ABSTRACT
Five species of Penicillium (Penicillium chrysogenum, P. funiculosum, P. griseofulvum, P. implicatum and P. oxalicum) are implicated in seed-borne diseases. Here, we report the discovery of molecular markers based on the internal transcribed spacer regions of fungal ribosomal DNA (rDNA), which are described as primary DNA barcode markers of fungi, for rapid diagnosis and early detection of Penicillium spp. The present markers are expected to be useful for the prevention of seedling and systemic plant diseases associated with Penicillium spp. Our findings, which provide valuable insights into the taxonomy of Penicillium spp., should contribute to improve safety of agricultural produce, thereby protecting both humans and animals from harmful food contaminants such as mycotoxins. In addition, we examined the cellular fatty acid composition of five species of Penicillium. The species studied were found to possess similar fatty acid composition; however, they differed in terms of relative concentration. The principal fatty acids were oleic acid (C18:1) and linoleic acid (C18:2), comprising 80% or more of the total fatty acid composition of these species. These fatty acid profiles may be useful for characterization and identification of fungi. Data derived from the present study highlight the importance of using polyphasic methods for accurate species-level identification of Penicillium.
Introduction
The genus Penicillium, which utilizes a diverse range of substrates, is one of the most common species of fungi [Citation1]. Penicillium belongs to the phylum Ascomycota; however, its taxonomic characterization is still a matter of discussion. As such, the identification of most Penicillium species requires multidisciplinary approaches [Citation2]. Species-level identification is often very difficult challenging; in particular, numerous errors related to the identification of Penicillium have been reported in the literature. The main difficulties involved in Penicillium identification are related to various nomenclature schemes, strain variation and decisions based on minutiae [Citation3]. New varieties and species have been designated, only to be subsequently reclassified as members of existing taxa [Citation4]. Identifications are still performed according to morphology [Citation5], along with the recent application of chemical methods involving the use of secondary metabolites [Citation6] and extracellular enzymes [Citation7].
DNA- and RNA-based diagnostic methods have been extensively utilized in the field of plant pathology for the identification and classification of fungal and bacterial species [Citation8]. The use of PCR-based molecular methods, such as nested PCR [Citation9], PCR-enzyme immunoassay [Citation10] and PCR-based hybridization [Citation11], as well as PCR typing techniques such as RAPD profiling [Citation12], amplified fragment length polymorphism (AFLP) fingerprinting for P. commune [Citation13], microsatellite markers P. camemberti and P. roqueforti [Citation14], has been proposed for the identification of Penicillium spp. The DNA barcoding system, which utilizes using cytochrome c oxidase 1 (CO1), represents the most recently developed technology for this purpose [Citation15].
The internal transcribed spacer (ITS) regions of fungal ribosomal DNA (rDNA) have been utilized for the identification of P. chrysogenum and P. griseofulvum [Citation16], P. implicatum [Citation17], P. funiculosum [Citation18] and P. oxalicum. [Citation19] Amino acids [Citation20], carbohydrates [Citation21], fatty acids (FAs) [Citation22] and omega-3 polyunsaturated fatty acids have been utilized for the classification of Penicillium spp. based on chemotaxonomy [Citation23]. The fungal ITS region consists of three subregions: the ITS1and ITS2 (variable regions) and the 5.8S gene (highly conserved region) [Citation24]. The ITS region possesses numerous advantageous properties, such as high sensitivity for amplification, because of its high copy number in the fungal genome and rapid rate of evolution which result in a higher degree of sequence variation at the species level. Therefore, PCR-based techniques utilizing the ITS region generally enable greater taxonomic resolution between closely related species [Citation25]. PCR-based amplification of the ITS region provides a powerful method for the identification of fungal species, and is therefore referred to as the ‘fungal barcode’ [Citation26].
Integrated methods for the identification of fungi are based on the use of phylogenetic and chemotaxonomic markers [Citation27]. Chemotaxonomic markers used in the field of mycology include numerous compounds such as amino acids, FAs and carbohydrates [Citation28,Citation29]; in particular, FAs are increasingly being used as a chemotaxonomic tool for the identification and classification of fungi [Citation30]. Numerous researchers have used FAs as chemical markers and auxiliary tools for identifying and differentiating between important fungal species such as Fusarium [Citation30], Aspergillus [Citation31], and Alternaria [Citation32].
