ABSTRACT
Vulvovaginal candidiasis (VVC) is the most common vaginal infection. It is considered to be the reason for 15%–30% of all vulvovaginal symptoms. A diagnosis based only on clinical signs and/or on a syndrome-based approach, recommended by some authors and health institutions, is in many cases incorrect. There is no indisputable diagnostic method that guarantees high specificity, sensitivity and predictive value. There is no pathognomonic clinical feature of VVC. The aim of the present study was to assess the role of selected clinical and mycological indicators in the diagnosis of VVC using the standard complex scoring system. The research is retrospective and was conducted in several stages to give a more precise and detailed assessment of the examined clinical and microbiological indicators. Clinical, microbiological and statistical methods were used. The results showed the lowest level of compliance between clinical and laboratory diagnosis in VVC: 67.65%. We did not observe a statistically significant relation between the density of the vaginal smear, inflammation, odour test and VVC (p > 0.05). The results showed a moderate association between the positive yellow swab test and VVC (p < 0.05). A significant positive association was established between VVC and presence of microscopic signs of invasiveness (p < 0.001). The most common isolate was Candida albicans: 72.81% of all vaginal Candida isolates.
KEYWORDS:
Introduction
Vulvovaginal candidiasis (VVC) is a common vaginal infection (VI) depending on the characteristics of the examined population and the geographical region in which the research is conducted [Citation1–6]. It is considered to be the cause of 15%–30% of all vulvovaginal symptoms [Citation7,Citation8]. The condition often occurs during pregnancy [Citation2,Citation7]. There are also a number of infections accompanying candidiasis [Citation9]. The diagnosis based only on clinical signs and/or on a syndrome-based approach, although recommended by certain authors and health institutions, is in many cases incorrect. There is no indisputable diagnostic method that guarantees high specificity, sensitivity and predictive value. There is no pathognomonic clinical feature of VVC. The lack of fast, simple and inexpensive diagnostic tests continues to result in misdiagnosis [Citation3,Citation10,Citation11]. Unfortunately, some specialists still comply with the understanding of a significant diagnostic advantage of the macroscopic view of the so-called ‘typical’ vaginal discharge and bloodshot mucosa. Targeted research over the past years proved that it is unreasonable to rely on clinical characteristics. A significant problem for clinicians is the differential diagnosis versus cytolytic vaginosis (CV) and herpetic vulvovaginitis [Citation12,Citation13]. Evidence accumulated over the past two or three decades demonstrates the advantages of quantitative mycological indicators in VVC diagnosis and the need for a complex scoring system for assessment. According to Odds et al. [Citation14–16], low-level positive fungal cultures do not correlate well with VVC diagnosis. It is well known that wet mounts and microscopic staining techniques for examination of vaginal discharge have low sensitivity in differentiating between fungal infection and fungal colonization [Citation11]. Moreover, many women, more often than not, resort to self-treatment [Citation17]. The statements on the role of the polymorphonuclear inflammatory response as a criterion for a vaginal fungal infection are highly contradictory [Citation5,Citation6,Citation18–20]. Judging by practice, even a microscopic finding of mycological morphological elements (budding, hyphae, pseudohyphae and germ tubes) is not reliable enough. Many Candida species are not able to form such elements for different reasons. Even in cases in which there are mycological elements present, they are not always seen on the microscopic preparation. The reason is that a number of factors that alkalize the vaginal environment also inhibit hyphae formation, while this is considered a certain sign of invasion and infection. There is a large body of data about the effect of such inhibiting factors in the vagina, e.g. blood, semen, spermicides, cosmetic products, estrogen levels, etc. The clinical practice, therefore, needs a complex, quantitative diagnostic approach that should include clinical (subjective and objective) as well as mycological (microscopic and cultural) indicators [Citation11].
The aim of the present study was to assess the role of some clinical and mycological indicators (density rate, odour test, yellow test and inflammation) in diagnosing VVC using the standard complex scoring system.
Subjects and methods
This retrospective study was conducted in several stages to make a more precise and detailed assessment of the examined clinical and microbiological indicators.
Patients and stages of research
Stage I
In this stage, we defined the common compliance levels between the clinical (only based on symptoms and signs) and microbiological diagnoses, as well as the level of compliance between the clinical and final microbiological diagnoses in cases of VVC, bacterial vaginosis (BV), trichomoniasis and other cervicovaginal infections (OCVI). A total of 98 non-pregnant women in reproductive age, patients of the specialized clinic for obstetrics and gynaecology for pre-hospital medical care at Consultative Diagnostic Center (CDC)–5, Plovdiv, with chronic vaginal complaints were examined. The patients were with various vaginal symptoms and with clinical findings of VI. In the primary data collection sheet (PDCS), the clinician was asked to make a preliminary note of the most probable clinical diagnosis according to them, based only on data about the medical history, physical status, examination with speculum and microscopic characteristics of the vaginal discharge of the patient. The diagnoses based on symptoms registered by the clinician in the PDCS were limited to two. A microbiological examination according to the original and the modified quantitative methods described below was held after the clinical examination.
