ABSTRACT
Generally, uterine leiomyosarcoma is easily diagnosed. However, uterine smooth muscle tumours which show atypical histological features and unusual growth patterns may mimic malignancy and may not be easily diagnosed. In this study, our aim is to show the expressions of Aquaporin3, Aquaporin7 and Aquaporin9 in uterine smooth muscle tumours, and to investigate if aquaglyceroporins can be used as diagnostic and prognostic markers to start rapidly an appropriate treatment for patients with these tumours in order to extend the survival time. We determined that there had been 74 patients diagnosed with uterine smooth muscle tumours. We divided patients into four groups based on the diagnosis: bizarre leiomyoma, smooth muscle tumour of uncertain malignant potential, leiomyosarcoma and leiomyoma. Aquaporin3, Aquaporin7 and Aquaporin9 were detected by using monoclonal anti-Aquaporin3, anti-Aquaporin7 and anti-Aquaporin9 antibodies, respectively. In leiomyosarcoma group, we observed a statistically significant relation of Aquaporin3 expression with survival time, grade, stage, mitotic index and Ki-67 score. A significant relation of both Aquaporin7 and Aquaporin9 expressions with survival time, grade, stage was not statistically detected in leiomyosarcoma group. The decrease of Aquaporin3 expression can be used as important diagnostic and prognostic marker. Aquaporin7 and Aquaporin9 expressions cannot be used as diagnostic and prognostic markers.
Introduction
Uterine leiomyomas are the most common cause of solid pelvic masses in women of reproductive age, forming 20%–40% of cases [Citation1]. On the other hand, leiomyosarcoma (LMS) is a rare type of malignant tumour of the uterus, which accounts for 1%–3% of female malignancies [Citation2]. Uterine LMS recurs frequently and has a poor prognosis; nearly 35% of the five-year survival rate depends on tumour staging [Citation3]. A method which is a combination of microscopic features is sufficient to differentiate between uterine LMS and leiomyoma that includes presence of necrosis, degree of cytological atypia, mitotic activity as well as the relationship of the tumour with surrounding normal structures. Atypical bizarre leiomyoma (BLM) can be identified with this method, but these tissue samples may be diagnosed as malignancy inadvertently. These kinds of tissue samples are subsequently diagnosed as smooth muscle tumour of uncertain malignant potential (STUMP). Cellular morphology should not be analysed with only haematoxylin–eosin stained slides by light microscopy because it may lead to erroneous and inconclusive diagnoses. Many immunohistochemical markers, such as P16, P21, CD44 and Ki67, were investigated to improve differential diagnosis of all uterine smooth muscle tumours [Citation4–6].
Aquaporins (AQPs) are a class of small integral membrane proteins distributed widely in organisms, and 13 members (AQP0–12) of them have been identified in mammals [Citation7,Citation8]. Recently, it has been established that AQPs play key roles in tumour pathogenesis. Since the involvement of AQPs is obtained in cell migration and proliferation, AQPs are added into an expanding list of effectors in tumour biology [Citation9–11]. A subset of the AQP family, the aquaglyceroporins (AQP3, AQP7 and AQP9), transports both water and glycerol. It has been reported that, in humans and rodents, many tumour cells express AQPs. For some tumours, there are positive correlations between histological tumour grade and the amount of AQP expression as for AQP4 expression in diffuse astrocytomas [Citation11].
In this study, our aim is to show the expressions of AQP3, AQP7 and AQP9 in uterine smooth muscle tumours and to investigate if aquaglyceroporins can be used as diagnostic and prognostic markers to start rapidly the appropriate treatment for patients with these tumours in order to extend the survival time.
Materials and methods
Tissue collection and examination
We determined that there had been 74 patients diagnosed with uterine smooth muscle tumours between 2003 and 2014 in archives of the Pathology Department of the Medical Faculty, Dicle University. Patients were divided into four groups based on the diagnosis: BLM (n = 6), STUMP (n = 16), LMS (n = 26) and leiomyoma (n = 26). Information regarding patient age, post-operative treatment and clinical follow-up was retrieved from the Department of Gynaecology and Obstetrics and the Department of Medical Oncology. All uterine smooth muscle tumours were staged again according to the Federation of Gynecology and Obstetrics (FIGO) staging system, which was revised in 2009 [Citation12].
