discs were placed on the inoculated plates, for 2 h at 4 °C. The plates were incubated at 37 °C for 24 h for bacteria, and at 28 °C for 48 h for fungal strains. The diameters of the inhibition zones (DD) were measured in millimetres. Each test was carried out in triplicate, and the average was calculated for the inhibition zone diameters.
Minimal Inhibitory Concentration (MIC) was defined as the lowest concentration, which resulted in a reduction of >90% in the observed absorbance. A broth microdilution method was used to determine MIC. All tests were performed in Mueller–Hinton broth and Sabouraud dextrose broth, both supplemented with ethanol at a final concentration of 0.5% (v/v) for both bacteria and fungi, respectively. Serial doubling dilutions of the oils were prepared in a 96-well plate, ranging from 0.0273 to 14 mg/mL. The final concentration of each strain was adjusted to 5 × 106 cells/mL. Plates were incubated at 37 °C for 24 h for bacteria and 30 °C for 24 h for fungi. The micro-organism growth was indicated by the turbidity.[Citation2]
To determine Minimum Bactericidal Concentration (MBC) and Minimal Fungicidal Concentration (MFC), 100 μL of each dilution showing no growth was spread on Mueller–Hinton agar and Sabouraud agar, respectively. The inoculated Petri dishes were incubated at 37 °C for 24 h for bacterial cultures and 30 °C for 48 h for fungal cultures. The colony forming units were counted and compared to control dishes. MBC and MFC were defined as the lowest concentration that killed >99.9% of the initial inoculum.[Citation2] Each test was carried out in triplicates.
Antioxidant activity
For the evaluation of antioxidant activity of essential oil, superoxide radical scavenging activity, OH radical scavenging activity and reducing power ability were determined according to the methods of Qi et al., Singh and Oyaizu, respectively.[Citation11–Citation13] In both assays, the essential oil samples were pre-dissolved in ethanol and tested at various concentrations in parallel with butylated hydroxytoluene (BHT) as a positive control.
Superoxide radicals were generated in a phenazine methosulfate/nicotinamide adenine dinucleotide (PMS/NADH) system for being assayed in the reduction of nitroblue tetrazolium chloride (NBT). The reaction mixture, containing varying concentrations of essential oil, Tris-HCl (16 mmol/L, pH 8.0), NADH (338 µm), NBT (72 µm) and PMS (30 µm), was incubated at room temperature for 5 min and the absorbance was read at 560 nm against a blank. The capability to scavenge superoxide radicals was calculated using the following equation: scavenging effect (%) = [1 − A/A0] × 100%, where A0 is the absorbance of mixture solution without sample; A is the absorbance of the test sample mixed with reaction solution.
As for OH radical scavenging activity assay, the varying concentrations of essential oil were incubated with a solution containing phenanthroline (7.5 mmol/L, 1 mL), phosphate buffer (20 mmol/L, pH 7.4, 0.5 mL), FeSO4 (0.75 mmol/L, 1.5 mL) and H2O2 (3%, 0.5 mL) at 37 ° C for 1 h. The absorbance was measured at 536 nm using UV-2550 spectrophotometer (Shimadzu, Kyoto, Japan). The scavenging activity was calculated using the following equation: OH radical scavenging (or inhibition) rate (%) = (Asample − Ablank)/(Acontrol − Ablank) × 100%, where Asample, Acontrol and Ablank were defined as absorbances of the sample, control (without essential oil) and blank (without H2O2 and essential oil), respectively.
As for the reducing power of essential oil, different concentrations of essential oil (0.5–2.0 mg/mL) were suspended in distilled water and mixed with 2.5 mL of 0.2 mol/L phosphate buffer (pH 6.6), and 2.5 mL of 1% K3Fe(CN)6. The mixture was incubated at 50 °C for 30 min; 2.5 mL of 0.1% ferric chloride was added to the mixture and centrifuged at 3000 rpm for 10 min. The upper layer of the solution (2.5 mL) was mixed with distilled water (2.5 mL) and FeCl3 (0.5 mL, 0.1%), and the absorbance was measured at 700 nm.
Statistical analysis
Experimental results from the experimental design were statistically subjected to analysis of variance using SPSS (version 11.0, Chicago, IL, USA). Probability values <0.05 and <0.01 were regarded as statistically significant and highly significant, respectively.
