ABSTRACT
A direct-seeded rice cultivation system has been widely adopted in Asian countries. Optimum germination and vigorous seedling growth under submergence are key traits for the practice of direct seeding. We studied the post-germination seedling vigour in Vietnamese lowland rice accessions based on three bio-parameters, shoot elongation growth under five-day submergence in water-filled test-tubes, seedling recovery rate five days after transferring submerged seedlings to pots with soil and seedling survival rate 21 days after sowing seeds in nursery beds and immediate incubation under submergence. A large diversity was found in seedling vigour thus estimated among the accessions. Significantly high correlations were observed among all three bio-parameters, verifying the contribution of seedling vigour to the manifestation of submergence tolerance at this critical stage of rice development. To examine the roles of anoxia/hypoxia-responsive genes, the expression of 17 candidate genes was studied by reverse transcription polymerase chain reaction (RT-PCR) and compared between selected vigorous and non-vigorous groups of accessions. Transcripts of all but two genes showed marked accumulation in submerged seedlings. No differences, however, were found between the two contrasting groups. The observed common and coordinate expression of anoxia/hypoxia-induced genes suggests that they might assume roles in attaining baseline tolerance against submergence stress. It was also suggested that some unknown genetic factors are operating in determining cultivar/genotype-specific levels of submergence tolerance as assessed by post-germination seedling vigour.
Introduction
In the tropical Asian countries, flood submergence during the rainy season is a major constraint causing significant losses in rice production.[Citation1] Rice as a semi-aquatic plant has evolved a variety of adaptive mechanisms to endure submergence stress.[Citation2–13] It has been well established that the quiescence strategy plays a key role in attaining submergence tolerance at the vegetative stage. According to this strategy, tolerance against short-term (within two weeks) but complete submergence is associated with slow growth to conserve a high enough energy level until the floodwater recedes.[Citation14–18] Slow growth has been considered advantageous for rice plants under submergence because active shoot/internode elongation is an energy-consuming process and elongated seedlings tend to lodge as soon as the water level recedes.[Citation4,Citation17,Citation19,Citation20] The quiescence strategy is controlled by a major locus Submergence-1 (SUB1) on rice chromosome 9 that encodes ethylene-responsive-factors.[Citation18,Citation21–23] However, a tolerance-specific allele SUB1A cannot fully explain the differences in the level of submergence tolerance among rice accessions.[Citation24,Citation25] Submergence tolerance at the vegetative stage controlled by SUB1A is not associated with tolerance at germination and the post-germination early seedling stage.[Citation8,Citation10] Moreover, most cultivars/varieties of rice possessing the active SUB1A allele do not show tolerance to submergence at this critical stage of rice development.[Citation26]
The direct-seeded rice cultivation system has received much attention because of its low-input demand and expected economic benefits for the intensification of rice cultivation. Germination and post-germination seedling vigour are the most crucial traits for ensuring optimum and uniform stand establishment under unfavourable conditions.[Citation27,Citation28] Under submergence, in contrast to the phenotype that the quiescence strategy assumes, rice seedlings need to grow rapidly to reach above the water surface for gas exchange and resume active aerobic metabolism and photosynthesis.[Citation8,Citation9,Citation27,Citation29–33] Rapid shoot elongation at the post-germination seedling stage, therefore, can constitute an important trait enabling rice seedlings to escape from submergence stress. Rapid shoot elongation growth, however, should not result in spindly and weak growth but must be accompanied with high vigour, which is needed for submerged seedlings to promptly recover from damage during desubmergence.[Citation13,Citation16]
Precise and efficient phenotypic assay is a prerequisite for accurate evaluation of complex traits like submergence tolerance. The most widely used bioassay for submergence tolerance is the seedling recovery/emergence method, which was adopted in studies for high-resolution mapping and map-based cloning of the SUB1A locus.[Citation22,Citation34,Citation35] However, this method cannot evaluate submergence tolerance at germination and the early seedling stage.[Citation10,Citation36] A test-tube bioassay was developed as a method suitable for the evaluation of post-germination seedling vigour under submergence based on shoot elongation growth in water-filled test-tubes.[Citation37] Using this simple and rapid bioassay, diversity in seedling vigour under submergence could effectively be studied among 150 cultivars of japonica and 17 cultivars of indica, including two deep-water rice accessions.[Citation36] Several quantitative trait loci (QTLs) associated with seedling vigour under submergence could also be detected.[Citation38] In the meanwhile, the International Rice Research Institute (IRRI) in the Philippines developed a large-sale screening method for detecting QTLs associated with seedling survival under submergence.[Citation26] This method is based on the percentage germination and seedling survival based on the number of seedlings that reached the surface of the water after sowing dry seeds in nursery beds and immediate subjection to submergence stress. The method, therefore, reflects the overall submergence tolerance expressed at germination through the post-germination seedling stage. Using this method, additional QTLs have been identified by researchers in IRRI and have been further targeted for molecular cloning and breeding.[Citation12,Citation26,Citation39–41]