Uterine smooth muscle tumour sections stained with haematoxylin and eosin were re-examined to confirm diagnosis. Mitotic figures per 10 consecutive high-power fields (HPFs) were counted in only the most mitotically active areas. Cellular morphology was analysed microscopically to determine the extent and presence of nuclear atypia, coagulative tumour cell necrosis and vascular invasion. Tumour type and histopathological grade were assessed via the 2003 World Health Organization classification of tumours of the breast and female genital organs [Citation13]. LMS diagnosis was confirmed if the tumour demonstrated at least two of the following features: mitotic index ≥10 per 10 HPFs, moderate to severe cytological atypia and tumour cell necrosis [Citation14]. Criteria for diagnosis of BLM were as follows: (1) certain smooth muscle cell type; (2) presence of pleomorphic multinucleated giant tumour cells (containing at least 5% of the tumour) and (3) mitotic index <10 per 10 HPFs [Citation15]. While tumours with focal nuclear atypia which was -moderate to severe, a mitotic index <15 per 10 HPFs and the absence of tumour cell necrosis were classified as STUMP [Citation16].
Immunohistochemical staining
Four-micrometre sections were prepared from routinely processed paraffin blocks and mounted on positively charged slides. Sections were incubated at 57 °C for 60 minutes to remove the paraffin and rehydrate the samples. Immunohistochemical staining was performed with the automated Bench Mark XT immunohistochemical system (Ventana, AZ, USA). AQP3, AQP7 and AQP9 were detected by using a monoclonal anti-AQP3 antibody (Abcam, ab125219), anti-AQP7 antibody (Abcam, ab85907) and anti-AQP9 antibody (Abcam, ab85910).
Immunohistochemical staining assessment was modelled from the study by Özler et al. [Citation17]. Intensity and extent of staining were evaluated and scored for each sample. The distribution of AQP3, AQP7 and AQP9 immunoreactivity was semi-quantitatively scored by using a 0–4 scale for the percentage of stained cells. A score of 0 represented none to <5% of cells stained, 1+ was 6%–25% of cells stained, 2+ was 26%–50% of cells stained, 3+ was 51%–75% of cells stained and 4+ was 76%–100% of cells stained. Immunohistochemical staining intensity was graded from 0 to 3, with 0 as none, 1 as weak, 2 as moderate and 3 as strong. The combined scores were calculated by multiplying the extent and intensity scores. Finally, the combined scores were graded as: negative = 0, weak = 1 or 2, moderate = 3 or 4 and strong = 5–7.
Statistical method
The Chi square test and Fisher exact test were used to compare AQP3, AQP7 and AQP9 expressions between each uterine smooth muscle tumour type. Kolmogorov–Smirnov test was used to evaluate the data's distribution pattern. Kruskal–Wallis test was used for multiple comparisons of the combined scores among groups. For pairwise comparisons of the combined scores between groups, the Mann–Whitney test was used with the Bonferroni correction. The effects of AQP3, AQP7 and AQP9 expressions, clinical and pathological characteristics on general survival time of the patients were evaluated by the Kaplan–Meier method in univariate analysis and by the Cox regression method in multivariate analysis. Correlation coefficients were determined by the Spearman's rank correlation test. SPSS 18.0 statistical package (SPSS Inc., Chicago, IL, USA) was used to perform all calculations, and p values less than 0.05 were considered statistically significant.
