ABSTRACT
Combination therapy can be used for the treatment of fungal infections, especially for those caused by antifungal-resistant fungi. In the present study, in vitro interactions and mechanisms between tunicamycin (TM) and amphotericin B (AmB) against Candida spp. were evaluated. The nature of the interactions determined by spectrophotometric method in a checkerboard assay was interpreted using nonparametric models of fractional inhibitory concentration index (FICI). We also evaluated the potential activity of TM in association with AmB on selected virulence gene (MP65 and ERG3) in C. albicans and C. dubliniensis. It was found that TM can work synergistically with AmB against C. dubliniensis; the minimum inhibitory concentration of TM and AmB decreased about fourfold, with an FICI of 0.5. The effect of combination has also significantly decreased the expression level of MP65 and ERG3 in which these genes have long been recognized as virulence traits in the pathogenicity of Candida (P ˂ 0.05). Our results suggest that the combination has the ability to reduce the growth of Candida in vitro.
Introduction
Invasive fungal infections, such as candidiasis, represent a public health problem. Since its discovery in 1839, the genus Candida has been shown to be the causative agent of many infections in an increasing range of anatomical sites and clinical settings [Citation1]. The high global incidence and prevalence of oral candidiasis may be attributed to an increasing usage of broad-spectrum antibiotics, cytotoxic and corticosteroids [Citation2]. The antifungal agents that are available for the treatment of Candida spp. infections can be categorized into several chemical classes with different cellular targets and among those, the polyenes and tunicamycin. Polyenes such as amphotericin B (isolated from streptomycin spp.) bind ergosterol and disrupt the major lipid component of the fungal cell membrane, thus resulting in leakage of intracellular ions [Citation3]. While, tunicamycin is a nucleoside antibiotic produced by Streptomyces lysosuperficus that blocks N-linked glycosylation and the formation of N-glycosidic protein–carbohydrate linkages in eukaryotic cells. Specifically, tunicamycin stops the synthesis of N-linked oligosaccharide chains on mannoprotein [Citation4]. As the major components of Candida albicans cell wall are mannoprotein, the secretion of this material plays an important structural and functional role during host pathogen interactions [Citation4].
In recent years, intense research has been conducted, leading to a better understanding of the molecular mechanisms and there are several mechanisms have been documented to be involved in the resistance to the polyenes and tunicamycin. The recent discovery of drug-target enzyme alterations or the absences of the target enzyme are among mechanisms that involved in antifungal resistance which opens up a new therapeutic concept [Citation5,Citation6].
The changes in ergosterol content may contribute to the development of resistance to amphotericin B and there are two important enzymes that participate in ergosterol synthesis; (1) C-8 sterol isomerase-activity of these enzymes is regulated by ERG2 gene and (2) C-5 sterol desaturase enzyme which is encoded by ERG3 gene [Citation7]. Changes in MP65 gene which encodes a cell wall mannoprotein by tunicamycin also might affect cell wall organization and has been suggested as an attractive potential target for the development of antifungal drugs [Citation8]. Previous studies indicate that MP65 gene appears in cell wall mannoprotein synthesis (Mp65p) and stability [Citation8] which is then translated into fungal virulence.
But some of the most effective antifungal drugs are too toxic for continuous use or can only be administered intravenously [Citation9]. The increasing incidence of fungal infections without a satisfactory response to the current antifungal therapy and the slow development of new agents with novel mechanisms of action have produced significant interest on associations between antifungal agents [Citation10]. The ideal antifungal drug would be non-toxic, fungicidal and amenable to self-administration. Combination therapy is one approach that can be used to improve the efficacy of antimicrobial therapy. Thus, the potentiating of the antifungal effect by its combination with different classes of partner drugs would be very useful.
Therefore, the present study was designed to investigate the in vitro anti-candidal activity of tunicamycin and amphotericin B alone and in combination against five Candida species in planktonic form. In addition, their effect on cell wall mannosylation (MP65) and ergosterol biosynthesis (ERG3) gene expression was also examined.
Materials and methods
Candida strains and growth condition
Five strains of Candida species used in this study were purchased from The American Type Culture Collection (ATCC), USA. The species were C. albicans (ATCC 14053), C. glabrata (ATCC 90030), C. tropicalis (ATC 13803), C. parapsilosis (ATCC 22019) and C. dubliniensis (ATCC MYA-2975). Upon revival, each respective candida strain was stored in 20% v/v glycerol and kept in −70 °C. Short-term storage was also carried out for all strains and subcultured monthly on Yeast Peptone Dextrose (YPD) agar media (BD Difco, USA). The working stock cultures were maintained weekly using the same agar media and kept refrigerated at 4 °C throughout the experimental period for a maximum of two weeks to ensure viability of cells.