The present study aimed to develop molecular markers based on the ITS1 and ITS2 nucleotide sequences of Penicillium spp., in order to enable the rapid identification of Penicillium species of agricultural importance such as P. chrysogenum, P. funiculosum, P. griseofulvum, P. implicatum and P. oxalicum. In addition, the utility of FAs as chemotaxonomic markers for differentiating between species was evaluated.
Materials and methods
Fungal isolates
Twenty-five isolates of Penicillium spp. () were obtained from various sources. The Penicillium spp. isolates were identified based on their morphological characteristics and maintained for mycelial growth as described previously [Citation33].
Table 1. Isolates of Penicillium spp. used in the study.
Genomic DNA extraction
The Penicillium spp. isolates were inoculated and grown in double-layer (one solid and one liquid) media in 50-mm Petri dishes. A potato dextrose agar film was used as the solid medium for the base layer, with peptone yeast glucose as the liquid medium in the top layer. The mycelium was filtered and washed with sterilized double-distilled water. DNA was extracted as previously described [Citation34].
Identification of Penicillium spp. isolates
PCR conditions and primers for the PCR-based amplification of DNA extracted from the Penicillium spp. isolates are summarized in . PCR was performed using the PCR T Personal Thermo Cycler (Biometra, Göttingen, Germany). The amplified PCR products were sequenced using an automated ABI-Prism 377 DNA Sequencer (Applied Biosystems Inc., Foster City, CA, USA). The sequences were aligned using the MEGA 7.01 software against nucleotide National Center for Biotechnology Information (NCBI) for final identification. A phylogenetic tree was constructed using the neighbour-joining method [Citation38]. Identification at species-level was based on per cent similarity between ITS sequences [Citation39].
Table 2. Amplified regions and name primers of the five Penicillium species.
Fatty acid analysis
FA extraction and preparation of methyl esters were carried out according to a previously described method [Citation40]. FA methyl esters (FAMEs) were analysed by gas-liquid chromatography using the Perkin-Elmer Model 910 Gas Chromatograph (Perkin Elmer, Shelton, CT, USA) equipped with a flame ionization detector. Identification (qualitative and quantitative) of FAMEs was based on comparison of sample retention times with those of an authentic methyl standard (Sigma Co., St. Louis, MO, USA).
Results and discussion
Identification of Penicillium spp.
The Penicillium species were initially identified based on their morphological characteristics, as well as by phylogenetic analysis of their ITS1, ITS2 and 5.8S regions of rDNA.
P. chrysogenum
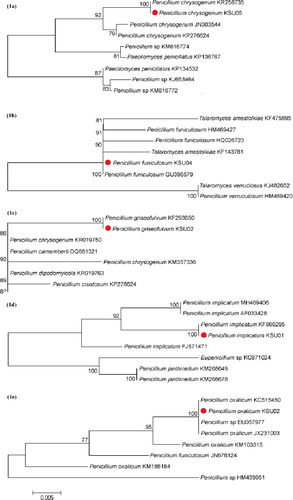
The phylogenetic tree ((a)) included nine isolates related to P. chrysogenum; three isolates were closely related to P. chrysogenum KSU05. According to the phylogenetic tree, P. chrysogenum KSU05 was classified into a clade comprising P. chrysogenum KR258735, P. chrysogenum JN303544 and P. chrysogenum KP276624. P enicillium chrysogenum KSU05 was found to be very closely related to P. chrysogenum KR258735, with an ITS sequence similarity of 99.9%.
P. funiculosum
The phylogenetic tree ((b)) included eight isolates related to P. funiculosum; six isolates were closely related to P. funiculosum KSU04. According to the phylogenetic tree, P. funiculosum KSU04 was classified into a clade comprising Talaromyces amestolkiae KF475895, P. funiculosum HM469427, P. funiculosum HQ026723, T. amestolkiae KF143781 and P. funiculosum GU396579. P enicillium funiculosum KSU04 was found to be very closely related to P. funiculosum GU396579, with an ITS sequence similarity of 99.9%.