At germination and the post-germination seedling stage, dramatic changes occur in plant energy-generating metabolism.[Citation42] Submergence stress is generally confounded with low-oxygen stress that causes a state of anoxia/hypoxia (anaerobiosis) and strongly disturbs normal aerobic metabolism.[Citation7,Citation9–11,Citation43] As oxygen serves as an electron acceptor in mitochondrial oxidative phosphorylation to produce adenosine triphosphate (ATP), anoxia/hypoxia induces a critical shortage of ATP due to the limited availability of oxygen. Rice is a semi-aquatic plant and can cope with anoxia/hypoxia better than most other cereals.[Citation3,Citation5,Citation44] Glycolysis and ethanol fermentation are the major pathways for energy generation during seed germination and early seedling growth under submergence.[Citation12,Citation45] Ethanol fermentation fuels anaerobic growth and occurs in two steps: decarboxylation of pyruvate to acetaldehyde, the process being catalysed by pyruvate decarboxylase (PDC), followed by reduction of acetaldehyde to ethanol with the concomitant oxidation of NADH to NAD+, which is catalysed by alcohol dehydrogenase (ADH). Acetaldehyde produced by PDC is toxic [Citation46] and thus needs to be converted to acetic acid by acetaldehyde dehydrogenase (ALDH). In the low-oxygen signalling for the induction of ADH, increased cytosolic Ca2+ acts as a second messenger in maize.[Citation47,Citation48] Paralogous rice genes, OsHREFs, encoding hypoxia-responsive proteins with EF-hand (helix-loop-helix) motifs may relate to the enhanced Ca2+ response.[Citation49] Other anoxia/hypoxia-responsive rice genes, OsB12D1 encoding a mitochondrion-located protein [Citation50] and SLENDERRICE (SLR1) and SLENDERRICE-like (SLRL1) encoding repressor proteins of gibberellin signalling,[Citation51,Citation52] have also been reported to be induced under submergence.
We herein report our study on the contribution of seedling vigour to submergence tolerance based on three different bio-parameters and the expression of anoxia/hypoxia-responsive genes under submergence at germination and the post-germination early seedling stage in Vietnamese lowland rice accessions. The study is a sequel to the previous reports [Citation36–38,Citation53,Citation54] pursuing an establishment of MAS (marker-assisted-selection) method for the selection of submergence tolerant superior genotypes in direct-seeded rice cultivation.
Materials and methods
Plant materials and bioassay methods for evaluating seedling vigour under submergence
A set of 148 Vietnamese lowland rice accessions obtained from the National Gene Bank of Plant Resource Center of Vietnam (Table S1 in the Online Supplementary Appendix) were subjected to the test-tube bioassay according to [Citation37]. Nipponbare and Kasalath were used as a high and a low seedling vigour japonica and indica control, respectively. Seeds were surface-sterilized in 1% sodium hypochlorite (NaClO) solution for 10 min and rinsed with distilled water three times. The seeds were then allowed to germinate in wet glass Petri dishes (70-mm-diameter) in a dark incubator at 28 ºC for three days. The seeds were washed every day before germination. For each accession, 10 germinating seeds at the pigeon breast stage with normal coleoptile growth were transferred to a glass test-tube filled with 10 cm deep distilled water. The uncovered test-tubes were incubated at 28 ºC in darkness without changing the water for five days. The shoot (coleoptile) lengths were measured from the base to the tip of the shoots at the end of the five-day stress period.
A set of selected 40 accessions that showed shoot length longer than 2.66 cm after the test-tube bioassay were transferred to normal conditions in pots with soil and were incubated at 28 ºC with a 16 h photoperiod for additional five days, and the seedling recovery rate (%) was measured. In parallel, the same set of 40 accessions was subjected to the seedling survival method according to Angaji et al.[Citation26] Ten dry seeds per accession were sown in soil with a depth of 5 mm in nursery beds. After sowing, the nursery beds were immediately submerged with a water depth of 10 cm, the level of which was maintained for three weeks. After the three-week incubation at 28 ºC with a 16 h photoperiod, the seedling survival rate was scored based on the percentage of seedlings that reached the surface of the water.
Data analysis
All experiments were laid out following a randomized complete block design with three replications. Differences within replications and among accessions were compared by the analysis of variance test using Microsoft Excel 2010. Computed F-value was used to test the significance of the treatment effect. The mean values of accessions were compared by least significant differences calculated using the Analyze-it + General 1.68 software. Probability levels lower than 0.05 were held to be significant. Coefficients of determination (R2) were calculated and compared among the three parameters of seedling vigour, i.e. shoot length, seedling recovery rate and seedling survival rate, according to Gomez and Gomez [Citation55].
