ABSTRACT
Sodium benzoate (SB) is one of the most widely used additives in food products in the world. The aim of this study was to assess the effect of three different concentrations of SB on the DNA breakage in liver cells and on the micronuclei formation and the mitotic index in lymphocytes of pregnant rats and their fetuses, as well as to evaluate the effects of SB on the fetus development. The results showed that general genomic injuries were present in almost all the liver cell samples obtained from the SB group compared with the control (non-treated) group. This indicates that SB usage may cause DNA damage and increase micronuclei formation. We recommend that pregnant women should avoid consuming foodstuffs containing SB as an additive.
Introduction
Safe storage of food and beverages for a prolonged time is one of the oldest human needs. It is achieved by different conservation methods which improve over time. In general, for preservation purposes, different chemical agents are used. Sodium benzoate (SB, E211), also known as benzoate of soda, is a food additive used as a preservative for its ability to effectively kill most yeasts, bacteria and fungi [Citation1]. SB is a bacteriostatic and fungistatic agent in acidic environments which, after being absorbed into cells, interacts with the anaerobic energy production pathways and suppresses the development of food-spoilage microorganisms [Citation2]. Owing to this feature, SB is broadly used in other fields and is one of the indispensable components of pharmaceutical and cosmetic products [Citation3,Citation4]. In order to preserve a product, assuming it has been correctly prepared and adjusted to a pH of 4.5 or less, an approximate SB concentration of 0.1% is usually enough [Citation1].
Today, it is difficult to imagine a food product without any food additives. Even in small concentrations, preservatives affect the health of long-term consumers, which may present as a migraine, nausea, vomiting, diarrhoea, rhinitis, bronchospasm, anaphylaxis and hyperactivity in children. What is more, there may also be induced damage at the molecular level, including chromosome damage. Because of reports of such side effects of these chemicals, especially in the gastrointestinal system, the allowable concentration is placed under the regulation of the Food and Drug Administration (FDA) [Citation5].
The International Programme on Chemical Safety found no adverse effects of SB on humans at doses of 647–825 mg/kg of body weight per day [Citation6] and the acceptable dose of SB declared in 2003 by the Turkish Ministry of Health is 500–1000 mg/kg [Citation7]. Although SB is widely used as a preservative in several products like pickles, sauces, milk and meat products and fruit juices, there is also a high demand in the soft drink industry due to the usage of high-fructose corn syrup in carbonated beverages [Citation8]. Benzoic acid, which is a precursor of SB, is found naturally in cranberries, prunes, greengage plums, cinnamon, ripe cloves and apples [Citation9]. Benzoates have been detected in groundwater, but not in drinking water [Citation6]. In the literature, there is only a limited number of studies that have been conducted on SB. According to Nair [Citation10], although previous works of other researchers reported some toxic effects of benzoates, no genotoxic effects of benzyl alcohol, benzoic acid and sodium benzoate have been found in studies on rats and mice. The results from carcinogenicity studies have also been found negative [Citation10]. However, Türkoğlu et al. [Citation11] have also reported some genotoxic effects of antimicrobial additives on root tips of Allium cepa.
Since there is scarce information about the genotoxic effects of preservatives, and an insufficient number of in vivo studies in mammals, testing the potential of SB to induce genomic alterations in mitotic cells of pregnant rats can help to increase our knowledge of the biological effect of SB from a different point of view. The detection of chromosome fragments or whole chromosomes without migration potential during cell division (micronuclei) using the micronucleus test [Citation12] is one of the methods for determination of the possible genotoxic effect of SB.
Thus, the aim of this study was to evaluate the effects of three different concentrations of SB (1) on the weight gain, food and water intake of pregnant rats; (2) on the micronuclei (MN) formation and mitotic index (MI) in T-lymphocytes of pregnant rats and their fetuses; (3) on the fetal body weight, perinatal mortality and malformations; and (4) the potential of SB to induce DNA breaks in the liver cells of pregnant rats and their newborns.
Materials and methods
Wistar rats (n = 16) reared at Experimental Research and Application Center, Erciyes University, were used in this study. The experimental protocol was approved by the Institutional Animal Ethics Committee before the beginning of the experiments. The rats were cared for at all times according to the institutional guidelines of Erciyes University. Twelve-week-old (∼210 g) rats (n = 12) were given SB by gavages continuously from the morning of gestation day (gd) 0 to the morning of gd 20 (rats) at doses of 0, 0.5, 1 and 1.5 mg/mL. The substance was diluted with distilled water. SB was acquired from Sigma–Aldrich, Steinheim, Germany (Cat no.: B-3375; chemical formula C6H5COONa, molecular weight 144, CAS no.: 532-32-1). Distilled water was used in the twelve-week-old (∼210 g) rats (n = 4) as a control group. The endpoints used for evaluation of the potential maternal toxicity during SB treatment included food and water consumption and body weight. The conscious animals were sacrificed by decapitation on gd 20. The endpoints used for evaluation of the potential developmental toxicity of SB were embryonal/fetal weight, structural malformations and variations observed during examinations. Live fetuses were weighed and examined for malformations and variations by standard techniques [Citation13–16].
