ABSTRACT
Genetic variations, and especially somaclonal variations, are undesirable in genetic transformation. In this study, random amplified polymorphic DNA (RAPD) and coupled restriction enzyme digestion-random amplification (CRED-RA) markers were used for detection of the variation in calli that were obtained from endosperm-supported mature embryo of rye on Murashige and Skoog (MS) medium containing different auxins (2,4-D, dicamba and picloram) at a range of different concentrations (2, 4, 6, 8, 10 and 12 mg/L). High level of auxins caused a reduction in the genomic template stability (GTS) value. While the highest GTS was observed in the calli maintained on MS medium with 2 mg/L dicamba (98.4%), the calli maintained on MS medium with 12 mg/L picloram were found to show the least GTS (81.7%) when RAPD patterns were analysed. Epigenetic changes were more frequent and variable than genetic changes when compared to RAPD and CRED-RA results. DNA hypermethylation was observed at higher concentrations of 2,4-D and picloram, whereas DNA hypomethylation was observed in dicamba. These results indicate that RAPD and CRED-RA techniques can be used for detection of somaclonal variation in in vitro cultures, which is a fundamental step in plant genetic transformation.
Introduction
Rye (Secale cereale L.) is second only to wheat (Triticum aestivum L.) as a grain used in bread-making [Citation1,Citation2], and it is very well adapted to submarginal conditions, including low fertility soils, drought and cold [Citation3]. Regeneration of a whole plant from in vitro cultured cells is an important step in the genetic manipulation of plants [Citation4]. In vitro culture is believed to destabilize the genetic and epigenetic programme of intact plant tissue [Citation5]. Genetic variations that occur in plants regenerated from cell and tissue cultures are called somaclonal variation. Although somaclonal variation could be useful for crop breeding programmes as a source of beneficial variation, it is not desirable in plant transformation studies [Citation6]. Therefore, in plant transformation studies, somaclonal variation should be detected during the early stage of plant tissue culture.
Genetic changes may involve chromosomal and point mutations, activation of transposable elements, rearrangement of DNA, ploidy changes and epigenetic variations such as DNA methylation [Citation6,Citation7]. These changes induced by in vitro culture depend on plant species, genotype, the type of explant and the culture media, plant growth regulators, duration of the culture and in vitro regeneration system [Citation8,Citation9]. The most common approach for cereal regeneration is indirect somatic embryogenesis. Unfortunately, callus-mediated methods such as indirect somatic embryogenesis induce somaclonal variation [Citation9]. In addition, plant growth regulators used in in vitro culture are powerful agents of somaclonal variations.
Somaclonal variations can be detected easily by morphological, cytological, biochemical and molecular markers. Detection of genetic polymorphism based on random amplified polymorphic DNA (RAPD) has found successful application in describing somaclonal variability in rye [Citation10], barley [Citation11] and wheat [Citation12]. Coupled restriction enzyme digestion-random amplification (CRED-RA) has been used for the identification of DNA methylation, which is a type of epigenetic variation. Temel et al. [Citation11] demonstrated the utility of this method in the determination of DNA methylation.
The aim of this study was to detect the effects of different types of auxins and their concentrations on the somaclonal variation in rye mature embryo culture.
Materials and methods
Plant growth and treatment conditions
Endosperm-supported mature embryos of the rye cultivar Aslım 93 were used as explants. Mature seeds were surface sterilized first by washing in 70% ethanol for 5 min, rinsed with sterile water and then with 1% solution of sodium hypochlorite with two drops of TWEEN 20 for 20 min and subsequently seeds were rinsed with sterile water three times and incubated at 4 ˚C for 24 h in sterile distilled water. Imbibed seeds were prepared as described by Aydin et al. [Citation13] for endosperm-supported mature embryo culture. Ten prepared seeds in four replications were placed in furrow downwards in Petri dishes containing MS (Murashige and Skoog Basal Medium, M5519, Sigma-Aldrich, St Louis, MO, United States) medium supplemented with 20 mg/L sucrose, 2 g/L phytagel and different concentrations (2, 4, 6, 8, 10 and 12 mg/L) of auxins (2,4-D, dicamba and picloram). The media pH was adjusted to 5.8 with sodium hydroxide (NaOH) before autoclaving for 20 min at 121 ˚C and 105 kPa. Vitamins and auxin solutions were filter-sterilized and subsequently added to the autoclaved media. The Petri dishes were incubated at 25 ± 1 ˚C for 21 d in darkness for callus induction from endosperm-supported mature embryos.