Preparation of the standard Candida cell suspension and antifungal agents
Each Candida strain was respectively cultured on YPD agar media (BD Difco, USA) at 37 °C for 18 h. To prepare the yeast inoculum, a loopful of the respective candidal growth was transferred into YPD broth and incubated for 24 h at 37 °C in a rotary shaker (Labnett 211DS) at a speed of 75 rpm. The turbidity of the respective suspension was adjusted to an optical density (OD550nm) of 0.144 which is equivalent to 1 × 106 cells/mL.
A stock solution of 500 µg/mL of tunicamycin (TM) was prepared in 5% DMSO and stored at 4 °C until used.
A stock solution of 250 µg/mL of amphotericin B (AmB) was used in combination with TM. AmB was also used in the study as the positive control. The median minimum inhibitory concentrations (MIC50) of the antifungal were determined according to the microdilution method of the Clinical Laboratory Science Institute (CLSI) [Citation11].
The Kirby–Bauer susceptibility test
The antifungal activity of TM and AmB alone and in combination was carried out based on the disc-diffusion concept of Kirby–Bauer susceptibility test. Candida yeast cell suspension (1.0 × 106 cells/mL) was swab evenly over a Mueller Hinton agar (MHA) plate. Then, sterile paper discs impregnated with different concentration of TM and AmB were placed on the agar surface, i.e. 500 µg/mL for TM combined with 250 µg/mL for AmB. Throughout this experiment, a blank disc impregnated with sterile distilled water represented as negative control and another disc impregnated with TM and AmB alone represented as the positive control were included. The volume of the combined antifungal agents, positive and negative controls impregnated onto the discs was standardized to 100 µL. The susceptibility of each Candida species was determined by the diameter and pattern of the inhibited zones surrounding the discs.
Determination of minimum inhibitory concentrations (MIC50)
The 50% minimum inhibitory concentrations (MIC50) of antifungal agents alone against the Candida sp. were determined according to the microdilution method of CLSI using 96-well microtiter plates with some modifications [Citation11]. Each well contained 10 µL of the respective 1.0 × 106 cells/mL Candida sp., 100 µL of YPD broth and 100 µL of antifungal agent. YPD broth without test agents was included as an agent-free control and used as medium blank in our study. All plates were incubated at 37 °C for 24 h, after which the growth was determined spectrophotometrically at 550 nm by means of a microplate reader (PowerWave 200, Bio-Tek Instruments, Winooski, VT, USA). The MIC50 were considered to be the concentration that inhibited 50% of fungal growth and was used for further analysis. All data obtained were analysed and reported as the median of at least three independent tests. The effects of combinations were confirmed by the checkerboard method.
Assessment of drug synergy against oral planktonic Candida spp.
Synergistic effects of TM in combination with AmB against five planktonic Candida spp. were quantitatively determined using the checkerboard microdilution method as described in the previous study [Citation12]. Different concentrations of antifungal combination were prepared in a microtiter plate as described earlier. The growth was determined spectrophotometrically at 550 nm by means of a microplate reader. The concentration of antifungal combination that inhibited 50% growth of the Candida spp. was recorded as the median MIC (MIC50) value. The negative and positive control was performed using sterile distilled water and amphotericin B, respectively. The MIC values of combined TM + AmB used to determine the fractional inhibitory concentration (FIC), i.e. the ratio of the MIC of an agent used in combination to the MIC50 of the agent used alone. The FIC index (∑FIC, the sum of individual FICs) was calculated using the formula: ∑FIC = MIC50(Acomb)/MIC50(Aalone) + MIC50(Bcomb)/MIC50(Balone). ∑FIC < 0.5 indicates synergy; 0.6–0.9 indicates partial synergy; 1.0–4.0, indifference and >4, antagonism [Citation13].
Real-time PCR
C andida albicans and C. dubliniensis were selected for the further study to explore the gene expression profiling based on FIC index obtained from our study.