Reverse transcription polymerase chain reaction (RT-PCR)
RT-PCR was used for assessment of the transcript levels of anoxia/hypoxia-related genes. Four representatives each of vigorous and non-vigorous accessions were selected based on shoot elongation growth by the test-tube bioassay. Nipponbare and Kasalath were used as vigorous and non-vigorous controls. Pre-germinated seeds were subjected to submergence under the same conditions in water-filled test-tubes, and also grown in pots with soil under normal conditions. After five days, seedling tissues were collected and frozen with liquid nitrogen. RNA extraction was done using Trizol reagent (Invitrogen, California, USA). RNA concentration was determined using a spectrophotometer (NanoDrop 2000, Thermo Fisher Scientific, Wilmington, MA, USA). For RT-PCR analysis, the first strand complementary DNA (cDNA) was synthesized from 1 μg of DNaseI-treated RNA with oligo-dT primers using ReverTra Ace kit (TOYOBO, Osaka, Japan). The gene-specific primer sets were designed and synthesized by Life Technologies (Invitrogen, California, USA) (). The rice ubiquitin gene was used as an internal control. For each sample, 4 μL of cDNA template was added to 16 μL of reaction mixture containing 1 μL of each forward and reverse primer, 2 μL of PCR buffer (10X DreamTaq buffer, Thermo Scientific, Foster, CA, USA containing 20 mmol/L MgCl2), 1 μL of 2.5 mmol/L deoxynucleoside triphosphates, 10 μL of MiliQ-water (Merck Millipore, Darmstadt, Germany) and 1 μL (5 U/μL) DreamTaq DNA Polymerase (Thermo Scientific, USA) to make a 20 μL PCR mixture. Negative control containing all the reaction components except for reverse transcriptase was included in the amplification to ensure no genomic DNA contamination. RT-PCR was performed in 0.2mL PCR tube strips using the Eppendorf thermocycler (Eppendorf AG, Hamburg, Germany). Different amplification cycles were applied to different paralogs. RT-PCR products together with 100 bp DNA ladder (Nacalai Tesque, Kyoto, Japan) were separated by electrophoresis in 0.9% agarose gel. After electrophoresis, the gel was stained with ethidium bromide in the darkness for 30 min. The gels were scanned on the high performance ultraviolet transilluminator (UVP, USA) and photo images were saved on a computer.
Table 1. List of primer sets for RT-PCR analysis.
Results and discussion
Contribution of seedling vigour to submergence tolerance and its diversity in Vietnamese lowland rice germplasm
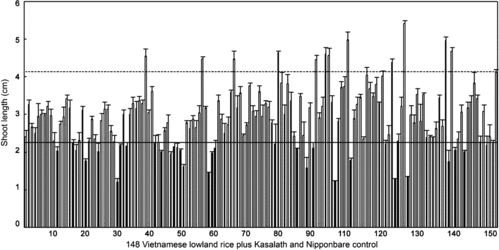
Rapid and vigorous shoot growth is a critical trait for ensuring the survival of submerged rice seedlings, particularly at the early seedling stage.[Citation8,Citation9,Citation27,Citation29–32,Citation36,Citation37] Submergence greatly hinders the post-germination seedling vigour and prevents stressed seedlings from prompt recovery during desubmergence.[Citation13] Seedling vigour under submergence and recovery during desubmergence are both complex traits expressed as the sum of all attributes needed to support normal and vigorous seedling growth.[Citation27,Citation56] To evaluate the contribution of seedling vigour to submergence tolerance at this critical stage in Vietnamese lowland rice, we first screened a set of 148 accessions including local and modern cultivars by adopting the test-tube bioassay.[Citation37] shows an example of the bioassay for shoot elongation growth. Large diversity among the accessions was found in seedling vigour evaluated based on shoot elongation growth (, Table S1 in the Online Supplementary Appendix). Twelve accessions showed significantly longer shoot lengths than the vigorous japonica control Nipponbare (4.13 ± 0.08 cm), whereas 32 accessions showed significantly shorter shoot lengths than the non-vigorous indica control Kasalath (2.25 ± 0.08 cm) at the 1% significance level. An overall average shoot length under submergence in 148 accessions was 2.92 ± 0.15 cm. Most of the accessions collected from the Red River Delta, Central Delta and Mekong River Delta regions showed more vigorous shoot elongation growth than Kasalath, and their mean shoot length was equivalent to that of 17 indica accessions (2.93 ± 0.19 cm) but much lower than that of 150 japonica accessions (4.20 ± 0.22 cm) previously studied.[Citation36]
Figure 1. Test-tube bioassay for evaluation of seedling vigour based on shoot elongation growth. Nipponbare: high seedling vigour control; Kasalath: low seedling vigour control; four others: tested rice accessions (see Table S1 in the Online Supplementary Appendix).