Stage II
In the second stage, we evaluated the correlation between a number of clinical characteristics (density rate of the vaginal smear, yellow test, clinical signs of inflammation and odour test) and infectious vaginitis and vaginoses that are most prevalent. A total of 280 vaginal smears of non-pregnant, non-menopausal women with chronic vaginal symptoms, patients of the specialized clinic for obstetrics and gynaecology at CDC–5, Plovdiv, were examined. To this end, the clinician, in advance and independently of the laboratory, registered the studied clinical characteristics observed during the examination in the PDCS. Standard templates used for comparison and evaluation of microscopic indicators, such as ‘density of the vaginal smear’ and ‘yellow test’, were determined in advance.
Stage III
A total of 607 women with clinical and microbiological signs of VVC, patients of the specialized clinic for obstetrics and gynaecology (experimental group), were examined, as well as 1524 women without clinical or microbiological signs of VVC (control group). The aim, at this stage, was to identify the potential association between microscopic signs of invasiveness and the degree of polymorphonuclear inflammatory response with VVC.
Clinical methods
We used the medical history data (subjective complains) like the macroscopic characteristics of the vaginal discharge, vulvovaginal pruritus, external dysuria and superficial dyspareunia. The objective gynaecological status included inspection of the vulvovaginal area, genitalia and perineum; examination of the pelvic organs; examination with speculum; other clinical and gynaecological examinations and transvaginal ultrasound.
Standard high vaginal cotton swabs were taken. The yellow swab test was performed as described by Donders et al. [Citation21]. Briefly, the test was considered positive if a high vaginal cotton swab taken for culture appeared clearly yellow when held in front of a white paper towel. Non-convincing slight yellowish coloration was scored as ‘intermediate’ [21].
The odour test was based on the subjective sensation of a ‘foul smell’ [Citation5,Citation21,Citation22].
Microbiological (quantitative and qualitative) methods
Microscopic examination included Gram staining and methylene-blue staining (Löffler's solution) of vaginal smears. The results were assessed according to the scoring system of Nugent et al. [Citation22] for BV; the scoring system of Donders et al. [Citation21] adapted for aerobic vaginitis (AV); the original complex scoring system for VVC [Citation23]; a complex of microbiological criteria for diagnosing CV [Citation12], presence of long serpiginous lactobacilli at vaginal lactobacillosis (VLB) and trichomonal vaginitis (TV).[Citation24,Citation25]
The density rate indicates the density of the vaginal smear. It was scored on the basis of three pre-prepared standards as low rate (1+), moderate (2+) or high rate (3+). The density was reported by two independent experts.
The culture conditions for primary isolation and identification of clinically important aerobes and facultative anaerobic microorganisms included inoculation on 5% blood agar, eosin methylene-blue agar, CHROMagar™ Candida (Liofilchem Diagnostica, Roseto degli , Italy) and thioglycollate medium.
Identification was done using rapid tests for presumptive diagnosis of significant aerobic isolates of vaginal secretion (VS): catalase, oxidase production, odour test, type of haemolysis; biochemical tests (including urease and nitrate); CAMP test; plasmocoagulase tube test; in vitro tests for determination of sensitivity to certain antimicrobial agents (optochin, bacitracin, ampicillin, trimethoprim, cefoxitin, ciprofloxacin, vancomycin); immunochromatographic test (T. vaginalis); presumptive identification of clinically significant fungal isolates from cultures of CHROMagar™ Candida; assimilation tests for precise identification of Candida spp. and the most common types of yeast (аpi® 20C AUX, bioMérieux SA, Marcy I Etoile, France) with follow-up result assessment after 48 and 72 h of incubation at 30 °C.
Data analysis
Descriptive statistics included analysis of qualitative variables (relative part with standard deviation (±SD)). Chi-squared test (with statistical power, Sp ) and correlation analysis were also performed. Statistical analysis was done using SPSS v13.0 for Windows.