Results and discussion
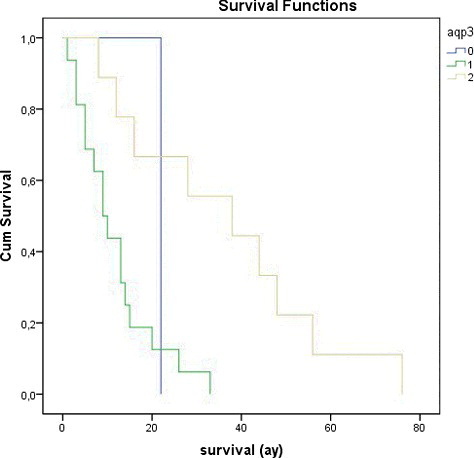
LMS is a rare malignancy of uterus that arises from the smooth muscle of uterine wall [Citation18]. Women patients with LMS mostly have no symptoms or a rapidly enlarging pelvic mass [Citation19]. Usually, the LMS is diagnosed by pathological examination of hysterectomy or myomectomy specimen [Citation20]. The rate of LMS in hysterectomies performed for presumed uterine leiomyomas is approximately 0.1%–0.3% [Citation21]. Overall, uterine LMS is easily diagnosed, thanks to histopathological features such as severe nuclear atypia, high mitotic rate usually exceeding 10 mitotic figures per 10 HPFs, necrosis and atypical mitotic figures. For uterine smooth muscle tumours which show atypical histological features and unusual growth patterns, the benign conditions like leiomyoma variants and STUMP should be included as differential diagnosis since they may mimic malignancy [Citation22]. In our study, we reviewed 74 patients with uterine smooth muscle cell tumour. Twenty-six (35.13%) of all uterine smooth muscle cell tumours were LMS ((a)), 16 (21.63%) of them were STUMP ((a)), 6 (8.11%) of them were BLM ((a)) and 26 (35.13%) of them were leiomyoma ((a)). The average age of the patients is 44.79 ± 11.83 years. Seventeen (65.4%) of LMS were grade 3, seven (26.9%) of them were grade 2 and two (7.7%) of them were grade 1. Seventeen (65.4%) of the LMS patients were stage 4, three (11.53%) of them were stage 3 and six (23.07%) of them were stage 1. Survival time of LMS patients varied between 1–76 months and average survival time was 20.53 ± 18.61. The clinical, histological and prognostic features of the patients are given in . A statistically significant relationship was observed to exist between Ki-67 score, mitotic index and survival time in all groups (p < 0.05). The immunohistochemical results are given in
Figure 1. (a) Histomorphological appearance of the leiomyosarcoma (H&E, 200×). (b) Weak expression of AQP3 in leiomyosarcoma tissue (Immunoperoxidase, 200×). (c) Strong expression of AQP7 in leiomyosarcoma tissue (Immunoperoxidase, 200×). (d) Moderate expression of AQP9 in leiomyosarcoma tissue (Immunoperoxidase, 200×).
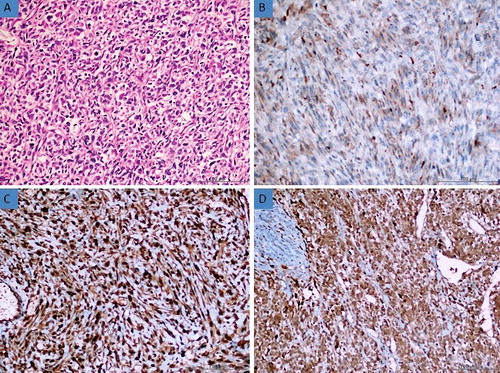
Figure 2. (a) Histomorphological appearance of the smooth muscle tumour of uncertain malignant potential (STUMP) (H&E, 200×). (b) Moderate expression of AQP3 in STUMP tissue (Immunoperoxidase, 200×). (c) Strong expression of AQP7 in STUMP tissue (Immunoperoxidase, 200×). (d) Strong expression of AQP9 in STUMP tissue (Immunoperoxidase, 200×).
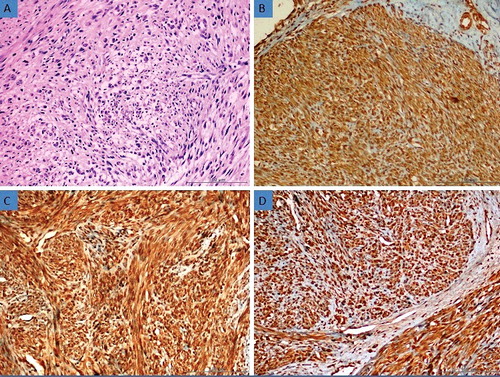
Figure 3. (a) Histomorphological appearance of the bizarre leiomyoma (H&E, 200×). (b) Strong expression of AQP3 in bizarre leiomyoma tissue (Immunoperoxidase, 200×). (c) Strong expression of AQP7 in bizarre leiomyoma tissue (Immunoperoxidase, 200×). (d) Strong expression of AQP9 in bizarre leiomyoma tissue (Immunoperoxidase, 200×).
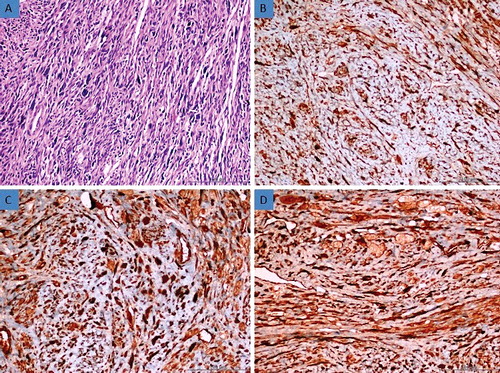
Figure 4. (a) Histomorphological appearance of the usual leiomyoma (H&E, 200×). (b) Strong expression of AQP3 in leiomyoma tissue (Immunoperoxidase, 200×). (c) Strong expression of AQP7 in leiomyoma tissue (Immunoperoxidase, 200×). (d) Strong expression of AQP9 in leiomyoma tissue (Immunoperoxidase, 200×).