Figure 2. Shoot lengths of 148 Vietnamese lowland rice accessions. Shoot lengths were measured by the test-tube bioassay in the dark.
We next subjected a set of 40 selected vigorous accessions to the recovery test. The mean shoot length of the selected accessions was 3.57 ± 0.16 cm. After transplanting the submerged seedlings into pots with soil and incubating under normal conditions, the bleached shoots rapidly recovered greening. The seedling recovery rate ranged from 45.3% to 93.3% (). All 40 accessions showed much higher seedling recovery rates (a mean of 75.1% ± 0.7%) than that of Kasalath (33.0% ± 0.6%). One accession, ‘Canh nong Bac Giang’, showed an even higher recovery rate (93.3% ± 0.58%) than that (92.0% ± 0.7%) of Nipponbare.
Figure 3. Correlations in seedling vigour evaluated by shoot length, seedling recovery rate and seedling survival rate.
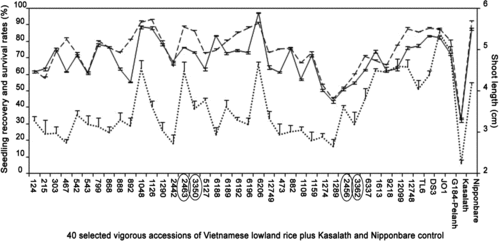
We further evaluated the seedling vigour according to the seedling survival rate 21 days after sowing seeds and immediate subjection to submergence [Citation26] using the same set of 40 accessions. The seedling survival rate ranged from 43.7% to 97.3% (). All 40 accessions showed much higher seedling survival rates (a mean of 69.7% ± 0.7%) than that (32.0% ± 0.8%) of Kasalath. Furthermore, ‘Re nuoc’ showed a higher rate (97.3% ± 0.67%) than that (87.7% ± 0.7%) of Nipponbare. For example, it was reported that only 0.23% of 8000 accessions from the IRRI Genetic Resources Center and breeding lines showed a seedling survival rate higher than 70%.[Citation26] Comparing to this extremely low frequency, the frequency of submergence tolerant accessions in Vietnamese lowland rice was much higher.
We further examined the effectiveness of three bio-parameters employed in evaluating the seedling vigour under submergence. A significantly high value of the coefficient of determination (R2 = 0.76) was observed between the seedling recovery rate and the seedling survival rate ( and ). The shoot length showed considerably high but much lower magnitudes of correlation with both seedling recovery (R2 = 0.55) and seedling survival (R2 = 0.52). The shoot length under submergence, therefore, could explain only about half of the variability observed in seedling recovery and seedling survival. In our previous study, using 17 indica cultivars, including two deep-water rice and 11 japonica cultivars, we found a higher correlation (R2 = 0.72) between shoot length and seedling recovery [Citation36] than that obtained in this study using 40 Vietnamese lowland rice accessions. After re-evaluation of the previous data, however, we noted that the correlation in indica was much lower (R2 = 0.40) than that (R2 = 0.90) in japonica. The subspecies japonica showed much more vigorous shoot growth than indica under submergence.[Citation36,Citation37,Citation57] The observed insufficient levels of correlation between the shoot length and seedling recovery and seedling survival in indica most probably represent an indica subspecies-specific characteristic. Further study is needed to identify the genetic factors linking the shoot elongation growth with the seedling vigour under submergence that seem to be missing or inactive in indica rice.
Table 2. Coefficients of determination (R2)* among the three bio-parameters used for the evaluation of seedling vigour under submergence.
Putative QTLs associated with submergence tolerance have been identified at germination and the post-germination seedling stage in rice. In such QTL mapping studies, researchers in IRRI have adopted seedling survival as a phenotypic marker.[Citation12,Citation26,Citation39–41] Shoot length assessed by the test-tube bioassay has also been successfully used.[Citation38,Citation57] It is notable that all such QTLs have been found in japonica or traditional landraces of Graszmann's group II (aus) rice.[Citation58] This apparently indicates a potential value of japonica germplasm for improving seedling vigour under submergence in indica rice. Vietnamese lowland accessions such as ‘Canh nong Bac Giang’ and ‘Re nuoc’ might serve as promising indica targets for introgressing japonica QTLs.