P. griseofulvum
The phylogenetic tree ((c)) consisted of seven isolates related to P. griseofulvum; six isolates were closely related to P. griseofulvum KSU02. The phylogenetic tree indicated that P. griseofulvum KSU02 was classified into a clade consisting of seven isolates of Penicillium spp. P enicillium griseofulvum KSU02 was found to be very closely related to P. griseofulvum KF293650, with an ITS sequence similarity of 99.9%.
P. implicatum
The phylogenetic tree ((d)) consisted of eight isolates related to P. implicatum; four isolates were closely related to P. implicatum KSU01. The phylogenetic tree indicated that P. implicatum KSU01 was classified into a clade comprising P. implicatum MH469404, P. implicatum AF033428 and P. implicatum KF866295. P enicillium implicatum KSU01was found to be very closely related to P. implicatum KF866295, with an ITS sequence similarity of 99.9%.
P. oxalicum
The phylogenetic tree ((e)) included eight isolates related to P. oxalicum; four of these were closely related to P. oxalicum KSU02. According to the phylogenetic tree, P. oxalicum KSU02 was classified into a clade comprising P. oxalicum KC515450, Penicillium spp. EU057977 and P. oxalicum JX231003. P enicillium oxalicum KSU02 was found to be very closely related to P. oxalicum KC515450, with an ITS sequence similarity of 99.9%.
The rDNA genes of fungi contain 5.8S, 18S and 28S genes (highly conserved regions) and ITS1 and ITS2 regions (variable regions), which form the genetic basis for the organization of fungal species into taxonomic groups. The ITS regions, which are located in the rDNA gene complex, are arranged as follows: 18S-ITS1-5.8S-ITS2-28S [Citation41]. The ITS1 region exhibits a higher degree of variability than the ITS2 region [Citation42].
The ITS region exhibits major sequence variation between genera as well as species of fungi; however, a minor degree of sequence variation has been observed between isolates of the same species [Citation43]. ITS sequences are premium identical in several genera of fungi, such as Aspergillus [Citation44], Fusarium [Citation45] and Penicillium [Citation46], that are known to be pathogenic to plants. As of 2012, approximately 172,000 sequences of the fungal ITS region were utilized for the identification of 2500 genera and 15,500 species [Citation46].
Numerous applications of ITS sequences, such as the characterization of fungi [Citation47], phylogenetic research [Citation48], direct sequencing of DNA from environmental samples [Citation49] and investigation of the epidemiology and dynamics of emerging diseases [Citation50], have been reported. The present findings should contribute to the application of ITS regions as ‘standard barcodes’ for the taxonomic classification of fungi [Citation46].
AFLP fingerprinting, whose discriminatory power is higher than that of M13 fingerprinting and RAPD, has revealed the existence of 55 AFLP groups among 321 P. commune isolates, which were identified based on their phenotypic characteristics [Citation13]. Microsatellites have been shown to be powerful for species-specific barcoding of Penicillium genera [Citation14]. The identification of Penicillium species using ITS sequences has been shown to enable superior species-level resolution, thereby providing a potential solution to the challenges related to the taxonomic classification of this genus [Citation51].
Fatty acid profiles
FA profiles for each Penicillium spp. are summarized in ; the chain lengths of the FAs present in each Penicillium species ranged from 14 to 20 carbons. Most fungi possess a similar FA composition, with varying concentrations of FAs. The most common and abundant FAs extracted were oleic acid (C18:1) and linoleic acid (C18:2), which comprised 80% or more of the total peak area for the five Penicillium species studied.
Table 3. Fatty acid profiles of the five Penicillium species.
Cellular FA composition is now routinely used for the identification and differentiation of micro-organisms [Citation22]; in particular, FA profiles have recently been used as a chemotaxonomic tool for the identification and classification of bacteria [Citation52]. Additionally, FA-based characterization is increasingly being used to distinguish between numerous species of fungi such as Fusarium spp., [Citation30] Aspergillus spp., [Citation31] Penicillium spp., [Citation53] and Alternaria spp. [Citation32].