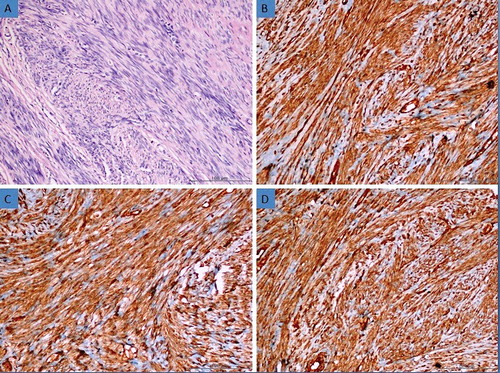
Table 1. The clinical, histological and prognostic features of the patients.
Table 2. The immunohistochemical results.
AQPs are transmembrane proteins which play a very important role in transcellular and transepithelial water movement [Citation23]. AQP1, AQP2, AQP4, AQP5 and AQP8 are primarily water-selective, whereas AQP3, AQP7, AQP9 and AQP10 called aquaglyceroporins also transport glycerol and other small solutes [Citation24]. Recently, it has been reported that aquaglyceroporins are expressed in many tumour cells [Citation11].
AQP3 is a typical aquaglyceroporin that plays a major role in fluid homeostasis in normal tissues [Citation25]. Recently, it has been shown that AQP3 expression might be related to development of several carcinomas including gastric carcinoma [Citation26,Citation27], oesophageal and oral squamous cell carcinoma [Citation28], cervical carcinoma [Citation29] and cancers of the thyroid [Citation30], pancreas [Citation31], prostate [Citation32,Citation33], ovary [Citation34] and skin [Citation35,Citation36]. In our study, AQP3 expression was observed in all tissue samples examined in LMS group except in one case. Weak AQP3 expressions were detected in 16 (61.53%) of the LMS ((b)) and moderate AQP3 expressions in 9 (34.62%) of the LMS. Strong staining with AQP3 was not detected in any of the LMS cases. AQP3 expression was observed in all tissue samples examined in STUMP, BLM and leiomyoma groups. Of the STUMP cases, weak AQP3 expressions were detected in 2 (12,5%), moderate in 12 (75%) and strong in 2 cases (12.5%) ((b)); however, of the BLM cases, moderate AQP3 expressions were observed in three (50%), and strong AQP3 expressions in three cases (50%) ((b)). In 5 (19.23%) of leiomyoma, moderate, and in 21 (80.77%), strong ((b)) AQP3 expressions were detected. In none of the BLM and the leiomyoma, weak staining with AQP3 was determined. In some of the pairwise comparisons of the groups, a statistically significant relationship of AQP3 expressions was detected (p < 0.05) (). In some of the LMS, in which AQP3 expressions increased, survival time rose as well, suggesting a statistically significant relationship between AQP3 expression and survival time (p < 0.05) (). At the same time, a significant relationship was statistically found between AQP3 expression and grade, stage, mitotic index and Ki-67 score in LMS (p < 0.05). Thus, we can say that this lack of expression can be used as an important marker in differential diagnosis of LMS. Additionally, we think that the decrease of AQP3 expression could be used as an important prognostic marker.
Table 3. p Values for the comparison of uterine smooth muscle tumours in terms of AQP3 combined scores.
AQP7 is considered to represent the gateway for the efflux of lipolysis-derived glycerol from adipocytes, whereas AQP9 is considered to play a role in the influx of circulating glycerol into the hepatocytes [Citation37]. Lacroix et al. [Citation38] reported that AQP7 expression increases in thyroid follicular carcinoma. Moreover, Warth et al. [Citation39] reported an increase of AQP9 expression in glioma. Jablonski et al. [Citation40] reported decline of AQP9 expression in hepatocellular carcinoma.