Genomic DNA extraction
After 21 d of incubation in callus induction medium, genomic DNA was isolated from calli, using cetyl-trimethylammoniumbromide buffer as recommended by Monsanto [Citation14].
RAPDs and CRED-RAs procedures and polymerase chain reaction (PCR) methods
RAPD–PCR amplification was carried out using 16 primers [OPW-20 (5'-TGTGGCAGCA-3'), OPW-13 (5'-CACAGCGACA-3'), OPA-1 (5'-CAGGCCCTTC-3'), OPA-2 (5'-TGCCGAGCTG-3'), OPA-4 (5'-AATCGGGCTG-3'), OPA-12 (5'-TCGGCGATAG-3'), OPA-13 (5'-CAGCACCCAC-3'), OPH-18 (5'-GAATCGGCCA-3'), OPY-6 (5'-AAGGCTCACC-3'), OPY-11 (5'-AGACGATGGG-3'), OPY-15 (5'-AGTCGCCCTT-3'), OPY-16 (5'-GGGCCAATGT-3'), OPB-8 (5'-GTCCACACGG-3'), OPW-4 (5'-CAGAAGCGGA-3'), OPW-7 (5'-CTGGACGTCA-3') and OPW-8 (5'-GACTGCCTCT-3')] (Operon Technologies, Alameda, CA, United States). PCR amplifications were carried out in a total volume of 25 μL containing 50 ng of genomic DNA, 1× PCR buffer, 400 µmol/L of deoxynucleoside triphosphates, 10 pmol of primer, 2.5 mmol/L MgCl2 and 1 U Taq DNA polymerase (D4545, Sigma-Aldrich, St Louis, MO, United States). Amplifications were performed in a thermocycler (SensoQuest Labcycler Gradient, SensoQuest, Goettingen, Germany) with the following conditions: 95 ˚C for 5 min (initial denaturation), 94 ˚C for 1 min, 36 ˚C for 1 min, 72 ˚C for 2 min for 42 cycles with a final extension at 72 ˚C for 15 min.
Genomic DNA samples (50 ng) from each treatment were separately digested with HpaII and MspI restriction endonucleases (which cut the sequence 5´-C/CGG-3´ with different sensitivity to cytosine methylation; MspI cuts if the inner C is methylated, whereas HpaII cannot cleave in the presence of methyl groups) as specified by the manufacturer (Promega Corp., Madison, WI, United States). After checking the digestion on agarose gel, 1 µL of each digestion product was amplified with eight random primers (OPY-11, OPY-6, OPA-13, OPH-18, OPW-7, OPW-8, OPW-4 and OPW-20). The amplification conditions for CRED-RA were the same as those described for RAPD analysis.
Electrophoresis
RAPD and CRED-RA PCR products were analysed by electrophoresis using 1% agarose gel in 1× Tris-borate-ethylenediaminetetraacetic acid buffer with constant voltage of 70 V for 2 h and visualized by staining with ethidium bromide under ultraviolet (UV) light. DNA ladder (GeneRuler, Thermo Fisher Scientific Inc., Waltham, MA, United States) used in the electrophoresis (BIO-RAD, Sub-Cell® Model 96 Cell, Hercules, CA, United States) was of 100 bp.