RNA extraction cDNA formation by reverse transcriptase
Total RNA from planktonic cells was isolated using the RiboPure yeast kit (Ambion, Inc.) according to the manufacturer's instructions. RNA concentrations and purity were determined by measuring the absorbance at 260 and 280 nm (ND-2000 spectrophotometer, NanoDrop Technologies) (Thermo Scientific, USA). Equal amounts of RNA (2 μg in 20 μL reactions) were reverse transcribed with specific primers using the VILO Superscript reverse transcriptase (Invitrogen).
Quantitative PCR (qPCR) using the listed primer
Sequences of C. albicans and C. dubliniensis genes were downloaded from the Candida Genome Database: http://www.Candidagenome.org. The optimal conditions for choosing efficient primers included: biasing the primers towards the 3' end of the gene, restricting the amplicon size between 50 and 200 base pairs, maintaining the GC content of the primers at a range of 40%–60% and keeping the difference in melting. Primer3 software was used to design ERG3 primers. Primers for MP65 and ACT1 were obtained from the study of Sandini et al. () [Citation8]. The actin producing gene, ACT1 was employed in the current investigation as the housekeeping gene.
Real-time PCR was performed in 96-well plates using the ABI 7500 fast real-time PCR machine (Applied Biosystems, Rotkreuz, Switzerland) using SYBR® Select Master Mix kit (Bio-Rad, USA). Two microliters of diluted cDNA samples and 20 µL of mastermix (containing the primers) were added to the plates. Real-time PCR reaction was performed at 95 °C for 5 min, followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C. Control samples were included on each plate to ensure that multiple plates could be compared. Expression of genes was calculated using the 2−ΔΔCT method [Citation14].
Statistical analysis
All results were computed and expressed as means ± standard deviation (SD) from three determination performed in triplicate (n = 9). Statistical analysis was performed using SPSS software. Analysis of variance (One-Way ANOVA) was used to compare the significant difference in gene expression between the groups. A P-value of < 0.05 was considered as statistically significant.
Results and discussion
The Kirby–Bauer susceptibility test
Candida is a true opportunistic pathogen that under certain circumstances is able to invade tissues normally resistant to infection. Infections of Candida cause a wide spectrum of diseases. However, the slow developments of new agents with novel mechanisms of action have produced significant interest on associations between antifungal agents. The selection of the ATCC reference strains C. albicans (ATCC 14053), C. parapsilosis (ATCC 22019), C. tropicalis (ATC 13803), C. dubliniensis (ATCC MYA-2975) and C. lusitaniae (ATCC 64125) was based on various reports of the prevalence of Candida species in the oral cavity [Citation15]. Although the reference strains were isolated originally from blood, similar strains have also been reported to be present in the oral cavity [Citation15].
A combination of antifungal drugs was screening for their effectiveness against five different Candida spp. From the initial screening with disc-diffusion assay, TM in combination with AmB exhibited considerable anti-candidal activity than TM alone. When a 500-µg/mL of TM was combined with 250-µg/mL of AmB, C. albicans exhibited antifungal activity with 18 mm inhibition zone (). The antifungal activity of this combination showed the neutral behaviour, leaving this activity unchanged. Against C. glabrata, inhibition zone of antifungal activity was 19.5 mm, whereas against C. parapsilosis, the antifungal activity showed a lowest activity which is 15 mm. Against C. tropicalis, combination of TM + AmB expressed of 19 mm inhibition zone. However, against C. dubliniensis, it showed a high antifungal activity as expressed by inhibition zone of 20 mm (). To quantify the indicated synergistic effects of TM and AmB, we evaluated the combinations in a checkerboard microdilution assay.
Figure 1. Effect of TM in combination with AmB on growth of Candida sp. Growth inhibition zones on MHA.
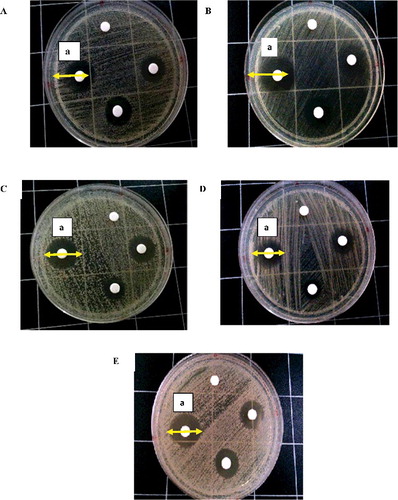
Table 1. Oligonucleotide sequences of MP65, ERG3 and ACT1 gene.