Results and discussion
Chemical composition of essential oil
The chemical compositions of essential oil from tobacco flower bud are shown in . Ten compounds representing 55.0% of essential oil were identified. The major comprised organic compounds were β-cembrenediol (12.24%), carotol (8.55%), isolimonene (7.37%), thunbergol (4.88%) and 9,12-octadecadienoic acid (4.09%). In another study, Zhang et al. indicated that the major constituent of the essential oil of discarded tobacco leaves was neophytadiene and nicotine,[Citation1] whereas, other studies found solanone and neophytadiene to be the most abundant constituents of oriental tobacco leave oil. These differences might have been derived from local, climatic and seasonal factors.[Citation5,Citation6]
Table 1. Chemical compositions of tobacco flower bud essential oil.
There are a lot of flavour components in the essential oil from tobacco flower bud, such as phenylethyl alcohol, β-damascenone and caryophyllene with floral flavour, 2-pentyl-furan and nonanal with fruity flavours, alpha-farnesene with oolong tea flavour and 1,3,6,10-cyclotetradecatetraene and β-cembrenediol with tobacco flavour.[Citation14,Citation15]
Antimicrobial activity
According to the results demonstrated in , tobacco flower bud essential oil exhibited moderate antimicrobial activity against the tested species in relation to penicillin. The essential oils showed the antimicrobial activity against both type of micro-organisms (MIC 7–14 mg/mL and MBC 7–14 mg/mL for bacteria and MIC 3.5–14 mg/mL and MFC 7–14 μg/mL for fungi. In our study, most of the antimicrobial activity in essential oils from tobacco flower bud appears to be associated with indole and β-cembrenediol.[Citation4,Citation16] Su et al. examined the antimicrobial activity of essential oils from Diospyros discolor and the antimicrobial compounds were determined to be alpha-cadinol, tau-cadinol, tau-muurolol and (2Z,6E)-farnesol.[Citation17] Pansanit and Pripdeevech investigated the antimicrobial activity of volatile components in essential oils from Trachelospermum jasminoides flowers and found citronellol as the major compounds.[Citation18]
Table 2. Antimicrobial activity of the essential oils from tobacco flower bud using MIC and MBC test.
Antioxidant activity
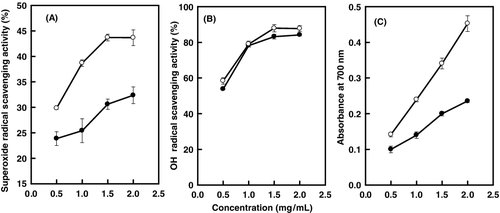
The antioxidant activities of tobacco flower bud essential oil were evaluated by three established in vitro systems, including superoxide radicals, hydroxyl radicals and reducing power ability. As shown in , the essential oil demonstrated concentration-dependent antioxidant activity with the concentration (0.5–1.5 mg/mL for scavenging activities and 0.5–2.0 mg/mL for reducing power ability). The results showed that the superoxide radical scavenging activities and reducing power ability of BHT were stronger than those of the essential oil ((A,C)). The OH radical scavenging activities of BHT and essential oil were similar at every concentration point ((B)). Our findings revealed that the hydroxyl groups and other functional groups, such as COOH, -O- and C=O, can donate electrons to reduce the radicals to a more stable form or react with the free radicals to terminate the radical chain reaction in the chemical compounds of essential oil, such are trans-chrysanthemal, caryophyllene, 6,10,14-trimethyl-2-pentadecanone, therefore the strong antioxidant activity could be attributed to them.[Citation19] Lee et al. investigated the antioxidative effects of volatile components in essential oils from Chrysanthemum indicum Linn flowers and found that thymol had the highest antioxidant capacity.[Citation20] Aliboudhar and Tigrine-Kordjani studied the effect of the extraction technique on the antioxidant property of Anacyclus clavatus flowers essential oil and found that artemisia ketone was the common and major constituent.[Citation21] The results indicated that these differences were derived from the plant classification.
Conclusions
The results in this study proved that the essential oil derived from tobacco flower bud possesses remarkable OH radical scavenging activities. The essential oil revealed higher antibacterial effects than anti-fungal activity. The results obtained expanded the possibilities for application of essential oil from tobacco flower bud not only as aroma product, but even as natural antimicrobial and antioxidant agent.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Zhang X, Gao H, Zhang L, et al. Extraction of essential oil from discarded tobacco leaves by solvent extraction and steam distillation, and identification of its chemical composition. Ind Crops Prod. 2012;39:162–169.