The cellular FA composition of 18 species of Penicillium was studied to investigate the utility of FAs as a taxonomic tool. Most fungi studied displayed the same FA composition but differed in terms of the relative concentration of the FAs. The principal FAs identified were palmitic acid (16:0), oleic acid (18:1) and linoleic acid (18:2). The proportion of unsaturated FAs was 68.5%–78.5%. Multivariate analyses of data showed that it is possible to differentiate between some species belonging to different Penicillium groups. The level of agreement of long chain FAs with morphological taxonomy was acceptable [Citation53,Citation54].
Cellular FA composition was utilized as a taxonomic tool to discriminate between different Aspergillus species. The most important FAs identified were palmitic acid (C16:0), stearic acid (C18:0), oleic acid (C18:1) and linoleic acid (C18:2), which collectively represented 95% of the total FA content of Aspergillus. Multivariate data analysis demonstrated that FA analysis is a useful tool for differentiating between species belonging to the genus Aspergillus. Therefore, analysis of FA composition may serve as a useful tool for the species-level identification of filamentous fungi [Citation31].
The cellular FA composition of nine species of Fusarium was analysed. The FA profiles of most Fusarium species studied (seven out of nine) consisted of the same FAs; however, differences were observed between the FA profiles of the some of the species. Lauric acid, myristic acid, palmitoleic acid, palmitic acid, heptadecanoic acid, oleic acid, stearic acid, linoleic acid, arachidic acid and behenic acid were detected in all Fusarium species investigated. However, caprylic acid, capric acid, tridecanoic acid, pentadecanoic acid and linolenic acid were not detected in some of the Fusarium species. The FA profiles may be useful for characterization and identification of fungi [Citation30].
Conclusions
The taxonomic classification of Penicillium spp. has historically been controversial. However, the present results strongly suggest that polyphasic methods, such as the use of molecular and biochemical markers, represent powerful and effective taxonomic tools for the identification and classification of the genus Penicillium.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding this Research Group (RG-269).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Pitt JI , Samson RA , Frisvad JC . List of accepted species and their synonyms in the family Trichocomaceae. In: Samson RA , Pitt JI , editors. Integration of modern methods for Penicillium and Aspergillus classification. Amsterdam: Harwood Academic Publishers; 2000. Chapter 1, p. 9–50.
- Grimm LH , Kelly S , Krull R , et al. Morphology and productivity of filamentous fungi. Appl Microbiol Biotechnol. 2005;69:375–384.
- Onions AHS , Bridge PD , Paterson RR . Problems and prospects for the taxonomy of Penicillium . Microbiol Sci. 1984;1:185–189.
- Frisvad JC , Filtenborg O , Lund F , et al. The homogeneous species and series in subgenus Penicillium are related to mammal nutrition and excretion. In: Samson RA, Pitt JI , editors. Integration of modern methods for Penicillium and Aspergillus classification. Amsterdam: Harwood Academic Publishers; 2000. Chapter 5, p. 265–284.
- Raper KB , Thom C . A manual of the Penicillia . Baltimore (MD):Williams and Wilkins; 1949.
- Frisvad JC , Thrane U , Filtenborg O . Role and use of secondary metabolites in fungal taxonomy. In: Frisvad JC , Bridge PD, Arora DK , editors. Chemical fungal taxonomy. New York (NY): CRC Press; 1998. Chapter 12, p. 289–331.
- Filtenborg O , Frisvad JC , Thrane U . Moulds in food spoilage. Int J Food Microbiol. 1996;33:85–102.
- Versalovic J , Swanson DS , Musser JM . Nucleic acid sequencing studies of microbial pathogens: insight into epidemiology, virulence, drug resistance, and diversity. In: Persing DH , editor. PCR protocols for emerging infectious diseases. Washington (DC): ASM Press; 1996. Chapter 4, p. 59–88.
- Zeng H , Li X , Chen X . Identification of Penicillium marneffei in paraffin-embedded tissue using nested PCR. Mycopathologia. 2009;168:31–35.
- Lindsley MD , Hurst SF , Iqbal NJ , et al. Rapid identification of dimorphic and yeast-like fungal pathogens using specific DNA probes. J Clin Microbiol. 2001;39:3505–3511.
- Vanittanakom N , Merz WG , Sittisombut N , et al. Specific identification of Penicillium marneffei by a polymerase chain reaction⁄hybridization technique. Med Mycol. 1998;36:169–175.