Blood and bone marrow samples of pregnant rats and two of their fetuses were cultured as proposed by Sharma and Sharma [Citation17]. The MI was determined by using 2% aceto-orcein [Citation17]. Each treatment was replicated three times and scoring was done from the three different samples of each replicate. A minimum of 1000 mitotic cells were counted on each slide. The MI was computed for each treatment as the number of dividing cells per 100 cells and the MN formation were scored in the mitotic cells (Olympus BX50 microscope, Olympus, Tokyo, Japan). Data were analyzed by using analysis of variance (ANOVA) and Duncan's mean range (DMR) test in SPSS (Version 22). For the MN analysis, the slides were immersed in 5 N HCL for 20 min at room temperature. They were then washed with tap water, and the slides were kept in Schift solution for 90 min in the dark. Following this procedure, they were passed through sodium bisulphite solution (1.0 g sodium bisulfite, 10 mL 5 N HCl, and 210 mL of distilled water) three times for 2 min each. The slides were washed with tap water three times for 30 min each. The randomization and scoring of slides were done by only one researcher. After scanning of 1500–3000 cells with the 600× objective, the 1000× objective (Olympus BX50 microscope, Olympus, Tokyo, Japan) was used for examination of the identified MN cells. As a reference, the Sarto et al. [Citation18] guideline was used for MN identification. Cases of nuclear blebbing (micronucleus-like structure connected with the main nucleus with a bridge) were not considered. Only MN which were smaller than 1/3 or larger than 1/16 of the diameter of the main nucleus were assumed to have resulted from chromosome breakage and were, thus, calculated. The cells with two or more MN were classified as multinucleated cells and were also scored as one micronucleus [Citation19]. In addition, the DNA damage to liver cells of the mothers and their newborn rats after SB exposure was examined. Briefly, 25–50 mg liver tissue was taken from a rat and one of its newborns. A standard genomic DNA extraction kit was used (Roche, Mannheim, Germany) according to the manufacturer's instructions. DNA samples were eluted in RNase/DNase-free water. Prior to analysis, the DNA samples were stored at −80 °C. The fragments were resolved by electrophoresis in a 2% agarose gel containing 1× TBE buffer (90 mmol/L Tris base, 90 mmol/L boric acid, 2 mmol/L ethylenediaminetetraacetic acid), followed by staining with ethidium bromide. Electrophoresis was run in 1× TBE buffer at 100 V for 1 h (PowerPac Basic 300 V, 400 mA, 75 W, Bio-Rad and Owl Easycast™ B2, Thermo Scientific) and gels were visualized by using UVP EC3 Chemi HR 410 Imagining system and UVP VisionWorksLS Image Acquisition and Analysis Software version 6.8 [Citation20].
Results and discussion
Maternal effects: the effects of SB on the weight gain, food and water intake of pregnant rats
As a first step in our experiments, the effects of three different concentrations of SB on the weight gain, food and water intake of pregnant rats were evaluated. Summarizing across all exposure groups, the maternal effects of SB treatment had little effect on the body weight gain in the mothers, which showed an insignificant decrease of 0.4%. The corrected body weight gain (i.e. gestational weight gain minus the gravid uterine weight) was not affected. Exposure to SB from gd 0 to 20 resulted in no significant change in the food and water intake of the mothers (). There were no deaths or clinical signs of toxicity.
Table 1. Summary of maternal and embryonal/fetal responses of Wistar rats to sodium benzoate administered on gestational days 0–20.