Minimal inhibitory concentration (MIC50) of Candida species against TM and AmB
The inhibitory activities of TM and AmB alone against a series of oral-associated Candida spp. were investigated (). Planktonic growth of Candida spp. was susceptible to different antifungal drugs at varying concentrations with the MIC50 values of TM ranging from 7.8 to 15.6 µg/mL. The MIC50 of AmB against the Candida species were lower than those of TM, ranging from 1.95 to 7.8 µg/mL. It has been found that, C. glabrata were the least susceptible to both respective antifungal of TM and AmB.
Table 2. Effect of TM and AmB alone and in combination against oral Candida spp. on agar diffusion assay.
Table 3. Susceptibility of planktonic Candida species to tunicamycin (TM) and amphotericin B (AmB) alone and in combination (MIC50).
Based on MIC50 results, AmB demonstrated greater effectiveness and showed the best performance against Candida spp. than TM. AmB has a high fungicidal potential, while TM exhibited a more moderate fungicidal ability. The differences between the fungicidal effect of TM and AmB may be caused by their distinct action mechanisms. Therefore, with the aim to improve the antifungal efficacy of TM, we decided to use it in combination with the conventional drug AmB.
The effect of antifungal combination on the planktonic growth of Candida species
In this study, the phenomenon of the interactions between tunicamycin and amphotericin B was also determined qualitatively by checkerboard microdilution method. The FICI model is the most commonly used approach to study the interaction between antifungal drugs and has been used to interpret the data. Checkerboard analysis has determined that the combination of TM and AmB having MIC50 of synergistic, partial synergistic and indifference effect against five planktonic Candida sp. with ∑FIC index ≥ 0.5 and these data ranged from 1.95 to 7.8 µg/mL and 0.98 to 3.9 µg/mL, respectively. The MIC50 results of antifungal combination shown in allow us to note that the yeast classified as non-albicans had MIC lower than C. albicans. These data demonstrate that non-albicans strains are more sensitive to the tested combination.
MIC50 of TM and AmB in combination against C. albicans were reduced to 7.8 and 3.9 µg/mL, respectively (), which showed a decreased about twofold dilution. However, this combination was found to display indifference effect with the FIC index of 1.0.
For C. glabrata, the MIC50 of TM and AmB in combination were brought down to 1.95 and 0.98 µg/mL, respectively, which showed a decreased of about fourfold dilution compared by itself. FIC index for the combination was calculated to be 0.75, which indicated that combination is partial synergistic.
Against planktonic of C. parapsilosis, MICs of TM and AmB in combination were reduced to 3.9 and 1.95 µg/mL, respectively. The MIC concentration decreased about two and fourfold for TM and AmB, respectively. In the combinations, partial synergistic interaction was also observed in C. parapsilosis with the FIC index of 0.75.
Combination of TM and AmB showed 50% inhibition (MIC50) of planktonic growth of C. tropicalis at concentration of 3.91 and 1.95 µg/mL, respectively. The MIC50 values decrease fourfold for TM and two fold for AmB. However, partial synergistic was observed for this combination against C. tropicalis with the FIC index of 0.75.
The MIC50 of combinations for C. dubliniensis were 3.9 and 1.95, respectively, which decrease about fourfold. Synergistic was observed in C. dubliniensis with the FIC index of 0.5. Thus, it showed that the combination of TM with AmB is a good combination against planktonically cells of C. dubliniensis since it showed the best results among all species.
Although the mechanism of interaction between TM and AmB is not known, it is possible that the loss of cell wall integrity/disruption of cell wall by TM allowing or facilitates the cellular uptake of AmB. In order to search the mechanism of synergism between TM and AmB, their individual antifungal modes of action need to be considered.
Real-time PCR (qPCR) detection of gene expression level
C andida albicans and C. dubliniensis were selected for further study to explore the gene expression profiling based on FIC index obtained from our study. The highest activity of antifungal combination was shown against C. dubliniensis. In contrast, the lowest activity of this combination was displayed against C. albicans.
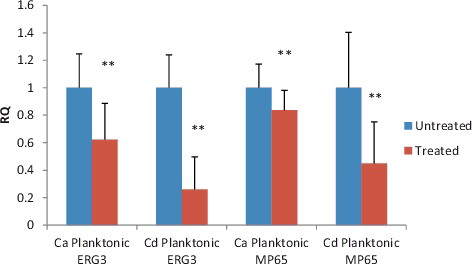
The mannoprotein (MP65) and ergosterol (ERG3) biosynthesis gene have been recognized as virulence traits in the pathogenicity of Candida species. Thus, we investigate the effect of combination on MP65 and ERG3 expression using real-time PCR assay. From the analysis, we found that MP65 and ERG3 gene in C. albicans and C. dubliniensis were down-regulated after exposure to the combination. Strong down-regulation of MP65 and ERG3 was observed in C. dubliniensis than in C. albicans. Statistical significant differences in the expression of ERG3 and MP65 were observed between cells exposed to combination and untreated cells in both species.