- Geisen R , Cantor MD , Hansen TK , et al. 2001. Characterizationof Penicillium roqueforti strains used as cheese starter cultures by RAPD typing. Int J Food Microbiol. 2001;65:183–191.
- Lund F , Nielsen AB , Skouboe P . Distribution of Penicillium commune isolates in cheese dairies mapped using secondary metabolite profiles, morphotypes, RAPD and AFLP fingerprinting. Food Microbiol. 2003;20:725–734.
- Giraud F , Giraud T , Aguileta G . Microsatellite loci to recognize species for the cheese starter and contaminating strains associated with cheese manufacturing. Int J Food Microbiol. 2010;137:204–213.
- Seifert KA , Samson RA , deWaard JR , et al. Prospects for fungus identification using CO1 DNA barcodes, with Penicillium as a test case. PNAS. 2007;104(10):3901–3906.
- Demirel R , Sariozlu NY , İlhan S . Polymerase chain reaction (PCR) identification of terverticillate Penicillium species isolated from agricultural soils in Eskişehir Province. Braz Arch Biol Technol. 2013;56(6):980–984.
- Paz Z , Komon-Zelazowska M , Druzhinina IS , et al. Diversity and potential antifungal properties of fungi associated with a Mediterranean sponge. Fungal Divers. 2010;42:17–26.
- Sun J , Najafzadeh MJ , Zhang J , et al. Molecular identification of Penicillium marneffei using rolling circle amplification. Mycoses. 2011;54:751–759.
- Larena I , Melgarejo P . Envelopment of a method for detection of the biocontrol agent Penicillium oxalicum strain 212 by combining PCR and a selective medium. Plant Dis. 2009;93(9):919–928.
- Bazzolli DMS , Ribon ABO , Queiroz MV , et al. Molecular characterization and expression profile of pectin-lyase-encoding genes from Penicillium griseoroseum . Can J Microbiol. 2006;52:1070–1077.
- Barreto-Bergter E , Figueiredo RT . Fungal glycans and the innate immune recognition. Front Cell Infect Microbiol. 2014;4:1–17.
- Devi P , Shridhar MP , Souza LD , et al. Cellular fatty acid composition of marine-derived fungi. Indian J Mar Sci. 2006;35(4):259–310.
- Tsurkan Y , Karpenyuk T , Guschina I , et al. Identification of newly-isolated microorganisms containing valuable polyunsaturated fatty acids. J Biotechnol Res. 2015;6:14–20.
- Nilsson RH , Ryberg M , Abarenkov K , et al. The ITS region as a target for characterization of fungal communities using emerging sequencing technologies. FEMS Microbiol Lett. 2009;296(1):97–101.
- Michaelsen A , Flavia P , Katrin R , et al. Application of molecular techniques for identification of fungal communities colonising paper material. Int Biodeter Biodegr. 2006;58(3–4):133–141.
- Schoch CL , Seifert KA , Huhndorf S , et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. P Natl Acad Sci USA. 2012;109:6241–6246.
- Samson R , Visagie CM , Houbraken J , et al. Phylogeny, identification and nomenclature of the genus Aspergillus . Stud Mycol. 2014;78:141–173.
- Frisvad JC , Larsen TO , de Vries R , et al. Secondary metabolite profiling, growth profiles and other tools for species recognition and important Aspergillus mycotoxins. Stud Mycol. 2007;59:31–37.
- Zain ME . Biochemical markers in taxonomy of the genus Fusarium . Res J Agric Biol Sci. 2010;6(1):1–7.
- Zain ME , Bahkali AH , Al-Othman MR . Developments in using fatty acids in fungal chemotaxonomy. Afr J Microbiol Res. 2013;7(38):4638–4645.
- Fraga ME , Santana DM , Gatti MJ , et al. Characterization of Aspergillus species based on fatty acid profiles. Mem Inst Oswaldo Cruz. 2008;103(6):540–544.
- Hashem A , Abd-Allah EF , Al-Huqail AA , et al. Report and characterization of Alternaria alternate (FR.) KEISSLER on Avicennia marina (FORSK.) VIERH forests of industrial Yanb'a city, Saudi Arabia. Pak J Bot. 2014;46(2):725–734.