Effects in mothers and their fetuses: MN formation and MI in T-lymphocytes from bone marrow and peripheral blood
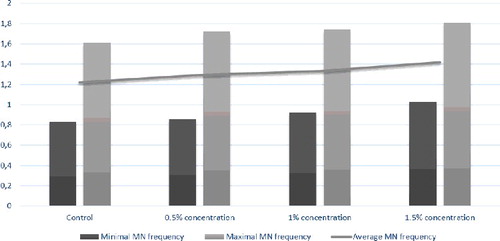
The induction of MN is regarded as a qualitative measure of genotoxicity for a series of mutagens and carcinogens. MN formation is associated with loss of genetic material. In our study, we did not document any significant MN formation after SB treatment in pregnant rats throughout the whole pregnancy. The average MN frequency in T-lymphocytes of pregnant rats and their fetuses was not statistically different (p > 0.05) between the groups (). There was no notable contrast in the MI in T-lymphocytes following treatment with all the tested concentrations either. However, MN have been observed after treatment with different food additives in several reports [Citation21,Citation22].
Embryonal/fetal effects
The perinatal mortality significantly increased in the 1% and 1.5% SB dose group (48% resorptions per litter) compared to the control group (0% resorptions per litter), which was accompanied by a decrease in the live litter size. The average fetal body weight per litter was not reduced in all SB-treated groups. No malformed fetuses were detected at any of the applied doses of SB.
Effects in mothers and newborns: the potential of SB to induce DNA damage in liver cells
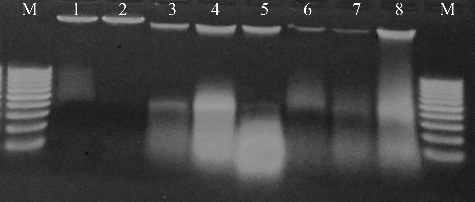
In our study, the agarose gel electrophoresis results showed increased DNA smears correlated with the increased doses of SB both in the mothers and in their newborns (). The observed expansion of the DNA migration distance with the increase in SB dose indicated concentration-dependent induction of DNA breaks in maternal and fetal liver tissues under the influence of SB. These results are in accordance with the reports of other authors. For example, Ishidate et al. [Citation23] detected chromosomal damage in Chinese hamster fibroblast cell line treated with SB in vitro and also observed inhibition of DNA synthesis in rat liver cells following application of 100 µg/mL of SB. Pongsavee [Citation2] also studied the in vitro effects of SB on a human lymphocyte cell line and described evidence of serious DNA damage. According to his results, the MN formation increased proportionally to the applied SB concentration and the treatment duration. Pongsavee observed chromosome breakage to occur (sister chromatid separation and chromosome gaps) at a dose of 2.0 mg/mL of SB at 24- and 48-hour incubation time, which was not observed in control groups. Based on this evidence that SB triggers micronucleus formation and induces chromosome breaks, Pongsavee [Citation2] suggested that this food additive agent has mutagenic and cytotoxic effects in lymphocytes.
Figure 2. Representative electrophoretogram of genomic DNA isolated from liver cells of a mother and one of her newborns following treatment with different concentrations of SB. M, DNA ladder (100–1500 bp, Roche, Mannheim, Germany); Lanes 1, 3, 5, 7, DNA from liver cells of the mother following treatment with 0%, 0.5%, 1%, and 1.5% SB, respectively; Lanes 2, 4, 6, 8, DNA liver cells of a newborn following treatment with 0%, 0.5%, 1%, and 1.5% SB, respectively.
In another study, Zengin et al. [Citation24] investigated the in vitro effects of SB and potassium benzoate (PB) on cultured human peripheral lymphocytes. They found a decrease in MI and an increase in chromosomal aberrations, sister chromatid exchanges and MN in groups treated with SB and PB in comparison with the control group. Also, significant DNA damage was indicated in SB-treated groups but not in PB-treated groups, providing further evidence for a cytotoxic, mutagenic and clastogenic activity of SB.
The total percentage of aberrations generally increases the longer the treatment period is and the higher the clastogen concentrations are [Citation8]. JECFA (Joint FAO/WHO Expert Committee on Food Additives) has allocated an acceptable daily intake (ADI) for benzoic acid and SB of 0–5 mg/kg body weight [Citation25]. Consequently, more extensive investigations on the SB effect on DNA and RNA need to be carried out. In general, the database for SB is still limited. Furthermore, the documentation of these studies in most cases is insufficient. Additional information is required in order to evaluate whether SB has genotoxic potential.
Conclusions
According to the findings of the present in vivo study, SB showed a concentration-dependent genotoxic effect in liver tissue. The results indicating induction of DNA breaks in rat liver cells under the influence of SB intake is disturbing and must be an alarming signal for the food industry which must be cautious with the dosage of this preservative agent. This matter should be strictly controlled by governments and further cytogenetic research on the potential clastogenicity and genotoxicity of food preservatives in vivo should be performed.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Chipley JR . Sodium benzoate and benzoic acid. In: Davidson PM , Sofos JN , Branen AL , editors. Antimicrobials in food. New York (NY): CRC Press; 2005. p. 11–48.