Results and discussion
Compliance between preliminary clinical and final microbiological diagnosis
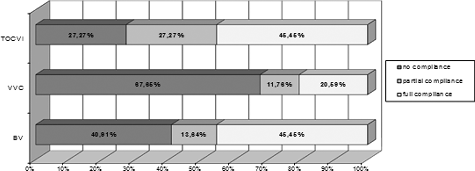
The levels of compliance between the preliminary clinical diagnosis and the final one confirmed by microbiological examination are shown in . There was full compliance only in 36 (36.73%) of the patients who participated in Stage I of the study.
Table 1. Levels of compliance between the clinical diagnosis (based on symptoms and signs) and the microbiological diagnosis.
In this study, the diagnosis based only on data from clinical examination and medical history was incorrect in 46% of the cases. The lowest level of compliance was observed in VVC (67.65% lack of compliance), followed by BV and the group of TV/OCVI, with 40.91% and 27.27% lack of compliance, respectively (). This study showed that there was the lowest level of compliance between diagnosis based only on clinical findings and quantitative mycological diagnosis in VVC (20.6%), as opposed to BV and TV/OCVI (45.45%).
Figure 1. Levels of compliance between clinical and microbiological diagnosis in the most common vaginitis and vaginosis.
According to Schwiertz et al. [Citation26], the errors in the diagnosis of BV and VVC are high. They reported that the cases of misjudgement of VVC (77%) are more numerous than those of BV (61%).
Correlation between the density of the vaginal smear and vaginal infections
Positive association (p < 0.05) was observed between higher density of the vaginal smear and cases of BV and CV (). In our previous study [Citation27], we did not find a connection between the density of the vaginal smear in VLB, AV or VVC (p > 0.05). The latter observation is in contrast to the understanding of a vast group of clinical practitioners who believe that VVC is always associated with dense, ‘curd-like’ vaginal discharge [Citation5,Citation10,Citation13].
Table 2. Density of vaginal smears.
Association between yellow test and vaginal infections
Our previous results [Citation27] showed strong association between a positive yellow test result and cases of AV (p < 0.001), a moderate association between a positive yellow test and VLB, CV and VVC (p < 0.05), and no association between the yellow test result and BV (p > 0.05). Donders et al. [Citation21] have reported similar findings.
Association between signs of inflammation and vaginal infections
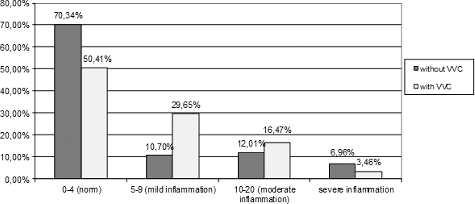
A statistically significant difference was shown between the signs of inflammation in the group with CV and the control group (р < 0.05). In the experimental group, there were CV signs of inflammation in only 7.69% of the cases versus 22.12% in the control group. The study showed a statistically significant relation between AV and the presence of inflammation (p < 0.01). There were objective signs of inflammation in 35.14% of the patients in the experimental group with AV, whereas, in the control group, there were registered signs of inflammation in only 12.5%. In BV, VVC and VLB cases, a statistically significant difference between the study group and the controlled group of patients was not established (p > 0.05) [Citation27].
Among experts, there is no consensus on the diagnostic significance of inflammatory signs for VVC. Eckert et al. [Citation8] believe that the establishment of clinical signs of inflammation during the examination is significant for VVC diagnosis. Other authors recognize that the clinical symptoms are non-specific and do not allow to distinguish VVC with certainty from other types of vaginitis and vaginosis [Citation28–30].
Association between odour test and vaginal infection
Our previous results [Citation23,Citation27] confirmed significant association between BV and positive odour test (p < 0.05). No statistically significant relation was established between positive odour test and VLB, CV, AV and VVC (p > 0.05) [Citation27].
The role of the whiff test (a test which is performed by adding 10% solution of KOH on the slide with vaginal discharge) in BV diagnosis is well known and often discussed, but specialists rarely make reference to the odour test, and even less to the difference between these two subjective clinical signs, the whiff test and the odour test.
Aetiology of VVC and association with microscopic signs of invasiveness and the degree of polymorphonuclear inflammatory reaction
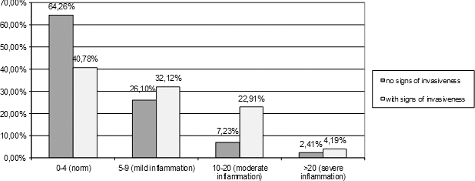
According to our previous study [Citation23], the most common isolate was Candida albicans: 72.81% of all vaginal Candida isolates (383/526). Samal et al. [Citation28] reported a 72.58% rate of prevalence of C. albicans and 27.42% of non-albicans Candida spp. C. albicans is the most common isolate in patients with VVC, according to other reports as well [Citation31,Citation32]. In our study, the analysis of the data from Stage III of the study showed positive association between VVC and the presence of microscopic signs for invasiveness (budding, germ tubes, hyphae/pseudohyphae) assessed by Gram and Löffler staining (r = 0.120; p < 0.001) (). There was also positive association between VVC and no and/or low-degree inflammatory reaction of polymorphonuclear cells (r = 0.156; p < 0.001) ().