Analysis
RAPD patterns were evaluated using the Total Lab TL120 computer software (Non-linear Dynamics, Total Lab Ltd., Newcastle Upon Tyne, United Kingdom). The genomic template stability (GTS, %) was calculated as follows: GTS = 100 − (100 × a/n), where a is the average number of polymorphic bands detected in each treated sample, and n is the number of total bands in the control sample. Polymorphisms in RAPD profiles included disappearance of a normal band and appearance of a new band compared to the control sample. The average was calculated for each experimental group. To compare the sensitivity of each parameter, changes in these values were calculated as a percentage of their control (set to 100%). The average number of polymorphisms (%) was calculated for each dose to realize CRED–RA analysis. To calculate the polymorphisms (%), the following formula was used: polymorphisms (%) = 100 × a/n [Citation15].
Results and discussion
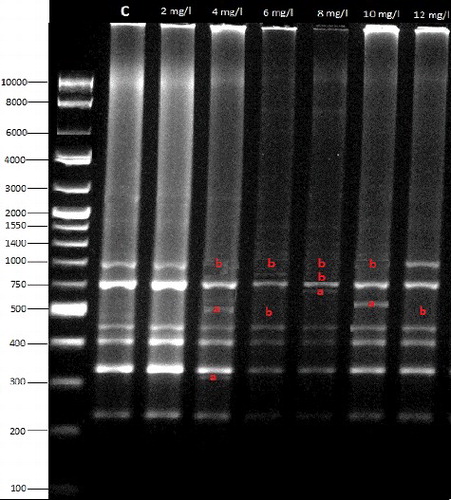
In the present study, somaclonal variation in endosperm-supported mature embryo culture of rye was detected using RAPD and CRED-RA analyses. The level of polymorphic bands varied with different primers in the RAPD patterns. Polymorphic bands were detected using nine primers (OPW-20, OPW-13, OPA-2, OPA-4, OPA-13, OPY-11, OPY-15, OPB-8 and OPW-8) and the maximum polymorphic bands were amplified with primer OPA-4 (six extra bands and five missing bands) (), whereas minimum polymorphic bands were amplified with primer OPA-13 (an extra band and a missing band) and OPY-15 (two extra bands) (). The sizes of polymorphic bands ranged from 116 (for OPA-2) to 1284 bp (for OPY-11). RAPD polymorphisms form either due to a nucleotide base change that alters the primer-binding site, or to an insertion or deletion within the amplified region. Our results indicated that RAPD was effective to detect variations that appear as a result of tissue culture. RAPD technique has been successfully used for assessment of the genetic relationship in many plant species [Citation2]. Furthermore, RAPD–PCR, in which different regions of the genome are amplified, has been widely used for identification of genetic changes in tissue culture, since it allows better analysis of genetic changes [Citation16]. RAPD markers also reveal more genetic variation than inter simple sequence repeat markers do [Citation17].
Table 1. Molecular sizes (bp) of bands (+: appearance/−: disappearance) in RAPD profiles.
The changes in the RAPD pattern were also measured as GTS (which is a qualitative measurement reflecting the changes in RAPD patterns) in relation to the pattern shown in the control plants. According to the average concentration of the used auxin, the highest GTS value was observed in the calli formed on MS medium supplemented with dicamba (96.1%). It was followed by 2,4-D (91.7%) and picloram (87.6%) auxins (). High levels of auxins, especially 2,4-D and picloram, caused a reduction in the GTS value. The polymorphism and GTS values were obviously dependent on the types and the concentrations of auxins. The maximum and the minimum GTS values were determined in the calli formed in the medium containing 2 mg/L of dicamba (98.4%) and 12 mg/L picloram (81.7%), respectively ().
Table 2. Average GTS (%) values in RAPD profiles.