Contribution of anoxia/hypoxia-responsive genes to seedling vigour under submergence
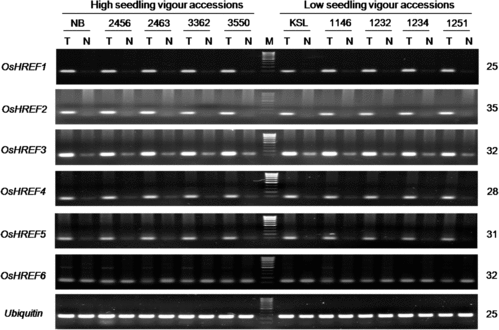
A considerable progress has been made towards understanding the physiological and molecular mechanisms of submergence tolerance at germination and the post-germination seedling stage in rice.[Citation7,Citation9–12,Citation16] Submergence stress induces expression of an array of anoxia/hypoxia-responsive genes and thus they have been considered as important players in attaining submergence tolerance. Under submergence, rice seeds can go into a metabolic state of anaerobiosis by shifting ATP generation from oxidative phosphorylation to ethanol fermentation.[Citation45] Cytoplasmic ADH encoded by two paralogous genes, ADH1 and ADH2, and mitochondria-localized ALDH encoded by ALDH2a play important roles in submergence stress responses in rice.[Citation59,Citation60] PDC is encoded by at least four paralogous genes in rice. Among them, expression of three genes, PDC1, PDC2 and PDC4, was induced under submergence.[Citation61–64] Ca2+ has been known as an important second messenger required for the induction of ADH1,[Citation47,Citation48] and small hypoxia-responsive EF-hand proteins (HREFs) encoded by six paralogous genes, OsHREF1–6, act as cytosolic Ca2+ sensors in response to anoxia/hypoxia in rice.[Citation49]
We studied the induction and transcript accumulation of these anoxia/hypoxia-responsive genes by RT-PCR analysis using vigorous and non-vigorous accessions. With respect to ADH, ALDH and PDC, marked induction and accumulation of transcripts of all six genes were observed under submergence (). No cultivar differences in their amounts, however, were found after different amplification conditions were tried. We next studied the transcript levels of all six OsHREF paralogs. All transcripts but OsHREF6 showed marked induction and accumulation under submergence (), which was in agreement with the previous report.[Citation49] No detectable differences in their transcript levels were found between the vigorous and non-vigorous groups. We further studied two other anoxia/hypoxia-responsive genes, OsB12D1 [Citation50] and SLRL1 [Citation51,Citation52]. Strong induction of these genes occurred under submergence, but no differences were detected between the two groups (). In a previous study using vigorous Nipponbare and FR13A and non-vigorous Kasalath and IR42, we observed marked induction of ADH and ALDH genes under submergence but no vigour-associated differences.[Citation54] These results suggest that the studied anoxia/hypoxia-responsive genes are not the determinants of cultivar-specific levels of submergence tolerance at the post-germination seedling stage in rice. The common and coordinate up-regulation of these facultative genes under submergence rather suggests that they play important roles in rendering a baseline level of submergence tolerance.
Figure 4. Induction of ADH1, ADH2, ALDH2a, PDC1, PDC2 and PDC4 genes in rice seedlings under submergence.
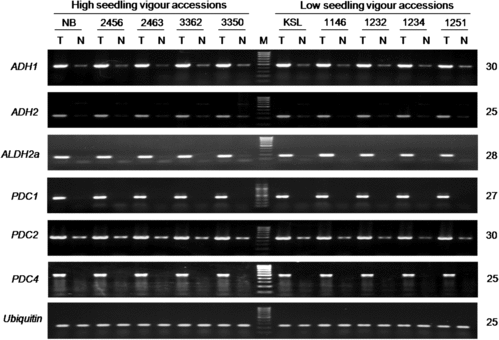
Figure 6. Induction of OsB12D1, SLRL1, SUB1A and SNORKEL1 and SNORKEL2 genes in rice seedlings under submergence.
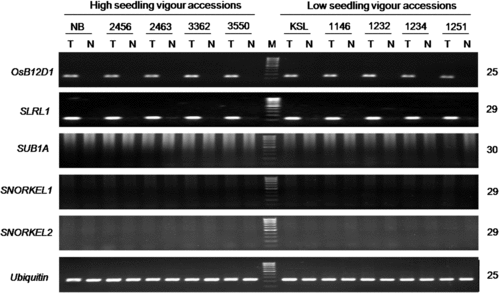
A major achievement in the study of submergence tolerance has been the discovery of two ethylene-responsive genes, SUB1 and SNORKEL, which respectively regulate two principal strategies that rice has evolved to acquire submergence tolerance. SUB1A encoding proteins with a variable cluster of two or three tandem-repeats of group VII of ethylene responsive factor (ERF-VII) play a key role for submergence tolerance in the quiescence strategy.[Citation22] SNORKEL1 (SK1) and SNORKEL2 (SK2), which also encode proteins containing ERF-VII motifs,[Citation65] are the key genes in the escape strategy. However, it has been noted that SUB1A can only partly explain differences in the level of submergence tolerance among cultivars, particularly at the early seedling stage.[Citation8,Citation24,Citation26] We previously showed that expression of SUB1A required light illumination and that the light-induced expression and transcript accumulation did not hinder shoot elongation of young seedlings under submergence in submergence tolerant and vigorous indica cultivar FR13A as well as several deep-water rice accessions.[Citation36] Expression of SNORKEL genes was reported to be restricted in some deep-water rice and wild relatives of rice.[Citation65] We now showed that neither SUB1A nor SNORKEL transcripts were present in the seedlings of Vietnamese lowland rice (). Taken together, these results support the suggestion that some unknown genetic factor(s) other than SUB1A and SNORKEL is operative at germination and the post-germination early seedling stage in rice.