Many practitioners [Citation18,Citation19,Citation33,Citation34] believe that VVC is always accompanied with strong polymorphonuclear inflammatory reaction, but this concept was not confirmed by the results in our study. De Souza Bonfim-Mendonça et al. [Citation35] comment that, in VVC episodes, neutrophils are known to be markedly recruited to the site of infection and play a key role in the host defence by killing microbes [Citation36]. We observed moderate and severe inflammation, respectively, in only 16.47% and 3.46% of the women with VVC ().
There are no dependable clinical characteristics to support the diagnosis of VVC. Such problems adversely affect the medical/diagnostic process and the reproducibility of results from laboratory and clinical diagnosis of vaginal candidiasis. Solving these problems is essential to improvement of the quality of diagnosis and the treatment effect. We suggest that there is a need to change the way some diagnostic criteria of VVC are interpreted and evaluated. VVC diagnosis calls for a complex examination and should be based on mandatory clinical and quantitative mycological indicators: discharge, pruritus, microscopic morphological yeast cells and positive yeast culture.
Conclusions
This study showed that there was lowest level of compliance between diagnosis based only on clinical findings and quantitative mycological diagnosis in VVC (20.6%), as opposed to BV and TV/OCVI (45.45%). No statistically significant difference was established between VVC and higher density of the vaginal smear, objective signs of inflammation and positive odour test (p > 0.05). No positive association was found between VVC and high-degree inflammatory reaction of polymorphonuclear cells in Gram and Löffler staining. Since there are still no dependable clinical characteristics to support the diagnosis of VVC, we suggest that it should be based on complex examination of mandatory clinical and quantitative mycological indicators.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Boselli F , Chiossi G , Garutti P , et al. Preliminary results of the Italian epidemiological study on vulvovaginitis. Minerva Ginecol. 2004;56:149–153.
- Hernandez B , Boza A , Leon E , et al. Sexually transmitted diseases in pregnancy. Rev Cub Obstet Ginecol. 1998;24:28–33.
- Kantardjiev T. Etiological diagnosis and etiotropic treatment of mycoses. Sofia :NCIPD; 2012. Bulgarian. ISBN 978-954-92298-3-7.
- Kent HL . Epidemiology of vaginitis. Am J Obstet Gynecol. 1991;165:1168–1176.
- Egan ME , Lipsky MS . Diagnosis of vaginitis. Am Fam Phys. 2000;62:1095–1104.
- Kuzmanov A , Filipova I , Ivanova Z , et al. [Vaginal candidiasis and other infections of women's genital tract]. Medicart. 2014;8:26–28. Bulgarian.
- Berg AO , Heidrich FE , Fihn SD , et al. Establishing the cause of genitourinary symptoms in women in a family practice. Comparison of clinical examination and comprehensive microbiology. JAMA. 1984;251:620–625.
- Eckert LO , Hawes SE , Stevens CE , et al. Vulvovaginal candidiasis: clinical manifestations, risk factors, management algorithm. Obstet Gynecol. 1998;92:757–765.
- Redondo-Lopez V , Meriwether C , Schmitt C , et al. Vulvovaginal candidiasis complicating recurrent bacterial vaginosis. Sex Transm Dis. 1990;17:51–53.
- Sobel J. Vulvovaginal candidosis. Lancet. 2007;369:1961–1971.
- Spiegel CA. Vaginitis/vaginosis. Clin Lab Med. 1989;9(3):525–533.
- Cibley LJ , Cibley LJ , Baldwin D . Diagnosing candidiasis, a new cost-effective technique. J Reprod Med. 1998;43:925–928.
- Cibley LJ , Cibley LJ . Cytolytic vaginosis. Am J Obstet Gynecol. 1991;165:1245–1249.
- Odds F , Webster C , Riley V , et al. Epidemiology of vaginal Candida infection: significance of numbers of vaginal yeasts and their biotypes. Eur J Obstet Gynecol Reprod Biol. 1987;25:53–66.
- Odds F , Webster C , Mayuranathan P , et al. Candida concentrations in the vagina and their association with signs and symptoms of vaginal candidosis. J Med Vet Mycol. 1988;26:277–283.