Somaclonal variation is genotype-specific and its rate is variable between and within species. de la Puente et al. [Citation18] reported that rye is a species that has a high rate of somaclonal variation. On the other hand, plant growth regulators that are used in the culture medium are known to be one of the causes of somaclonal variation [Citation2]. Our study showed that a high concentration of auxin leads to variation. The reason behind this variation may be the stress inherent in cellular deprogramming induced by the synthetic hormones such as 2,4-D [Citation19]. Higher concentrations of the auxins 2,4-D or dicamba, which can stimulate somaclonal variation, are required for the induction of somatic embryogenesis in rye [Citation20]. Induction of calli using 2,4-D at high concentrations was confirmed to cause somaclonal variation in line with the studies of Gesteira et al. [Citation21] and Jin et al. [Citation22]. Furthermore, dicamba and 2,4-D are well-known mutagens in vivo with proven mutagenic effect, and the frequency of induction of point mutations (A → G) was estimated by using specially designed transgenic Arabidopsis plants [Citation23].
Epigenetic variability may be caused by DNA methylation, DNA amplification, histone modification and activation of transposable elements (transposons). These may influence the gene transcription [Citation24]. The evaluation of epigenetic modifications in in vitro has mostly focused on the analysis of DNA methylation, since this is one of the best described epigenetic mechanisms [Citation25]. The results of the CRED-RA assay were presented as the average percentage of polymorphism caused by DNA methylation for each auxin concentration. DNA hypermethylation was observed in 2,4-D and picloram, except for dicamba, when CRED-RA patterns were analysed (). Eight oligonucleotide primers produced specific and stable results. It was observed that the HpaII polymorphism was higher than the MspI polymorphism and, based on the primers means, the MspI polymorphism ranged from 6.6% to 17.3%, from 16.2% to 29.0% and from 7.3% to 19.6% for dicamba (), 2,4-D and picloram, respectively (). In our study, DNA hypermethylation (2,4-D and picloram) and hypomethylation (dicamba) were detected. Similarly, Yildirim et al. [Citation15] reported that dicamba caused DNA hypomethylation in Phaseolus vulgaris. Also, our results indicated that CRED-RA was an effective method to determine the methylation of DNA. This is in agreement with the report by Temel et al. [Citation11].
Table 3. Number of CRED-RA bands and polymorphism per cent.
Our results showed that the epigenetic changes were more frequent and variable than the genetic changes. This result is in agreement with the observations reported by Linacero et al. [Citation10]. This suggests that not only genetic changes, but also epigenetic changes should be determined for in vitro plant regeneration systems used in genetic transformation.
Conclusions
In this study, genetic and epigenetic polymorphisms were observed, when RAPD and methylation sensitive CRED-RA patterns were analysed. Increased auxin concentration led to a decrease in the GTS value. DNA hypermethylation occurred at higher concentrations of 2,4-D and picloram, whereas DNA hypomethylation was observed in dicamba. These results suggested that dicamba is more suitable than 2,4-D and picloram in genetic transformation studies in which mature embryos are used as explants in rye.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bushuk W . Rye production and uses worldwide. Cereal Food World. 2001;46(2):70–73.
- Bairu MW , Aremu AO , Van Staden J . Somaclonal variation in plants: causes and detection methods. Plant Growth Regul. 2011;63(2):147–173.
- Popelka JC , Xu JP , Altpeter F . Generation of rye (Secal e cereale L.) plants with low transgene copy number after biolistic gene transfer and production of instantly marker-free transgenic rye. Transgenic Res. 2003;12(5):587–596.
- Ma R , Pulli S . Factors influencing somatic embryogenesis and regeneration ability in somatic tissue culture of spring and winter rye. Agric Food Sci. 2004;13(4):363–377.
- Neelakandan AK , Wang K . Recent progress in the understanding of tissue culture-induced genome level changes in plants and potential applications. Plant Cell Rep. 2012;31(4):597–620.
- Linacero R , Rueda J , Esquivel E , et al. Genetic and epigenetic relationship in rye, Secale cereale L., somaclonal variation within somatic embryo-derived plants. In Vitro Cell Dev Biol-Plant. 2011;47(5):618–628.
- Kaeppler SM , Kaeppler HF , Rhee Y . Epigenetic aspects of somaclonal variation in plants. Plant Mol Biol. 2000;43(2–3):179–188.