Transcriptional response of MP65 and ERG3 gene in planktonic C. albicans
Gene expression in C. albicans treated with median MIC (MIC50) of combination was compared to gene expression in untreated and results are shown in . All experiments were performed at least from three independent biological samples in duplicates. The expression level of genes encoding enzymes involved in ergosterol biosynthesis (ERG3) and gene encodes for putative β-glucanase mannoprotein (MP65) in C. albicans were significantly down-regulated after 1 h exposure (P value ≤ 0.05). The fold changes of ERG3 and MP65 genes in planktonic state were 0.622 and 0.883, respectively.
Down-regulation of MP65 and ERG3 in C. albicans showed the effectiveness of combination and this finding was in contrast with result from FIC index which indicates that the interaction of combination was indifference. Even though the checkerboard method is relatively simple to perform and the results are easily interpreted, however, it also has some limitations to detect the changes in susceptibility end points that permit interpretations of synergy or antagonism [Citation16]. As emphasize in other published literature, the use of real-time PCR provides a more specific and sensitive test with respect to the current microbiology, biochemical and serological diagnostic method [Citation17].
Cell wall of C. albicans is the initial site of host–pathogen interactions and is an obvious target for the development of antifungal and vaccines. TM in association with AmB reduced the expression level of MP65 gene by preventing the synthesis of mannoprotein (Mp65p) where these mannoproteins are important components in the cell wall organization. Apart from that, tunicamycin is also well-known endoplasmic-reticulum-stressing (ER) agents that lead to the accumulation of unfolded protein in the ER by inhibiting N-linked glycosylation which is important for proper protein folding.[Citation18]. Cumulative evidence suggests that ER stress induces cell death. For instance, intense or prolonged ER stress induced by tunicamycin leads to the activation of apoptosis pathways in mammalian cells [Citation19]. However, since apoptotic pathways are not entirely conserved in fungi [Citation20], the ER stress occurred is likely to be different. One possibility is that it involves simple pathways due to the toxic accumulation of unfolded proteins that disrupts the cell survival. The ER is involved in important cellular functions, including the processing of secreted proteins, membrane synthesis and calcium storage. The vast majority of secretory and transmembrane proteins are folded and assembled in the ER lumen before being delivered to the cell surface or outside the cell [Citation21]. Increased demand of protein secretion overloads the ER with unfolded proteins, causing a condition termed ER stress. However, until now, the mechanistic features and pathophysiological importance of ER stress-induced cell death in any of the Candida species remain unclear.
Perturbation of membrane structure and function was also evident from the ergosterol levels that were significantly reduced in C. albicans when grown in the presence of combination which proves that combination obliterates membrane veracity. Reduced in ergosterol content in the cell membrane causes accumulation of toxic ergosterol precursors and inhibit cell growth. These results also support the idea from previous study by Martel et al. [Citation22], that suppress in ERG3 gene expression or loss of function in Erg3p when exposed to antifungal combination results in disorganized membrane with increased permeability leads to the leakage of cytosolic components and, therefore, fungal death.
Transcriptional response of MP65 and ERG3 gene in planktonic C. dubliniensis
We also investigated the expression level of ERG3 and MP65 genes in C. dubliniensis (). Our result showed that the relative quantification of MP65 and ERG3 expression displayed significant down-regulation (P < 0.0001) compared to untreated control after 1 h treatment with antifungal combination in concentrations based on MIC50. Our finding has confirmed the efficacy in preventing the growth of C. dubliniensis in planktonic state which could be related to the down-regulated expression of ERG3 and MP65 gene. Hence, it could be proposed that combination has the ability to inhibit drug resistance of C. dubliniensis by affecting gene expression in mannoprotein and ergosterol biosynthesis. The fold changes of ERG3 and MP65 genes in planktonic state were 0.260 and 0.45, respectively. These results were in accordance with our FIC index which reported that the combination has synergistic effect against C. dubliniensis. Besides that, C. dubliniensis has a low capacity to produce hyphae, resulting in lower levels of colonization and tissue invasion compared to C. albicans. Different sites of interaction are possible and can even lead to synergistic effects but the exact mechanisms behind the effect are still not clear.