In our study, AQP7 expression was observed in all tissue samples examined in all groups. In 8 (30.77%) of the LMS, moderate, and in 18 (69.23%), strong ((c)) AQP7 expressions were detected. In all of the STUMP ((c)), BLM ((c)) and leiomyoma ((c)), strong AQP7 expressions were observed. In none of the pairwise comparison of the groups, any statistically significant relationship of AQP7 expressions was detected (p > 0.05). Any statistically significant relationship was not detected between AQP7 expression and survival time, grade, stage, mitotic index and Ki-67 score in LMS (p > 0.05). AQP9 expression was observed in all tissue samples examined in all groups. In 15 (57.69%) of the LMS, moderate, and in 11 (42.31%), strong ((d)) AQP9 expressions were detected. In all of the STUMP ((d)), BLM ((d)) and leiomyoma ((d)), strong AQP9 expressions were observed. As for pairwise comparison of the groups, any statistically significant relationship of AQP9 expressions was detected in none of them (p > 0.05). Any statistically significant relationship between AQP9 expression and survival time, mitotic index and Ki-67 score was not detected in LMS AQP9 (p > 0.05). However, there was a statistically significant relationship obtained between AQP9 expressions and mitotic index and Ki-67 score (p < 0.05). So we can say that AQP7 and AQP9 cannot be used for differential diagnosis of uterine smooth muscle tumours and also they cannot be used as prognostic markers.
Conclusion
In our study, the decrease of AQP3 expression can be used in differential diagnosis of LMS from other uterine smooth muscle tumours, and since this decrease is directly proportional with survival time, it can also be used as a poor prognostic marker in patient follow-up. Although AQP7 and AQP9 are highly expressed in uterine smooth muscle tumours, they cannot be used as diagnostical and prognostic markers.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Wallach EE, Vlahos NF. Uterine myomas: an overview of development, clinical features, and management. Obstet Gynecol. 2004;104:393–406.2
- Tsukada H, Muramatsu T, Miyazawa M, et al. Long term prognostic implications of expression of glucose transporter-1 and hexokinase II in patients with stage I uterine leiomyosarcoma. Acta Histochem Cytochem. 2012;45:147–154.
- Amant F, Coosemans A, Debiec-Rychter M, et al. Clinical management of uterine sarcomas. Lancet Oncol. 2009;10:1188–198.
- Gökaslan H, Türkeri L, Kavak ZN, et al. Differential diagnosis of smooth muscle tumors utilizing p53, pTEN and Ki-67 expression with estrogen and progesterone receptors. Gynecol Obstet Invest. 2005;59:36–40.
- Poncelet C, Walker F, Madelenat P, et al. Expression of CD44 standard and isoforms V3 and V6 in uterine smooth muscle tumors: a possible diagnostic tool for the diagnosis of leiomyosarcoma. Hum Pathol. 2001;32:1190–1196.
- Ünver NU, Acikalin MF, Öner Ü, et al. Differential expression of P16 and P21 in benign and malignant uterine smooth muscle tumors. Arch Gynecol Obstet. 2011;284:483–490.
- Verkman AS, Mitra AK. Structure and function of aquaporin water channels. Am J Physiol Renal Physiol. 2000;278:13–28.
- King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol. 2004;5:687–698.
- Saadoun S, Papadopoulos MC, Hara-Chikuma M, et al. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–792.
- Hu J, Verkman AS. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB J. 2006;20:1892–1894.
- Verkman AS, Hara-Chikuma M, Papadopoulos MC. Aquaporins new players in cancer biology. J Mol Med (Berl). 2008;86:523–529.
- Prat J. FIGO staging for uterine sarcomas. Int J Gynaecol Obstet. 2009;104:177–178.
- Yang C, Wang S, Li CC, et al. Ovarian germ cell tumors in children: a 20-year retrospective study in a single institution. Eur J Gynaecol Oncol. 2011;32:289–192.
- Bell SW, Kempson RL, Hendrickson MR. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am J Surg Pathol. 1994;18:535–558.
- Downes KA, Hart WR. Bizarre leiomyomas of the uterus: a comprehensive pathologic study of 24 cases with long-term follow-up. Am J Surg Pathol. 1997;21:1261–1270.
- Kefeli M, Yildiz L, Kaya FC, et al. Fascin expression in uterine smooth muscle tumors. Int J Gynecol Pathol. 2009;28:328–333.
- Özler A, Evsen MS, Turgut A, et al. CD147 expression in uterine smooth muscle tumors, and its potential role as a diagnostic and prognostic marker in patients with leiomyosarcoma. J Exp Ther Oncol. 2014;10:325–330.
- Kaur K, Kaur P, Kaur A, Singla A. Uterine leiomyosarcoma: a case report. J Midlife Health. 2014;5:202–204.