- Mahmoud MA , Ali HM , El-Aziz ARM . Molecular characterization of aflatoxigenic and non-aflatoxigenic Aspergillus flavus isolates collected from corn grains. Genet Mol Res. 2014;13(4):9352–9370.
- Mahmoud MA Detection of Aspergillus flavus in stored peanuts using real-time PCR and the expression of aflatoxin genes in toxigenic and atoxigenic A. flavus isolates. Foodborne Pathog Dis. 2015;12(4):289–296.
- Dupont J , Dennetière B , Jacquet C , et al. PCR-RFLP of ITS rDNA for the rapid identification of Penicillium subgenus Biverticillium species. Rev Iberoam Micol. 2006;23(3):145–150.
- Samson RA , Seifert KA , Kuijpers AF , et al. Phylogenetic analysis of Penicillium subgenus Penicillium using partial β-tubulin sequences. Stud Mycol. 2004;49:175–200.
- Larena I , Melgarejo P . Development of a method for detection of the biocontrol agent Penicillium oxalicum strain 212 by combining PCR and a selective medium. Plant Dis. 2009;93(9):919–928.
- Saitou N , Nei M . The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 1987;4:406–425.
- Higgins KL , Arnold AE , Miadlikowska J , et al. Phylogenetic relationships, host affinity, and geographic structure of boreal and arctic endophytes from three major plant lineages. Mol Phylogenet Evol. 2007;42:543–555.
- Lepage G , Roy CC . Direct transesterifcation of all classes of lipids in a one-step reaction. J Lipid Res. 1986;27:114–119.
- Iwen PC , HinrichS SH , Rupp ME . Utilization of the internal transcribed spacer regions as molecular targets to detect and identify human fungal Pathogens. Med Mycol. 2002;40:87–109.
- Mullineux T , Hausner G . Evolution of rDNA ITS1 and ITS2 sequences and RNA secondary structures within members of the fungal genera Grosmannia and Leptographium . Fungal Genet Biol. 2009;46:855–867.
- Henry T , Peter C , Iwen S , et al. Identification of Aspergillus species using internal transcribed spacer regions 1 and 2. J Clin Microbiol. 2000;38(4):1510–1515.
- Kebeish RM , El-Sayed AS . Morphological and molecular characterization of L-methioninase producing Aspergillus species. Afr J Biotechnol. 2012;11(87):15280–15290.
- Datta S , Choudhary RG , Shamim M . Polymorphism in the internal transcribed spacer (ITS) region of the ribosomal DNA among different Fusarium species. Arch Phytopathology Plant Protect. 2011;44(6):558–566.
- Schoch CL , Seifert KA , Huhndorf S , et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. PNAS. 2012;109(16):6241–6246.
- Nilsson RH , Martin R , Kessy A . The ITS region as a target for characterization of fungal communities using emerging sequencing technologies. FEMS Microbiol Lett. 2009;296:97–101.
- Shenoy BD , Jeewon R , Hyde KD. Impact of DNA sequence-data on the taxonomy of anamorphic fungi. Fungal Divers. 2007;26:1–54.
- Oliver AK , Callaham MA , Jumpponen A . Soil fungal communities respond compositionally to recurring frequent prescribed burning in a managed southeastern US forest ecosystem. Forest Ecol Manag. 2015;345:1–9.
- Sulaiman IM , Emily J , Steven S , et al. Molecular identification of isolated fungi from unopened containers of Greek yogurt by DNA sequencing of internal transcribed spacer region. Pathogens. 2014;3:499–509.
- Tiwari KL , Jadhav SK , Kumar A . Morphological and molecular study of different Penicillium species. Middle East J Sci Res. 2011;7(2):203–210.
- Cody RB , McAlpin CR , Cox CR , et al. Identification of bacteria by fatty acid profiling with direct analysis in real time mass spectrometry. Rapid Commun Mass Spectrom. 2015;29:2007–2012.
- Silva TL , Sousa E , Pereira PT , et al. Cellular fatty acid profiles for the differentiation of Penicillium species. FEMS Microbiol Lett. 1998;164:303–310.
- Hashem A , Abd-Allah EF , Al-Qarawi AA . Biodiversity of seedborne mycobiota associated with Sesamum indicum L. J Pure Appl Microbiol. 2014;8(4):2845–2854.