According to qPCR results, amphotericin B in associations with TM showed a better activity in C. dubliniensis than C. albicans and it can be developed as a promising anti-biofilm strategy against C. dubliniensis.
Conclusion
In summary, analysis of the data obtained from this study demonstrated that the combination of TM + AmB could display the potential to inhibit the growth of Candida spp. and has the ability to synergize against C. dubliniensis. The synergistic effect offers the possibility to decrease the mannoprotein and ergosterol level by suppressing the expression of ERG3 and MP65 gene. Further studies are warranted to explore the mechanism behind the antifungal activity of tunicamycin and amphotericin B.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Beck-Sague C , Jarvis WR . Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. National nosocomial infections surveillance system. J Infect Dis. 1993;167:1247–1251.
- George ME , Sofia P , Thomas F. Patterson antifungal resistance in pathogenic fungi. Clin Infect Dis. 2002;35:1073–1080.
- Gupte M , Kulkarni P , Ganguli B . Antifungal antibiotics. Appl Microbiol Biotechnol. 2002;58(1):46–57.
- Pierce CG , Thomas DP , López-Ribot JL . Effect of tunicamycin on Candida albicans biofilm formation and maintenance. J Antimicrob Chemother. 2009; 63(3):473–479.
- Perea S , López-Ribot JL , Kirkpatrick WR , et al. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2001;45:2676–2684.
- Sanglard D , Ischer F , Koymans L , et al. Amino acid substitutions in the cytochrome P-450 lanosterol 14α-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. J Antimicrob Chemother. 1998;42:241–253.
- Sheikh N , Jahagirdar V , Kothadia S , et al. Antifungal drug resistance in Candida species. Eur J Gen Med. 2013;10:254–258.
- Sandini S , Stringaro A , Arancia S , et al. The MP65 gene is required for cell wall integrity, adherence to epithelial cells and biofilm formation in Candida albicans . BMC Microbiol. 2011;11:106.
- Onyewu C , Heitman J . Unique applications of novel antifungal drug combinations. Anti-Infect Agents Med Chem. 2007;6(1):3–15.
- Morio F , Pagniez F , Lacroix C , et al. Amino acid substitutions in the Candida albicans sterol Δ5, 6-desaturase (Erg3p) confer azole resistance: characterization of two novel mutants with impaired virulence. J Antimicrob Chemother. 2012;67:2131–2138.
- National Committee for Clinical Laboratory Standards . Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard NCCLS document M27-A2. Wayne PA NCCLS. 2002;22.
- Hudzicki J . Kirby-Bauer disk diffusion susceptibility test protocol. Am Soc Microbiol. 2009.
- Jeong N , Kim JY , Park SC , et al. Antibiotic and synergistic effect of Leu–Lys rich peptide against antibiotic resistant microorganisms isolated from patients with cholelithiasis. Biochem Biophys Res Commun. 2010;399:581–586.
- Pfaffl MW . A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45.
- Nordin MAF, Harun WHAW , Razak FA , et al. Growth inhibitory response and ultrastructural modification of oral-associated candidal reference strains (ATCC) by Piper betle L. extract. Int J Oral Sci. 2014;6:15–21.
- Keele DJ , DeLallo VC , Lewis RE , et al. Evaluation of amphotericin B and flucytosine in combination against Candida albicans and Cryptococcus neoformans using time-kill methodology. Diagn Microbiol Infect Dis. 2001;41:121–126.
- Morace G , Sanguinetti M , Posteraro B , et al. Identification of various medically important Candida species in clinical specimens by PCR restriction enzyme analysis. J Clin Microbiol. 1997;35:667–672.
- Miyazaki T , Kohno S . ER stress response mechanisms in the pathogenic yeast Candida glabrata and their roles in virulence. Virulence. 2014;5:365–370.
- Boyce M , Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 2006;13:363–373.
- Criscuolo BA , Krag SS . Selection of tunicamycin-resistant Chinese hamster ovary cells with increased N-acetylglucosaminyltransferase activity. J Cell Biol. 1982;94:586–591.
- Walter P , Ron D . The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086.
- Martel CM , Parker JE , Bader O , et al. Identification and characterization of four azole-resistant erg3 mutants of Candida albicans. Antimicrob Agents Chemother. 2010;54:4527–4533.