- Pongsavee M . Effect of sodium benzoate preservative on micronucleus ınduction, chromosome break, and Ala40Thr superoxide dismutase gene mutation in lymphocytes. BioMed Res Int. [Internet]. 2015 [cited 2016 Feb 10]; 2015:103512. Available from: http://www.hindawi.com/journals/bmri/2015/103512/.
- Boukarim C , Jaoudé SA , Bahnam R , et al. Preservatives in liquid pharmaceutical preparations. Drug Test Anal. 2009;1:146–148.
- Ikarashi Y , Uchino T , Nishimura T . Analysis of preservatives used in cosmetic products: salicylic acid, sodium benzoate, sodium dehydroacetate, potassium sorbate, phenoxyethanol, and parabens. Bull Natl Inst Health Sci. 2010;128:85–90.
- Tuormaa TE . The adverse effects of food additives on health: a review of the literature with a special emphasis on childhood hyperactivity. J Orthomol Med. 1994;9:225–243.
- Benzoic acid and sodium benzoate [Internet] . 2000. Geneva: World Health Organization. [cited 2016 Aug 8]. Available from: www.inchem.org/documents/cicads/cicads/cicad26.htm.
- The Ministry of Health of Turkey food codex instructions . Ankara (Turkey): The Ministry of Health of Turkey; 2003. p. 25324.
- Srour R . Benzoic acid and derivatives. Aromatic intermediates and derivatives. Paris; 1998. p. A.IV.1–A.IV.17 (unpublished report).
- Penney V , Henderson G , Blum C , et al. The potential of phytopreservatives and nisin to control microbial spoilage of minimally processed fruit yogurts. Innov Food Sci Emerg Technol. 2004;5:369–375.
- Nair B . Final report on the safety assessment of benzyl alcohol, benzoic acid, and sodium benzoate. Int J Toxicol. 2001;20(Suppl 3):23–50.
- Türkoğlu Ş . Genotoxicity of five food preservatives tested on root tips of Allium cepa L. Mutat Res. 2007;626:4–14.
- Aardema MJ , Kirsch-Volders M . The in vitro micronucleus assay. In: Choy WN , editor. Genetic toxicology and cancer risk assessment. 1st ed. New York: Marcel Dekker; p. 163–186.
- Wilson JG . Embryological considerations in teratology*. Ann N Y Acad Sci. 1965;123:219–227.
- Staples R , editor. Detection of visceral alterations in mammalian fetuses. Teratology. 1974;9:37A–38A.3
- Stuckhardt J , Poppe S . Fresh visceral examination of rat and rabbit fetuses used in teratogenicity testing. Teratog Carcinog Mutagen. 1984;4:181–198.
- Marr MC , Myers CB , George JD , et al. Comparison of single and double staining for evaluation of skeletal development: the effects of ethyleneglycol (EG) in CD rats. Teratology. 1988;37:476.
- Sharma AK , Sharma A . Chromosome techniques: theory and practice. Oxford: Butterworth-Heinemann; 2014.
- Sarto F , Finotto S , Giacomelli L , et al. The micronucleus assay in exfoliated cells of the human buccal mucosa. Mutagenesis. 1987;2:11–17.
- Sarto F , Tomanin R , Giacomelli L , et al. Evaluation of chromosomal aberrations in lymphocytes and micronuclei in lymphocytes, oral mucosa and hair root cells of patients under antiblastic therapy. Mutat Res. 1990;228(2):157–169.
- Aaij C , Borst P . The gel electrophoresis of DNA. Biochim Biophys Acta. 1972;269:192–200.
- Münzner R , Guigas C , Renner H . Re-examination of potassium sorbate and sodium sorbate for possible genotoxic potential. Food Chem Toxicol. 1990;28:397–401.
- Meng Z , Zhang L . Cytogenetic damage induced in human lymphocytes by sodium bisulfite. Mutat Res. 1992;298:63–69.
- Ishidate M , Sofuni T , Yoshikawa K , et al. Primary mutagenicity screening of food additives currently used in Japan. Food Chem Toxicol. 1984;22:623–636.
- Zengin N , Yüzbaşıoğlu D , Ünal F , et al. The evaluation of the genotoxicity of two food preservatives: sodium benzoate and potassium benzoate. Food Chem Toxicol. 2011;49:763–769.
- Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Sodium benzoate [Internet]. World Health Organization ; 1996. [cited 2007 Mar 28]. Available from: http://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=1098#.