- Vyroubalova S , Smehilova M , Galuszka P , et al. Genetic transformation of barley: limiting factors. Biol Plantarum. 2011;55(2):213–224.
- Eudes F , Acharya S , Laroche A , et al. A novel method to induce direct somatic embryogenesis, secondary embryogenesis and regeneration of fertile green cereal plants. Plant Cell Tiss Org. 2003;73(2):147–157.
- Linacero R , Alves EF , Vazquez AM . Hot spots of DNA instability revealed through the study of somaclonal variation in rye. Theor Appl Genet. 2000;100(3–4):506–511.
- Temel A , Kartal G , Gozukirmizi N . Genetic and epigenetic variations in barley calli cultures. Biotechnol Biotechnol Equip. 2008;22(4):911–914.
- Abouzied HM . Assessment of genetic diversity among wheat somaclonal variants lines using morphological traits and molecular markers. Afr J Biotechnol. 2011;10(66):14851–14861.
- Aydin M , Tosun M , Haliloglu K . Plant regeneration in wheat mature embryo culture. Afr J Biotechnol. 2011;10(70):15749–15755.
- Monsanto [Internet] . A recommended procedure for DNA extraction from plant tissues. St. Louis ( MO ): Monsanto Company; c2004 [ cited 2013 Feb 22]. Available from:www.monsanto.com/products/documents/dna-detection/dna_im.pdf
- Yildirim N , Agar G , Taspinar MS , et al. Protective role of humic acids against dicamba induced genotoxicity and DNA methylation in Phaseolus vulgaris L. Acta Agric Scand B-SP. 2014;64:1–9.
- Rawat JM , Rawat B , Mehrotra S , et al. ISSR and RAPD based evaluation of genetic fidelity and active ingredient analysis of regenerated plants of Picrorhiza kurroa . Acta Physiol Plant. 2013;35(6):1797–1805.
- Yuan XF , Dai ZH , Wang XD , et al. Assessment of genetic stability in tissue-cultured products and seedlings of Saussurea involucrata by RAPD and ISSR markers. Biotechnol Lett. 2009;31(8):1279–1287.
- de la Puente R , González AI , Ruiz ML , et al. Somaclonal variation in rye (Secale cereale L.) analyzed using polymorphic and sequenced AFLP markers. In Vitro Cell Dev Biol-Plant. 2008;44(5):419–426.
- Saieed NT , Douglas GC , Fry DJ . Induction and stability of somaclonal variation in growth, leaf phenotype and gas-exchange characteristics of poplar regenerated from callus-culture. Tree Physiol. 1994;14(1):1–16.
- Rakoczy-Trojanowska M . The effects of growth regulators on somaclonal variation in rye (Secale cereale L.) and selection of somaclonal variants with increased agronomic traits. Cell Mol Biol Lett. 2002;7(4):1111–1120.
- Gesteira AS , Otoni WC , Barros EG , et al. RAPD-based detection of genomic instability in soybean plants derived from somatic embryogenesis. Plant Breed. 2002;121(3):269–271.
- Jin SX , Mushke R , Zhu HG , et al. Detection of somaclonal variation of cotton (Gossypium hirsutum) using cytogenetics, flow cytometry and molecular markers. Plant Cell Rep. 2008;27(8):1303–1316.
- Filkowski J , Besplug J , Burke P , et al. Genotoxicity of 2, 4-D and dicamba revealed by transgenic Arabidopsis thaliana plants harboring recombination and point mutation markers. Mutat Res/Genet Toxicol Environ Mutagen. 2003;542(1):23–32.
- Mgbeze G , Iserhienrhien A . Somaclonal variation associated with oil palm (Elaeis guineensis Jacq.) clonal propagation: a review. Afr J Biotechnol. 2014;13(9):989–997.
- Miguel C , Marum L . An epigenetic view of plant cells cultured in vitro: somaclonal variation and beyond. J Exp Bot. 2011;62(11):3713–3725.