- Odds FC , Bernaerts R . CHROMagar Candida, a new differential isolation medium for presumptive identification of clinically important Candida species. J Clin Microbiol. 1994;32:1923–1929.
- Ferris DG , Nyirjesy P , Sobel JD , et al. Over-the-counter antifungal drug misuse associated with patient-diagnosed vulvovaginal candidiasis. Obstet Gynecol. 2002;99:419–425.
- Fidel PL Jr. Immunity in vaginal candidiasis. Curr Opin Infect Dis. 2005;18(2):107–111.
- Sobel JD. Vaginitis: vulvovaginal candidiasis. In: Hillard PJA , editor. The 5-minute obstetrics and gynecology consult. Philadelphia (PA ): Lippincott Wiliams & Wilkins; 2008. p. 208.
- Reece EA , Barbieri RL . Obstetrics and gynecology: the essentials of clinical care. Stuttgart : Georg Thieme Verlag; 2010. Chapter 30, Vaginitis and vulvitis; p. 293–304.
- Donders G , Vereecken A , Bosmans E , et.al. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. BJOG. 2002;109:34–43.
- Nugent RP , Krohn MA , Hillier SL . Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29(2):297–301.
- Dermendzhiev T. Studies on etiology and epidemiology of the infectious vaginitides and vaginosеs [dissertation]. Plovdiv : Medical University of Plovdiv; 2015. p. 160–171.
- Nayar R , Wilbur DC . The Bethesda system for reporting cervical cytology. In: Bibbo M , Wilbur D , editors. Comprehensive cytopathology. 4th ed. Philadelphia ( PA ): Saunders, Elsevier; 2014. p. 70–81.
- Gupta PK , McGrath C . Microbiology, inflammation and viral infections. In: Bibbo M , Wilbur D , editors. Comprehensive cytopathology. 4th ed. Philadelphia ( PA ): Saunders, Elsevier; 2014. p. 82–118.
- Schwiertz A , Taras D , Rusch K , et al. Throwing the dice for the diagnosis of vaginal complaints? Ann Clin Microbiol Antimicrob. [ Internet]. 2006 [ cited 2015 Nov 20];5:4. Available from: http://ann-clinmicrob.biomedcentral.com/articles/10.1186/1476-0711-5-4
- Dermendzhiev T , Lazarova G , Stanev S , et al. Associations between four different characteristics and vaginitides/vaginoses in women with chronic vaginal complaints. Trakia J Sci. 2014;12:233–237.
- Samal R , Vaithy A , Kotasthane D , et al. Prevalence of clinic-mycological profile of vulvovaginal candidiasis in tertiary care hospital. Int J Reprod Contracept Obstet Gynecol. 2015;4:1142–1147.
- Anderson MR , Klink K , Cohrssen A . Evaluation of vaginal complaints. JAMA. 2004;291:1368–1379.
- Landers DV , Wiesenfeld HC , Heine RP , et al. Predictive value of the clinical diagnosis of lower genital tract infection in women. Am J Obstet Gynecol. 2004;190:1004–1010.
- BC Centre for Disease Control . Non-certified practice decision support tool: vulvovaginal candidiasis. Vancouver : Provincial Health Services Authority; c2016 [ revised 2014 Dec; cited 2015 Nov 20]. Available from: http://www.bccdc.ca/resource-gallery/Documents/Communicable-Disease-Manual/Chapter%205%20-%20STI/STI_DST_NonCertified_VVC_20141210.pdf
- Živadinović R , Petrić A , Krtinić D. Vaginal candidiasis – gynecological aspect of the problem. Acta Med Medianae. 2014;53(4):46–53.
- Fidel PL . History and new insights into host defense against vaginal candidiasis. Trends Microbiol. 2014;12(5):220–227.
- Ratti BA , Godoy JS , de Souza Bonfim Mendonça P , et al. Microbicidal activity of neutrophils is inhibited by isolates from recurrent vaginal candidiasis (RVVC) caused by Candida albicans through fungal thioredoxin reductase. Cell Immunol. 2015;293:22–29.
- de Souza Bonfim-Mendonça P , Ratti BA , Godoy JR , et al. Beta-glucan induces reactive oxygen species production in human neutrophils to improve the killing of Candida albicans and Candida glabrata isolates from vulvovaginal candidiasis. PLoS One [Internet]. 2014 [ cited 2016 Aug 11];9(9):e107805. Available from: http://journals.plos.org/plosone/article?id=10.1371%2Fjournal.pone.0107805
- Fidel PL Jr , Cutler JE . Prospects for development of a vaccine to prevent and control vaginal candidiasis. Curr Infect Dis Rep. 2011;13:102–107.