- Yu JQ, Lei JC, Yu H, et al. Chemical composition and antimicrobial activity of the essential oil of Scutellaria barbata. Phytochemistry. 2004;65:881–884.
- Zhu SY, Yang Y, Yu HD, et al. Chemical composition and antimicrobial activity of the essential oils of Chrysanthemum indicum. J Ethnopharmacol. 2005;96:151–158.
- Hamdan DI, Mohamed ME, Abdulla RH, et al. Anti-inflammatory, insecticidal and antimicrobial activities and chemical composition of the essential oils of different plant organs from navel orange (Citrus sinensis (L.) Osbeck var. Malesy) grown in Egypt. J Med Plants Res. 2013;7:1204–1215.
- Alagic S, Stancic I, Palic R, et al. Chemical composition and antimicrobial activity of the essential oil of the oriental tobacco Yaka. J Essential Oil Res. 2002;14:230–232.
- Stojanovic G, Palic R, Alagic S, et al. Chemical composition and antimicrobial activity of the essential oil and CO2 extracts of semi-oriental tobacco, Otlja. Flavour Fragr J. 2000;15:335–338.
- Haouas D, Cioni PL, Halima-Kamel MB, et al. Chemical composition and bioactivities of three Chrysanthemum essential oils against Tribolium confusum (du Val) (Coleoptera: Tenebrionidae). J Pest Sci. 2012;85:367–379.
- Kim KR, Zlatkis A, Park JW, et al. Isolation of essential oils from tobacco by gas co-distillation/solvent extraction. Chromatographia. 1982;15:559–563.
- Palic R, Stojanovic G, Alagic S, et al. Chemical composition and antimicrobial activity of the essential oil and CO2 extracts of the oriental tobacco. Prilep Flavour Fragr J. 2002;17:323–326.
- Adams RP. Identification of essential oil components by gas chromatography/massspectrometry. 4th ed. Carol Stream (IL): Allured Business Media; 2009.
- Qi HM, Zhang QB, Zhao TT, et al. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int J Biol Macromol. 2005;37:195–199.
- Singh A. Physicochemical and physiological aspects. In: CRC handbook of free radicals and antioxidants in biomedicine. Boca Raton (FL): CRC Press Inc.; 1989. p. 123–126.
- Oyaizu M. Studies on product of browning reaction prepared from glucoseamine. Jpn J Nutr. 1986;44:307–315.
- Zhao M. Cigrette flavoring. Beijing: Chemical Industry Press; 2008.
- Liu J, Li Y, Ji X, et al. GC/MS analysis of oxidative degradation of lutein and its flavoring application in cigarette. J Zhengzhou Univ. (Nat Sci.). 2011;2:24–27.
- Guo L, Wu JZ, Han T, et al. Chemical composition, antifungal and antitumor properties of ether extracts of Scapania verrucosa Heeg. and its endophytic fungus Chaetomium fusiforme. Molecules. 2008;13:2114–2125.
- Su YC, Hsu KP, Wang EIC, et al. Composition, in vitro cytotoxic, and antimicrobial activities of the flower essential oil of Diospyros discolor from Taiwan. Nat Prod Commun. 2015;10:1311–1314.
- Pansanit A, Pripdeevech P. Constituents, antibacterial and antioxidant activities of essential oils from Trachelospermum jasminoides flowers. Nat Prod Commun. 2014;9:1791–1794.
- Leung PH, Zhao S, Ho KP, et al. Chemical properties and antioxidant activity of exopolysaccharides from mycelial culture of Cordyceps sinensis fungus Cs-HK. Food Chem. 2009;114:1251–1256.4
- Lee BH, Nam TG, Park WJ, et al. Antioxidative and neuroprotective effects of volatile components in essential oils from Chrysanthemum indicum Linn, flowers. Food Sci Biotechnol. 2014;24:717–723.2
- Aliboudhar H, Tigrine-Kordjani N. Effect of extraction technique on the content and antioxidant activity of crude extract of Anacyclus clavatus flowers and their essential oil composition. Nat Prod Res. 2014;28:2140–2149.