- Zivanovic O, Leitao MM, Iasonos A, et al. Stage-specific outcomes of patients with uterine leiomyosarcoma: a comparison of the International Federation of Gynecology and Obstetrics and American Joint Committee on Cancer Staging Systems. J Clin Oncol. 2009;27:2066–2072.
- Sait HK, Anfinan NM, El Sayed ME, et al. Uterine sarcoma. Clinico-pathological characteristics and outcome. Saudi Med J. 2014;35:1215–1222.
- Leibsohn S, d'Ablaing G, Mishell DR Jr, et al. Leiomyosarcoma in series of hysterectomies performed for presumed uterine leiomyomas. Am J Obstet Gynecol. 1990;162:968–974.
- D'Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol 2010;116:131–139.
- King IS, Agre P. Pathophysiology of the aquaporin water channels. Annu Rev Physiol. 1996;58:619–648.
- Skowronski MT. Distribution and quantitative changes in amounts of aquaporin 1, 5 and 9 in the pig uterus during the estrous cycle and early pregnancy. Reprod Biol Endocrinol. 2010;8:109.
- Litman T, Sogaard R, Zeuthen T. Ammonia and urea permeability of mammalian aquaporins. Handb Exp Pharmacol. 2009;190:327–358.
- Wang G, Gao F, Zhang W, et al. Involvement of aquaporin 3 in Helicobacter pylori-related gastric diseases. PLoS One. 2012;7:e49104.
- Zhou Y, Wang Y, Wen J, et al. Aquaporin 3 promotes the stem-like properties of gastric cancer cells via Wnt/GSK-3β/β-catenin pathway. Oncotarget. 2016;7:16529–16541.
- Kusayama M, Wada K, Nagata M, et al. Critical role of aquaporin 3 on growth of human esophageal and oral squamous cell carcinoma. Cancer Sci. 2011;102:1128–1136.
- Shi YH, Chen R, Talafu T, et al. Significance and expression of aquaporin 1, 3, 8 in cervical carcinoma in Xinjiang Uygur women of China. Asian Pac J Cancer Prev. 2012;13:1971–1975.
- Niu D, Kondo T, Nakazawa T, et al. Differential expression of aquaporins and its diagnostic utility in thyroid cancer. PLoS One. 2012;7:e40770.
- Liu W, Wang K, Gong K, et al. Epidermal growth factor enhances MPC-83 pancreatic cancer cell migration through the upregulation of aquaporin 3. Mol Med Rep. 2012;6:607–610.
- Ismail M, Bokaee S, Morgan R, et al. Inhibition of the aquaporin 3 water channel increases the sensitivity of prostate cancer cells to cryotherapy. Br J Cancer. 2009;100:1889–1895.
- Chen J, Wang Z, Xu D, et al. Aquaporin 3 promotes prostate cancer cell motility and invasion via extracellular signal-regulated kinase 1/2-mediated matrix metalloproteinase-3 secretion. Mol Med Rep. 2015;11(4):2882–2888.
- Ji C, Cao C, Lu S, et al. Curcumin attenuates EGF-induced AQP3 up-regulation and cell migration in human ovarian cancer cells. Cancer Chemother Pharmacol. 2008;62:857–865.
- Hara-Chikuma M, Verkman AS. Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by targeted aquaporin-3 gene disruption. Mol Cell Biol. 2008;28:326–332.
- Seleit I, Bakry OA, Al Sharaky D, et al. Evaluation of aquaporin-3 role in nonmelanoma skin cancer: an immunohistochemical study. Ultrastruct Pathol. 2015;39:306–317.
- Wakayama Y, Hirako S, Ogawa T, et al. Upregulated expression of AQP 7 in the skeletal muscles of obese ob/ob mice. Acta Histochem Cytochem. 2014;47:27–33.
- Lacroix L, Lazar V, Michiels S, et al. Follicular thyroid tumors with the PAX8-PPAR gamma1 rearrangement display characteristic genetic alterations. Am J Pathol. 2005;167:223–231.
- Warth A, Mittelbronn M, Hulper P, et al. Expression of the water channel protein aquaporin-9 in malignant brain tumors. Appl Immunohistochem Mol Morphol 2007;15:193–198.
- Jablonski EM, Mattocks MA, Sokolov E, et al. Decreased aquaporin expression leads to increased resistance to apoptosis in hepatocellular carcinoma. Cancer Lett. 2007;250:36–46.