Conclusions
Seedling vigour under submergence was assessed in 148 Vietnamese lowland rice accessions based on three bio-parameters, i.e. shoot elongation, seedling recovery and seedling survival. Significantly high correlations were found among them, confirming the contribution of seedling vigour in the manifestation of submergence tolerance at germination and the post-germination early seedling stage. The frequency of tolerant accessions was much higher than that reported in a survey of indica rice collection, indicating that highly vigorous Vietnamese lowland rice can provide a promising source for further improvement of indica rice. Transcript levels of eight anoxia/hypoxia-responsive genes were compared between vigorous and non-vigorous accessions. Dramatic induction of paralogs of six genes, i.e. ADH, ALDH, PDC, OsHREF, OsB12D1 and SLRL, was observed under submergence as compared to the aerobically grown normal seedlings in all tested cultivars. No cultivar/genotype-specific differences were found in their transcript levels under submergence. Further study is needed to find genetic factors determining cultivar/genotype specificity acting at this critical stage of rice development.
TBEQ_A_1204944_Suppl_mat.rar
Download (507.9 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Mackill DJ, Ismail AM, Singh US, et al. Development and rapid adoption of submergence-tolerant (Sub1) rice varieties. Adv Agronomy. 2012;115:299–352.
- Yamaguchi M, Aguilar AM, Vughan DA, et al. Rice (Oryza sativa L.) germplasm suitable for direct sowing under soil surface. Euphytica. 1993;67:177–184.
- Yamauchi M, Aragones DV, Casayuran PR, et al. Seedling establishment and grain yield of tropical rice sown in puddle soil. Agronomy J. 2000;92:275–282.
- Setter TL, Laureles EV. The beneficial effect of reduced elongation growth on submergence tolerance of rice. J Exp Bot. 1996;47:1551–1559.
- Setter TL, Ellis M, Laureles CV, et al. Physiology and genetics of submergence tolerance in rice. Ann Bot. 1997;79:67–77.
- Bailey-Serres J, Voesenek LACJ. Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol. 2008;59:313–339.
- Bailey-Serres J, Voesenek LACJ. Life in the balance: a signaling network controlling survival of flooding. Curr Opin Plant Biol. 2010;13:489–494.
- Ismail AM, Ella ES, Vergara GV, et al. Mechanisms associated with tolerance to flooding during germination and early seedling growth in rice (Oryza sativa). Ann Bot. 2009;103:197–209.
- Ismail AM, Johnson DE, Ella ES, et al. Adaptation to flooding during emergence and seedling growth in rice and weeds, and implications for crop establishment. AoB Plants [Internet]. 2012 [cited 2016 Apr 20]. Available from: http://dx.doi.org/10.1093/aobpla/pls019
- Magneschi L, Perata P. Rice germination and seedling growth in the absence of oxygen. Ann Bot. 2009;103:181–196.
- Bailey-Serres J, Fukao T, Gibbs DJ, et al. Making sense of low oxygen sensing. Trends Plant Sci. 2012;17:1360–1385.
- Miro B, Ismail AM. Tolerance of anaerobic conditions caused by flooding during germination and early growth in rice (Oryza sativa L.). Front Plant Sci [Internet]. 2013 [cited 2016 Apr 20];4:269. Available from: http://doi.org/10.3389/fpls.2013.00269
- Tamang BG, Fukao T. Plant adaptation to multiple stresses during submergence and following desubmergence. Int J Mol Sci. 2015;16:30164–30180.
- Ito O, Ella E, Kawano N. Physiological basis of submergence tolerance in rainfed lowland rice ecosystem. Field Crops Res. 1999;64:75–90.
- Ram PC, Singh BB, Singh AK, et al. Submergence tolerance in rainfed lowland rice: physiological basis and prospects for cultivar improvement through marker-aided breeding. Field Crops Res. 2002;76:131–152.
- Jackson MB, Ram PC. Physiological and molecular basis of susceptibility and tolerance of rice plants to complete submergence. Ann Bot. 2003;91:227–241.
- Das KK, Sarkar RK, Ismail AM. Elongation ability and non-structural carbohydrate levels in relation to submergence tolerance in rice. Plant Sci. 2005;168:131–136.
- Fukao T, Xu K, Ronald PC, et al. A variable cluster of ethylene response factor-like genes regulates metabolic and development acclimation responses to submergence in rice. Plant Cell. 2006;18:2021–2034.
- Suge H. Ethylene and gibberellin: regulation of internodal elongation and nodal root development in floating rice. Plant Cell Physiol. 1985;26:607–614.
- Ella ES, Kawano N, Yamauchi Y, et al. Blocking ethylene perception during submergence reduced chlorophyll degradation and improved seedling survival in rice. Funct Plant Biol. 2003;30:813–819.
- Fukao T, Bailey-Serres J. Plant responses to hypoxia – is survival a balancing act? Trends Plant Sci. 2004;9:449–456.
- Xu K, Xu X, Fukao T, et al. SubA is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature. 2006;442:705–708.
- Bailey-Serres J, Fukao F, Ronald P, et al. Submergence tolerant rice: SUB1's journey from landrace to modern cultivar. Rice. 2010;3:138–147.
- Perata P, Voesenek LACJ. Submergence tolerance in rice requires Sub1A, an ethylene-response-factor-like gene. Trends Plant Sci. 2007;12:43–46.
- Sarkar RK, Bhattacharjee B. Rice genotypes with SUB1 QTL differ in submergence tolerance, elongation ability during submergence and re-generation growth at re-emergence. Rice [Internet]. 2011 [cited 2016 Apr 20];5:7. Available from: http://dx.doi.org/10.1007/s12284-011-9065-z
- Angaji SA, Septiningsih EM, Mackill DJ, et al. QTLs associated with tolerance of flooding during germination in rice (Oryza sativa L.). Euphytica. 2010;172:159–168.
- Redona ED, Mackill DJ. Genetic variation for seedling vigour traits in rice. Crop Sci. 1996;36:285–290.
- Pandey S, Mortimer M, Wade L, et al. (editors). Direct seeding: research issues and opportunities. Proceedings of the International Workshop on Direct Seeding in Asian Rice Systems: Strategic Research Issues and Opportunities, 2000 Jan 25--28, Bangkok, Thailand. Los Baños (Philippines): International Rice Research Institute; 2002. p. 383.
- Kende H, Van der Knaap E, Cho H-T. Deepwater rice: a mode plant to study stem elongation. Plant Physiol. 1998;118:1105–1110.
- Cui KH, Peng SB, Xing YZ, et al. Molecular dissection of seedling-vigour and associated physiological traits in rice. Theor Appl Genet. 2002;105:745–753.
- Vriezen WH, Zhou Z, Van Der Straeten D. Regulation of submergence-induced enhanced shoot elongation in Oryza sativa L. Ann Bot. 2003;91:263–270.
- Voesenek LACJ, Colmer TD, Pierik R, et al. Tansley review. how plants cope with complete submergence. New Phytol. 2006;170:213–226.
- El-Hendawy S, Sone C, Ito O, et al. Traits associated with the escape strategy are responsible for flash flooding tolerance of rice during the emergence and seedling stages. Cereal Res Commun. 2015;43:525–536.
- Xu K, Mackill DJ. A major locus for submergence tolerance mapped on rice chromosome 9. Mol Breed. 1996;2:219–224.
- Xu K, Xu X, Ronald PC, et al. A high-resolution linkage map of the vicinity of the rice submergence tolerance locus Sub1. Mol Gen Gent. 2000;263:681–689.
- Vu HTT, Manangkil OE, Mori N, et al. Post-germination seedling vigour under submergence and submergence-induced SUB1A gene expression in indica and japonica rice (Oryza sativa L.). Aust J Crop Sci. 2010;4:264–272.
- Manangkil OE, Vu HTT, Yoshida S, et al. A simple, rapid and reliable bioassay for evaluating seedling vigour under submergence in indica and japonica rice (Oryza sativa L.). Euphytica. 2008;163:267–274.
- Manangkil OE, Vu HTT, Mori N, et al. Mapping of quantitative trait loci controlling seedling vigour in rice (Oryza sativa L.) under submergence. Euphytica. 2013;192:63–75.
- Septiningsih EM, Ignacio JC, Sendon PM, et al. QTL mapping and confirmation for tolerance of anaerobic conditions during germination derived from the rice landrace Ma-Zhan Red. Theor Appl Genet. 2013;126:1357–1366.
- Baltazar MD, Ignacio JCI, Thomson MJ, et al. QTL maping for tolerance of anaerobic germination from IR64 and the aus landrace Nanhi using SNP genotyping. Euphytica. 2014;197:251–260.
- Kretzschmar T, Pelayo MAF, Trijatmiko KR, et al. A trehalose-6-phosphate phosphatase enhances anaerobic germination tolerance in rice. Nature Plants. 2015;15124. doi: 10.1038/nplants.2015.124
- Weitbrecht K, Muller K, Lerbner-Metzger G. First off the mark: early seed germination. J Exp Bot. 2011;62:3289–3309.
- Gibbs J, Greenway H. Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct Plant Biol. 2003;30:1–47.
- Vartapetian BB, Jackson MB. Plant adaptations to anaerobic stress. Ann Bot. 1997;79:3–20.
- Perata P, Alpi A. Plant responses to anaerobiosis. Plant Sci. 1993;93:1–17.
- Tsuji H, Meguro N, Suzuki Y, et al. Induction of mitochondrial aldehyde dehydrogenase by submergence facilitates oxidation of acetaldehyde during re-aeration in rice. FEBS Lett. 2003;546:369–373.
- Subbaiah CC, Zhang J, Sachs MM. Involvement of intracellular calcium in anaerobic gene expression and survival of maize seedlings. Plant Physiol. 1994;105:369–376.
- Subbaiah CC, Bush DS, Sachs MM. Elevation of cytosolic calcium precedes anoxic gene expression in maize suspension cultured cells. Plant Cell. 1994;6:1747–1762.
- Otsuka C, Minami I, Oda K. Hypoxia-inducible genes encoding small EF-hand proteins in rice and tomato. Biosci Biotechnol Biochem. 2010;74:2463–2469.
- He D, Zhang H, Yang P. The mitochondrion-located protein OsB12D1 enhances flooding tolerance during seed germination and early seedling growth in rice. Int J Mol Sci. 2014;15:13461–13481.
- Itoh H, Shimada A, Ueguchi-Tanaka M, et al. Overexpression of a GRAS protein lacking the DELLA domain confers altered gibberellin responses in rice. Plant J. 2005;44:669–679.
- Fukao T, Bailey-Serres J. Submergence tolerance conferred by Sub1A is mediated by SLR1 and SLRL1 restriction of gibberellin responses in rice. Proc Natl Acad Sci USA. 2008;105:16814–16819.
- Vu HTT, Nguyen HTT, Tran KD, et al. Genetic diversity of Vietnamese lowland rice germplasms as revealed by SSR markers in relation to seedling vigour under submergence. Biotechnol Biotechnol Equip. 2016;30:17–25.
- Vu HTT, Manangkil OE, Mori N, et al. Submergence-induced ADH and ALDH gene expression in japonica and indica with contrasting levels of seedling vigour under submergence stress. Biotechnol Biotechnol Equip. 2009;23:1469–1473.
- Gomez KA, Gomez AA. Statistical procedures for agricultural research. 2nd ed. Toronto: Wiley; 1984. p. 363–366.
- Kawano N, Ito O, Sakagami JI. Morphological and physiological responses of rice seedlings to complete submergence (flash flooding). Ann Bot. 2009;103:161–169.
- Hsu S-K, Tung C-W. Genetic mapping of anaerobic germination-associated QTLs controlling coleoptile elongation in rice. Rice [Internet]. 2015 [cited 2016 Apr 20];8:38. Available from: http://dx.doi.org/10.1186/s12284-015-0072-3
- Glaszmann JC. Isozymes and classification of Asian rice varieties. Theor Appl Genet. 1987;74:21–30.
- Umeda M, Uchimiya H. Differential transcript levels of genes associated with glycolysis and alcohol fermentation in rice plants (Oryza sativa L.) under submergence stress. Plant Physiol. 1994;106:1015–1022.
- Nakazono M, Tsuji H, Li Y, et al. Expression of a gene encoding mitochondrial aldehyde dehydrogenase in rice increases under submerged conditions. Plant Physiol. 2000;124:587–598.
- Hossain MA, Huq E, Hodges TK. Sequence of a cDNA from Oryza sativa (L.) encoding the pyruvate decarboxylase 1 gene. Plant Physiol. 1994;106:799–800.
- Hossain MA, Huq E, Grover A, et al. Characterization of pyruvate decarboxylase genes from rice. Plant Mol Biol. 1996;31:761–770.
- Huq E, Hossain MA, Hodges TK. Cloning and sequencing of a cDNA encoding pyruvate decarboxylase 2 gene (accession no. U27350) from rice (PGR95-072). Plant Physiol. 1995;109:722.
- Rivoal J, Thind S, Pradet A, et al. Differential induction of pyruvate decarboxylase subunits and transcripts in anoxic rice seedlings. Plant Physiol. 1997;114:1021–1029.
- Hattori Y, Nagai K, Furukawa S, et al. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature. 2009;460:1026–1030.